Abstract
The efficacy of many chemotherapeutic agents can be attenuated by expression of the anti-apoptotic proteins Bcl-2, Bcl-XL and Mcl-1. Flavopiridol and dinaciclib are cyclin-dependent kinase 7 and 9 inhibitors that transcriptionally inhibit expression of Mcl-1. We have investigated the ability of flavopiridol and dinaciclib to sensitize a part of leukemia cell lines to vinblastine and paclitaxel. Both drugs acutely sensitized most of the leukemia lines to vinblastine, with 100% apoptosis in 4 h. Furthermore, dinaciclib sensitized freshly-isolated chronic lymphocytic leukemia cells to vinblastine. This rapid induction of apoptosis was attributed to vinblastine-mediated activation of JNK because (a) flavopiridol and dinaciclib failed to induce apoptosis when combined with non-JNK activating concentrations of vinblastine; (b) JNK inhibitors suppressed JNK activity and prevented apoptosis; (c) flavopiridol did not potentiate apoptosis induced by paclitaxel which does not activate JNK in these cells; and (d) Jurkat cells failed to activate JNK in response to vinblastine and were not sensitive to combinations of vinblastine and flavopiridol or dinaciclib. The rapid induction of apoptosis by this combination in multiple cell systems but not in normal lymphocytes provides justification for performing a clinical trial to assess the efficacy in patients.
Key words: vinblastine, flavopiridol, dinaciclib, Mcl-1, c-Jun N-terminal kinase
Introduction
Bcl-2 proteins are the key regulators of the mitochondrial (intrinsic) apoptotic pathway with a balance of pro- and anti-apoptotic proteins controlling cell survival. The anti-apoptotic family members, Bcl-2, Bcl-XL and Mcl-1, contribute to carcinogenesis and resistance to anticancer drugs. For example, one of the hallmarks in the development of chronic lymphocytic leukemia (CLL) is an upregulation of the Bcl-2 protein. As a result there is a drive to find new drugs that can effectively target these antiapoptotic Bcl-2 proteins.
The MEK inhibitor PD98059 can suppress Mcl-1 upregulation and sensitize ML-1 leukemia cells to vinblastine-mediated apoptosis.1 The microtubule-dissociating drug vinblastine characteristically induces apoptosis in a mitosis-dependent manner, a process taking greater than 12–24 h depending on cell type. However the combination of vinblastine with a MEK inhibitor induced apoptosis in ML-1 cells in 4 h, with apoptosis occurring in all phases of the cell cycle.2 Targeting Mcl-1 with shRNA also acutely sensitized ML-1 cells to vinblastine suggesting that other drugs that target Mcl-1 may be synergistic with microtubuleinterfering agents in the treatment of leukemia.3 However the MEK inhibitor did not suppress Mcl-1 in many other cells lines and did not sensitize them to vinblastine, thus we sought alternative ways to reduce Mcl-1 protein expression.
Cyclin-dependent kinases (CDKs) regulate cell cycle progression and their inhibition can lead to apoptosis of malignant cells. Flavopiridol (Alvocidib, HMR-1275) is a drug derived from a plant native to India that potently inhibits CDKs 1, 2, 4, 6, 7 and 9 with EC50 in the 20–300 nmol/L range4–10 and is a potent inducer of apoptosis in CLL cells.11–13 Flavopiridol inhibits global transcription via inhibition of CDK 7 (initiation) and CDK 9 (elongation), which are responsible for the phosphorylation of RNA polymerase II at Ser5 and Ser2 respectively. As a result, the levels of some short-lived proteins whose mRNA is also shortlived decreases very rapidly (e.g., Mcl-1).12,14 Flavopiridol is one of the most potent CDK 9 inhibitors to date, with phase 2 clinical trials in relapsed CLL showing 30 partial responses, three nodular responses and one complete response.15 Flavopiridol treatment selectively reduces Mcl-1 protein levels while Bcl-2 and Bcl-XL protein levels are unaffected suggesting that targeting Mcl-1 is sufficient to sensitize cells.10,12,16
As we hypothesize that the short-lived anti-apoptotic protein Mcl-1 protects ML-1 cells from vinblastine, we tested whether flavopiridol-mediated inhibition of Mcl-1 transcription would also sensitize ML-1 cells to vinblastine. Here we show that flavopiridol potently sensitizes ML-1 cells to vinblastine, with 100% of cell undergoing apoptosis within 4 h. Flavopiridol also reduced Mcl-1 levels in many other leukemia cell lines sensitizing them to vinblastine. Furthermore, dinaciclib (SCH 727965) a more selective inhibitor of CDKs with a reported improved therapeutic index over flavopiridol,17 also acutely sensitized multiple leukemia cell lines to vinblastine at concentrations lower than those required for flavopiridol. We also assessed the sensitivity of freshly-isolated CLL cells to dinaciclib alone and in combination with vinblastine.
Results
CDK inhibitor-mediated apoptosis in leukemia and lymphoma cell lines.
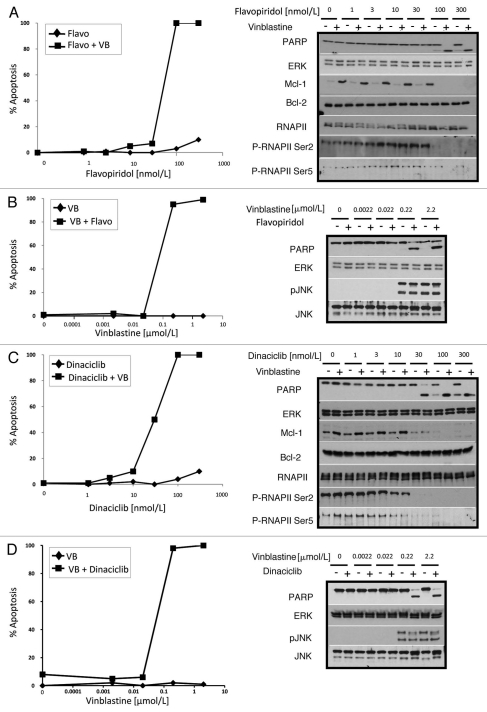
Individually the MEK inhibitor PD98059 and the microtubule-interfering agent vinblastine are minimally toxic to ML-1 leukemia cells over 24 h, however in combination they induce greater than 70% apoptosis in 4 h.2,3 This sensitization was attributed to the ability of PD98059 to suppress vinblastinemediated Mcl-1 induction. Here we investigated vinblastinemediated apoptosis in combination with other drugs that can reduce Mcl-1 protein levels such as the CDK inhibitors flavopiridol and dinaciclib. Flavopiridol alone had minimal toxicity to ML-1 cells at any concentration tested within 4 h. However flavopiridol (at 100 nmol/L or higher) in combination with vinblastine induced 100% apoptosis in 4 h (Fig. 1A). Apoptosis was observed both as chromatin condensation and cleavage of the caspase substrate poly(ADP-ribose) polymerase (PARP). Dinaciclib in combination with vinblastine induced apoptosis within 4 h at concentrations lower than those required for flavopiridol (30 nmol/L dinaciclib vs. 100 nmol/L flavopiridol; Fig. 1C). While dinaciclib appeared to have some toxicity as a single agent as seen by PARP cleavage, it is minor in comparison to the apoptosis induced by the dinaciclib and vinblastine combination. The ability of flavopiridol and dinaciclib to kill cells in combination correlates with each drug's ability to prevent phosphorylation at Ser2 of RNA polymerase II (RNAP II) and reduce levels of Mcl-1 protein. While ML-1 cells do not express Bcl-XL protein, the levels of Bcl-2 protein did not change upon treatment. Dinaciclib is designated as a CDK 9 selective inhibitor based on in vitro assays,17 yet it also inhibits CDK 7 (i.e., inhibits phosphoserine-5-RNAP II) at the same concentration as CDK 9 in ML-1 cells, suggesting that in vitro inhibitor assays are not always predictors of drug selectivity in cells. Vinblastine had no impact on RNAP II phosphorylation. We hypothesized that the dinaciclib-mediated apoptosis can be attributed to the inhibition of CDKs 7 and 9 and not the other CDK targets of dinaciclib (CDKs 1, 2). In fact concentrations of roscovitine that effectively inhibit CDKs 1/2 had no impact on RNAP II phosphorylation and did not sensitize cells to vinblastine (data not shown), reiterating the importance of CDK 7/9 inhibition. The effective concentration of vinblastine when combined with either flavopiridol or dinaciclib was also tested. ML-1 cells showed a minimum requirement of 0.22 µmol/L vinblastine to undergo apoptosis in combination with either CDK inhibitor (Fig. 1B and D).
Figure 1.
CDK inhibitors in combination with vinblastine alter protein expression and induce apoptosis. ML-1 cells were concurrently incubated with increasing concentrations of flavopiridol (A) or dinaciclib (C) in the presence (+) or absence (−) of 2.2 µmol/L vinblastine for 4 h. Additionally, ML-1 cells were concurrently incubated with increasing concentrations of vinblastine in the presence (+) or absence (−) of 100 nmol/L flavopiridol (B) or 100 nmol/L dinaciclib (D). Apoptosis was scored by chromatin condensation and results are expressed as the percentage of cells undergoing apoptosis. Cell lysates were also prepared and analyzed for protein expression.
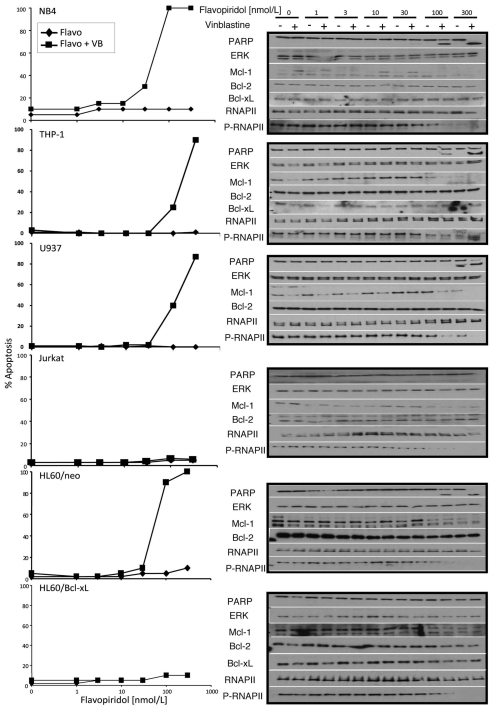
Several leukemia cell lines appear to rely on MEK for Mcl-1 regulation (e.g., ML-1, HL60/neo and AML4) and they are all sensitized to vinblastine by the MEK inhibitor PD98059.3 However as PD98059 did not suppress Mcl-1 in the other cells lines tested we could not assess the importance of Mcl-1. The current experiments target Mcl-1 via transcriptional repression and include the same panel of leukemia cell lines. NB4, THP-1, U937 and HL60/neo cells all underwent apoptosis in 4 h when exposed to vinblastine plus flavopiridol (Fig. 2). In the presence of 100–300 nmol/L flavopiridol, Mcl-1 protein levels were reduced in all cell lines in which it is normally expressed, while Bcl-2 and Bcl-XL protein levels were unaffected. In contrast, Jurkat and HL60/Bcl-XL cells were not sensitized to vinblastine by either flavopiridol (Fig. 2) or dinaciclib (data not shown) although both cell lines showed a decrease in phospho-RNAP II levels.
Figure 2.
Multiple leukemia cells lines are sensitized by combined flavopiridol and vinblastine treatment. NB4, THP -1, U937, Jurkat, HL60/neo and HL60/Bcl-XL cells were concurrently incubated with increasing concentrations of flavopiridol and 2.2 µmol/L vinblastine (+) or vehicle control (−) for 4 h. Lysates were analyzed for protein expression. Apoptosis was scored by chromatin condensation.
To confirm that the loss of both Mcl-1 and phosphorylation of RNAP II preceded apoptosis, experiments were repeated in ML-1 cells in the presence of the pan-caspase inhibitor z-VADfmk. z-VAD-fmk protected cells from apoptosis induced by the combination of vinblastine with flavopiridol or dinaciclib, however, RNAP II phosphorylation and Mcl-1 protein levels were still decreased (Fig. S1A). Similar results were seen in NB4, THP-1 and U937 cell lines (data not shown). Thus we conclude that decreased Mcl-1 protein levels are not a consequence of apoptosis induced by these drug combinations. In contrast, Mcl-1 protein levels decrease in response to vinblastine in Mino cells, which are acutely sensitive to vinblastine alone,3 and z-VAD-fmk protects these cells from apoptosis and Mcl-1 depletion suggesting that in this model, loss of Mcl-1 is a consequence of apoptosis (Fig. S1B).
Role of JNK in response to vinblastine.
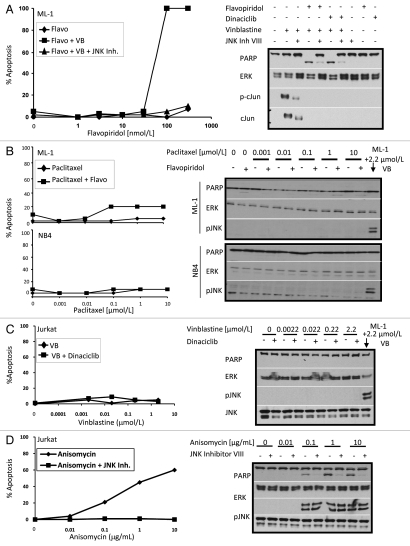
We previously showed that PD98059-sensitization of ML-1 cells to vinblastine is JNK-dependent.2,3 The lowest concentration of vinblastine that induced phosphorylation of JNK (0.22 µmol/L) is the same that induced apoptosis (Fig. 1B and D) suggesting that JNK activation also plays a role in CDK inhibitor-sensitization of vinblastine-treated ML-1 cells. To confirm the importance of JNK in this acute apoptosis, we showed that JNK inhibitor VIII prevented flavopiridol- or dinaciclib-mediated sensitization to vinblastine (Fig. 3A). Similar results were also obtained with another JNK 1/2 inhibitor SP600125 (data not shown), confirming a role for JNK activation in this acute induction of apoptosis. JNK inhibitor VIII also prevented apoptosis in NB4, THP-1, U937 and HL60/neo cells upon incubation with vinblastine in combination with flavopiridol or dinaciclib (data not shown).
Figure 3.
JNK activity is essential for sensitizing leukemia cells to CDK inhibitors. (A) ML-1 cells were pretreated for 30 min with 1 µmol/L JNK Inhibitor VIII followed by concurrent incubation with increasing concentrations of flavopiridol or dinaciclib and 2.2 µmol/L vinblastine for 4 h. Flavopiridol treated cells were assayed for chromatin condensation while all lysates were analyzed for protein expression, with the 100 nmol/L dose of flavopiridol and dinaciclib being shown. (B) ML-1 and NB4 cells were concurrently incubated with increasing concentrations of paclitaxel in the presence (+) or absence (−) of 100 nmol/L flavopiridol for 4 h. (C) Jurkat cells were concurrently incubated with increasing concentrations of vinblastine in the presence (+) or absence (−) of 100 nmol/L dinaciclib for 4 h. (D) Jurkat cells were pretreated for 30 min with 1 µmol/L JNK Inhibitor VIII followed by incubation with increasing doses of anisomycin for 2 h. Cell lysates were analyzed for protein expression. Apoptosis was scored by chromatin condensation.
Flavopiridol was ineffective at sensitizing cells to paclitaxel which correlates with the inability of this microtubule-interfering agent to activate JNK acutely (Fig. 3B). We attempted to identify other drugs that acutely activated JNK to test in combination with dinaciclib. Unfortunately most of these drugs were acutely toxic as single agents (i.e., etoposide, staurosporine) or failed to acutely activate JNK.
Jurkat cells lack any apparent JNK activation upon treatment with vinblastine (Fig. 3C) which may explain their failure to respond. To determine whether the Jurkat cells are unable to activate JNK, we used the protein synthesis inhibitor anisomycin which also induces ribotoxic stress and potently activates JNK.18 Anisomycin activated JNK and induced apoptosis within 2 h (Fig. 3D), while pretreatment with JNK inhibitor VIII prevented apoptosis. These results show that when JNK is activated, Jurkat cells are susceptible to apoptosis. Thus we conclude that Jurkat cells are resistant to combination treatment with flavopiridol or dinaciclib because of their inability to activate JNK in response to vinblastine.
Impact of drug removal on apoptosis induction.
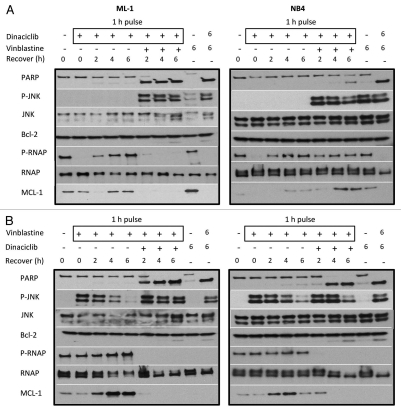
Incubation with the CDK inhibitors for 8 h induced apoptosis in the absence of vinblastine (data not shown). Meanwhile, previous reports suggest that short exposures to CDK inhibitors are sufficient for long-term efficacy.10,17,19 We therefore investigated the impact of removing the inhibitor after a short period of time. When ML-1 and NB4 cells were pulsed with dinaciclib for 1 h followed by incubation in media without drug, the levels of phospho-RNAP II started to recover in 2 h and returned to basal levels within 4 h (Fig. 4A). Mcl-1 levels also declined after the 1 h pulse with none present by 2 h, but recovered within 4 h of removing dinaciclib. ML-1 and NB4 cells also recovered fully from a 1 h pulse with flavopiridol alone (data not shown). The leukemia cells however did not recover from a 1 h pulse with dinaciclib followed by incubation in media containing vinblastine; 2 h of incubation in vinblastine is sufficient to induce apoptosis in 100% of ML-1 cells while NB4 cells are partially sensitized in 6 h (Fig. 4A). This rapid apoptosis in ML-1 cells can be attributed to the loss of Mcl-1 within this 2 h vinblastine incubation. Similar results were seen in response to a flavopiridol pulse followed by incubation in vinblastine (data not shown). These results suggest that the effects of dinaciclib and flavopiridol are reversible when used as single agents, but even a short exposure to either drug followed by a short exposure to vinblastine is sufficient to rapidly kill leukemia cells.
Figure 4.
Short drug exposures are sufficient to sensitize leukemia cells to dinaciclib and vinblastine. (A) ML-1 and NB4 cells were incubated with 100 nmol/L dinaciclib for 1 h, after which the drug was removed and cells were incubated in media alone (lanes 3-5) or media containing 2.2 µmol/L vinblastine (lanes 6–8) for 2, 4 or 6 h as indicated. Controls include lane 1: vehicle, lane 2: 1 h dinaciclib no post-incubation, lane 9: 6 h vinblastine only, and lane 10: 6 h concurrent vinblastine and dinaciclib. (B) ML-1 and NB4 cells were incubated with 2.2 µmol/L vinblastine for 1 h, after which the drug was removed and cells were incubated in media alone (lanes 3–5) or media containing 100 nmol/L dinaciclib (lanes 6–8) for 2, 4 or 6 h as indicated. Controls include lane 1: vehicle, lane 2: 1 h vinblastine no post-incubation, lane 9: 6 h dinaciclib only, and lane 10: 6 h concurrent vinblastine and dinaciclib. Cell lysates were prepared and the indicated proteins were measured by immunoblotting.
Reverse experiments were also done where ML-1 and NB4 cells were pulsed with vinblastine for 1 h then incubated in either media alone or media containing dinaciclib (Fig. 4B), or flavopiridol (data not shown). Incubation in media reveals that vinblastine-mediated JNK phosphorylation declines in 6 h with cells remaining viable, yet incubation in dinaciclib induces apoptosis as early as 2 h while phospho-JNK levels remain high (Fig. 4B). Exposure to vinblastine alone leads to an increase in Mcl-1 protein in ML-1 cells as expected. Mcl-1 protein levels also increased in response to vinblastine in NB4 cells, however these levels of Mcl-1 are far lower than those seen in ML-1 cells.3 This increase in Mcl-1 protein is maximal 4 h after a 1 h pulse with vinblastine in both cell lines but is prevented by post-incubation in CDK inhibitor. These results demonstrate that short exposure to vinblastine prior to CDK inhibitor acutely sensitizes 100% of ML-1 and NB4 leukemia cells.
Vinblastine enhances CDK inhibitor-mediated apoptosis in CLL.
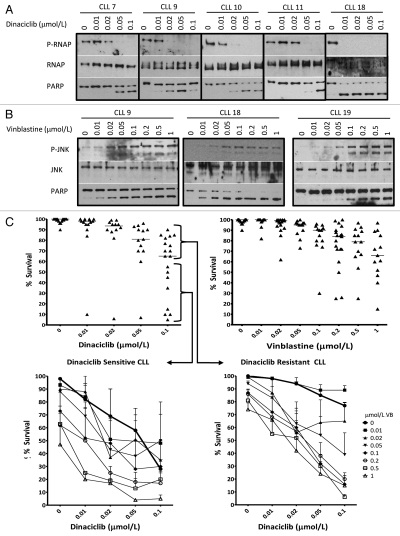
Flavopiridol has had some success in the clinic against relapsed CLL,15,20 while dinaciclib is reported to have a better therapeutic index.21 In order to be effective in patients, dinaciclib must inhibit RNAP II phosphorylation at therapeutically achievable concentrations. In freshly-isolated CLL cells, dinaciclib concentrations that completely reduced phospho-RNAP II varied from 0.01–0.05 µmol/L between patients (Fig. 5A). Loss of phospho-RNAP II correlated with apoptosis as assessed by PARP cleavage which appeared to be a slightly more sensitive indicator of apoptosis than chromatin condensation, perhaps because it occurred more rapidly. However, chromatin condensation provides a quantitative assessment of the number of cells undergoing apoptosis and was therefore used to assess the sensitivity of 19 CLL patient samples (Fig. 5C). A wide range of sensitivity was observed with 5–95% survival following a 6 h incubation with 0.1 µmol/L dinaciclib. One patient sample (CLL 18) showed extreme sensitivity to even 0.01 µmol/L dinaciclib. The median response at 0.1 µmol/L dinaciclib was around 60% survival, and for presentation of results below, we have defined those above or below the median as either resistant or sensitive to dinaciclib.
Figure 5.
Freshly-isolated CLL cells are sensitive to dinaciclib and vinblastine treatment. (A) Lymphocytes freshly-isolated from CLL were incubated with dinaciclib for 6 h. Cell lysates were prepared and analyzed for expression of P-RNAP II (Ser2), RNAP II and PARP with immunoblots from five representative CLL patients shown. (B) Cell lysates from vinblastine treated patient samples were analyzed for PARP, total JNK and phospho-JNK protein levels with representative immunoblots shown. Apoptosis was assessed by chromatin condensation and is indicated for each sample. (C) CLL cells isolated from 19 patients were incubated with increasing concentrations of dinaciclib (upper left) or vinblastine (upper right) for 6 h. CLL cells were also incubated with combinations of increasing concentrations of dinaciclib and vinblastine. The effect of drug combination therapy in CLL resistant to single agent dinaciclib are shown in the lower right (n = 11) while the effect in CLL sensitive to single agent therapy are shown in the lower left (n = 8). Apoptosis was scored by chromatin condensation and results are expressed as the percentage of cells that survived.
We have previously reported that CLL cells are often sensitive to vinblastine alone after only a 6 h incubation.3 Vinblastine induced JNK phosphorylation at 0.02–0.1 µmol/L in CLL (Fig. 5B). Similar to dinaciclib, the same 19 CLL samples display a range of sensitivity to single agent vinblastine (Fig. 5C). The median response at 1 µmol/L vinblastine induced apoptosis in about 40% of the cells (60% survival). The patient sample highly sensitive to dinaciclib was also the most sensitive to vinblastine.
All CLL patients are routinely characterized for ZAP70 and CD38 expression. We therefore compared these diagnostic parameters to the observed sensitivity to dinaciclib and vinblastine (Fig. S2). The majority of samples were negative for both markers, but no correlation was observed between phenotype and sensitivity.
Dinaciclib and vinblastine were co-incubated for 6 h, and the results are separately expressed for those deemed sensitive or resistant to dinaciclib alone (Fig. 5C). For the dinaciclib-resistant cells, we observed greater than additive effects when vinblastine was added. For example, whereas 0.1 µmol/L dinaciclib and 0.2 µmol/L vinblastine as single agents induced 23% and 14% apoptosis respectively, the combination induced 80% apoptosis. For dinaciclib-sensitive samples, the combined treatment markedly enhanced the levels of apoptosis at lower concentrations of dinaciclib. For example, whereas 0.1 µmol/L dinaciclib induced 72% apoptosis alone, the same level of apoptosis could be achieved using a 10-fold lower concentration of dinaciclib when combined with 0.2 µmol/L vinblastine. These results suggest that adding vinblastine to a CDK inhibitor may increase efficacy against this disease.
Vinblastine does not enhance CDK inhibitor-mediated apoptosis in normal lymphocytes.
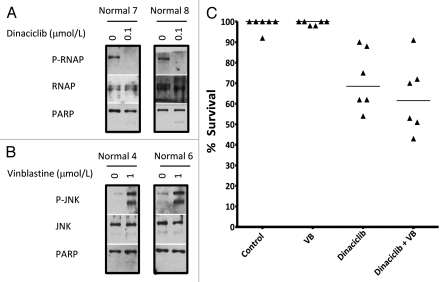
The enhanced apoptosis induced in CLL cells by combining dinaciclib and vinblastine would have little therapeutic value if it simultaneously killed all lymphocytes. We therefore obtained lymphocytes from individuals without cancer and assessed the impact of both drugs. Alone, dinaciclib inhibited RNAP II phosphorylation and vinblastine activated JNK (Fig. 6A and B). Dinaciclib induced variable apoptosis in normal lymphocytes with a maximum level of about 45% whereas vinblastine did not induce apoptosis (Fig. 6C). When combined, vinblastine had no further impact on the number of cells that underwent apoptosis (Student's t-test, p value = 0.39). Hence the sensitivity to the combination of dinaciclib and vinblastine appears to be unique to CLL samples.
Figure 6.
Vinblastine does not sensitize non-tumorigenic lymphocytes to dinaciclib. Freshly-isolated non-tumorigenic lymphocytes were incubated with 0.1 µmol/L dinaciclib (A) or 1 µmol/L vinblastine (B) for 6 h and lysates were analyzed for protein expression. (C) Lymphocytes were incubated with 0.1 µmol/L dinaciclib, 1 µmol/L vinblastine or both for 6 h and apoptosis was scored by chromatin condensation, with results expressed as the percentage of cells that survived.
Discussion
Resistance to currently used chemotherapeutic drugs has been attributed to many different mechanisms including overexpression of the anti-apoptotic Bcl-2 family proteins. We have previously shown that vinblastine alone acutely kills several mantle cell lymphoma lines and freshly-isolated CLL cells while other leukemic cell lines could be sensitized to vinblastine by either PD98059 or cycloheximide, with apoptosis occurring within 4–6 h.3 These results highlighted a novel mechanism of action of vinblastine whereby cells die at all phases of the cell cycle rather than the generally recognized mechanism of action that requires cells first accumulate in mitosis.
The transcript for the Mcl-1 anti-apoptotic protein has a short half-life while the protein itself is also short-lived. Thus Mcl-1 levels rapidly decrease upon inhibition of protein synthesis. An alternate means to inhibit Mcl-1 is to use an inhibitor of CDK 7 and/or CDK 9 such as flavopiridol or dinaciclib, thereby leading to inhibition of global transcription.7,22 Here we report that these CDK inhibitors sensitized many leukemia cell lines to vinblastine with apoptosis being induced rapidly, often with 100% of the cells dying within 4 h (Figs. 1 and 2). These CDK inhibitors were ineffective at inducing apoptosis within this time frame as single agents. Previous reports have shown that flavopiridol can kill cells in an acute time frame, but concentrations greater than 1 µmol/L were required,10,23 whereas the sensitization we observed occurred at 100 nmol/L.
Most of the cell lines used in this study were not sensitized to vinblastine by PD98059 and in these cells inhibition of MEK did not reduce Mcl-1 protein levels. The importance of Mcl-1 was further supported by shRNA knockdown of Mcl-1 protein levels in ML-1, in which Mcl-1 reduction sensitized cells to vinblastine.3 Although basal Mcl-1 protein levels were low in NB4 and CLL cells, the protein was still expressed and the cells were sensitive to dinaciclib plus vinblastine. While we focused on the loss of Mcl-1, dinaciclib and flavopiridol inhibit global transcription resulting in the loss of several short lived transcripts, some of which may also play a role in protecting these cells. Other anti-apoptotic proteins such as XIAP and BAG-1 have also been implicated as targets of flavopiridol as they also have short halflives.12,24 Jurkat and HL60/Bcl-XL cells were not sensitive to the combination of vinblastine plus flavopiridol or dinaciclib. We believe the lack of sensitivity in the Jurkat cells is due to the inability of vinblastine to activate JNK (Fig. 3), while the resistance in the HL60/Bcl-XL cells is likely due to the very high level of expression of Bcl-XL.3
Although vinblastine and paclitaxel are both microtubuleinterfering agents, their effect on JNK signaling is very different; only vinblastine is able to acutely activate JNK in these leukemia cell models (Figs. 1 and 3). Furthermore, the CDK inhibitors (which do not activate JNK) only sensitized cells to concentrations of vinblastine that activate JNK and failed to sensitize cells to paclitaxel. To confirm the involvement of JNK in the induction of apoptosis, two pan JNK inhibitors were utilized (JNK Inhibitor VIII and SP600125), both of which protected the cells. The fact that JNK appears to be involved in the apoptotic program induced by the combination of vinblastine and CDK inhibitor may imply that the combination involves an alternate pattern of signaling cascades to those required for apoptosis after G2/M arrest. This pathway appears to involve both caspase activity and mitochondrial disruption since sensitization was prevented by pretreatment with z-VAD-fmk or in cells overexpressing Bcl-XL. The target of JNK in this apoptosis remains to be determined, but is clearly not mediated by transcription which is inhibited in the CDK combination. Most likely the target of JNK is some member(s) of the Bcl-2 family. Several reports have shown that JNK activation results in phosphorylation and inactivation of Bcl-2 thus allowing for the pro-apoptotic players to prevail.25–28 JNK activation in response to ionizing radiation has also been shown to lead to the phosphorylation and inactivation of the antiapoptotic protein Bcl-XL,29 while following exposure to oxidative stress, JNK has been shown to phosphorylate and inactivate Mcl-1.30 It has also been shown that JNK-mediated phosphorylation of 14-3-3ζ or σ leads to the dissociation of Bax or Bad permitting translocation to the mitochondria where they can both activate the apoptotic cascade.31,32 Finally, JNK-mediated phosphorylation of BimEL on Ser-65 potentiates its pro-apoptotic function.33 Future studies will focus on the role of JNK in vinblastineinduced cell cycle-independent apoptosis.
While most of the results here show effective apoptosis induction in 4 h of concurrent drug treatment, we also tested the effectiveness of even shorter treatment schedules as this might more closely mimic a bolus administration in a patient (Fig. 4). Pulsing cells with dinaciclib followed by recovery in vinblastine killed 100% of ML-1 cells, but only partially killed NB4 cells. However, pulsing with vinblastine followed by recovery in dinaciclib induces apoptosis in 100% of ML-1 and NB4 cells. This difference in sensitivity between the sequences is likely due to phospho-RNAP II levels rapidly recovering after dinaciclib is removed. As a result Mcl-1 levels remain low for only the first 2 h of recovery which is sufficient to sensitize ML-1 cells, but not NB4 cells. Alternatively, pulsing with vinblastine and recovering in dinaciclib keeps phospho-RNAP II levels low allowing for Mcl-1 and other short-lived proteins to remain low and thus killing both ML-1 and NB4 cells.
Early clinical trials with flavopiridol as a single agent were based on in vitro experiments showing that long exposures (24–72 h) to 200–500 nmol/L flavopiridol induced apoptosis in several cancer cell lines as well as isolated CLL cells.7,10,11,14 This concentration was sufficient to inhibit CDKs, albeit CDK 7 and 9 were not initially recognized as targets. As a consequence initial clinical trials used a 72 h infusion of flavopiridol and concentrations of 200–500 nmol/L were attained in plasma.34–36 However, few responses were observed. Meanwhile, acute exposures to flavopiridol also induced rapid apoptosis in CLL cells ex vivo although concentrations of at least 1 µmol/L were required.10,23 This acute strategy (administration over only 4 h) was more recently tested in a clinical trial with remarkable success; most notable was an acute tumor lysis syndrome that demonstrated the efficacy of the drug but limited treatment.20 The results described above show that very short exposures to lower concentrations of flavopiridol or dinaciclib are sufficient to sensitize cell lines and CLL cells to vinblastine. As these concentrations can be achieved in patients, and the sensitivity appears fairly broadly applicable to many hematopoietic malignancies, this drug combination may have more success than single agent therapy. Importantly, vinblastine did not sensitize normal lymphocytes to dinaciclib suggesting selectivity of this drug combination for malignant cells. While dinaciclib alone did kill a fraction of normal lymphocytes ex vivo, this does not appear to reflect in vivo toxicity as clinical trials indicate that this drug is well tolerated when there is not a large tumor load that can lead to tumor lysis syndrome.
Currently used chemotherapeutic agents have not been particularly effective as single agents in models where anti-apoptotic Bcl-2 family proteins are overexpressed. Therefore the development of novel agents with selectivity against critical targets necessary for survival (i.e., Bcl-2, Mcl-1, Bcl-XL) provides an attractive rationale for combination therapy. In the current experiments, we show that a brief inhibition of transcription with flavopiridol or dinaciclib effectively suppressed Mcl-1. Vinblastine can then induce apoptosis through activation of JNK, which might be mediated by inhibition of Bcl-2. Importantly, our data provide a model whereby a cell cycle phase-specific agent such as vinblastine can be converted to a phase non-specific agent with the addition of the broad spectrum CDK inhibitors. This cell cycle phase-independent death is particularly evident in the ability of this combination to kill non-dividing CLL cells. This may be of particular relevance in the clinic where tumor cells at all phases of the cell cycle may be eliminated effectively with a therapeutic strategy that only requires an acute treatment. If this strategy can kill non-dividing tumor cells, it may be effective in preventing tumor recurrence.
Materials and Methods
The source of cell lines, materials and antibodies, as well as protocols for chromatin condensation and immunoblotting have been published.3 Flavopiridol was provided by Dr. W. D. Figg (National Cancer Institute). Dinaciclib was obtained from the Cancer Therapy Evaluation Program, National Cancer Institute. Additional antibodies included RNA Polymerase II, phospho-RNA Polymerase II Ser2, phospho-RNA Polymerase II Ser5 (Covance, Cat# MMS-126R, MMS-129R, MMS-134R) and phospho-Histone H3 (Cell Signaling, Cat# 9706). The phospho-RNAP II antibody selective for Ser2 was used unless otherwise stated.
The cell lines were maintained in RPMI-1640 containing 10% fetal bovine serum and incubated at 37°C in 5% CO2/95% humidified air; HL60/neo and HL60/Bcl-XL cells were maintained in media containing 500 µg/mL G418. Lymphocytes were obtained from consented patients at the Norris Cotton Cancer Center. Cells from 10 mL of blood were diluted in PBS and purified by centrifugation in Ficoll-Paque PLUS. Lymphocytes were collected, washed three times in PBS + 2 mmol/L EDTA and plated in RPMI-1640 plus 10% serum at 1 × 106 cells/mL. Cells were incubated immediately with drugs.
As required, JNK Inhibitor VIII, SP600125, zVAD-fmk or the appropriate vehicle control were added to the cells 30 min prior to the addition of vinblastine, paclitaxel or anisomycin and were maintained in the medium for the duration of the experiment.
Acknowledgments
This work was supported by grants from the National Institute of Health (CA50224; A.E.), (T32 09658; D.J.P.B. and B.L.S.) and Cancer Center Support (CA23108).
Supplementary Material
References
- 1.Townsend KJ, Zhou P, Qian L, Bieszczad CK, Lowrey CH, Yen A, et al. Regulation of MCL1 through a serum response factor/Elk-1-mediated mechanism links expression of a viability-promoting member of the BCL2 family to the induction of hematopoietic cell differentiation. J Biol Chem. 1999;274:1801–1813. doi: 10.1074/jbc.274.3.1801. [DOI] [PubMed] [Google Scholar]
- 2.Stadheim TA, Xiao H, Eastman A. Inhibition of extracellular signal-regulated kinase (ERK) mediates cell cycle phase independent apoptosis in vinblastine-treated ML-1 cells. Cancer Res. 2001;61:1533–1540. [PubMed] [Google Scholar]
- 3.Salerni BL, Bates DJ, Albershardt TC, Lowrey CH, Eastman A. Vinblastine induces acute, cell cycle phase-independent apoptosis in some leukemias and lymphomas and can induce acute apoptosis in others when Mcl-1 is suppressed. Mol Cancer Ther. 2010;9:791–802. doi: 10.1158/1535-7163.MCT-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Losiewicz MD, Carlson BA, Kaur G, Sausville EA, Worland PJ. Potent inhibition of CDC2 kinase activity by the flavonoid L86-8275. Biochem Biophys Res Commun. 1994;201:589–595. doi: 10.1006/bbrc.1994.1742. [DOI] [PubMed] [Google Scholar]
- 5.Chao SH, Fujinaga K, Marion JE, Taube R, Sausville EA, Senderowicz AM, et al. Flavopiridol inhibits P-TEFb and blocks HIV-1 replication. J Biol Chem. 2000;275:28345–28348. doi: 10.1074/jbc.C000446200. [DOI] [PubMed] [Google Scholar]
- 6.Chao SH, Price DH. Flavopiridol inactivates P-TEFb and blocks most RNA polymerase II transcription in vivo. J Biol Chem. 2001;276:31793–31799. doi: 10.1074/jbc.M102306200. [DOI] [PubMed] [Google Scholar]
- 7.Lam LT, Pickeral OK, Peng AC, Rosenwald A, Hurt EM, Giltnane JM, et al. Genomic-scale measurement of mRNA turnover and the mechanisms of action of the anti-cancer drug flavopiridol. Genome Biol. 2001;2:41. doi: 10.1186/gb-2001-2-10-research0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Azevedo WF, Jr, Canduri F, da Silveira NJ. Structural basis for inhibition of cyclin-dependent kinase 9 by flavopiridol. Biochem Biophys Res Commun. 2002;293:566–571. doi: 10.1016/S0006-291X(02)00266-8. [DOI] [PubMed] [Google Scholar]
- 9.Lu X, Burgan WE, Cerra MA, Chuang EY, Tsai MH, Tofilon PJ, et al. Transcriptional signature of flavopiridol-induced tumor cell death. Mol Cancer Ther. 2004;3:861–872. [PubMed] [Google Scholar]
- 10.Chen R, Keating MJ, Gandhi V, Plunkett W. Transcription inhibition by flavopiridol: mechanism of chronic lymphocytic leukemia cell death. Blood. 2005;106:2513–2519. doi: 10.1182/blood-2005-04-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byrd JC, Shinn C, Waselenko JK, Fuchs EJ, Lehman TA, Nguyen PL, et al. Flavopiridol induces apoptosis in chronic lymphocytic leukemia cells via activation of caspase-3 without evidence of bcl-2 modulation or dependence on functional p53. Blood. 1998;92:3804–3816. [PubMed] [Google Scholar]
- 12.Kitada S, Zapata JM, Andreeff M, Reed JC. Protein kinase inhibitors flavopiridol and 7-hydroxy-staurosporine downregulate antiapoptosis proteins in B-cell chronic lymphocytic leukemia. Blood. 2000;96:393–397. [PubMed] [Google Scholar]
- 13.Pepper C, Thomas A, Hoy T, Fegan C, Bentley P. Flavopiridol circumvents Bcl-2 family mediated inhibition of apoptosis and drug resistance in B-cell chronic lymphocytic leukaemia. Br J Haematol. 2001;114:70–77. doi: 10.1046/j.1365-2141.2001.02895.x. [DOI] [PubMed] [Google Scholar]
- 14.Ma Y, Cress WD, Haura EB. Flavopiridol-induced apoptosis is mediated through upregulation of E2F1 and repression of Mcl-1. Mol Cancer Ther. 2003;2:73–81. [PubMed] [Google Scholar]
- 15.Lin TS, Ruppert AS, Johnson AJ, Fischer B, Heerema NA, Andritsos LA, et al. Phase II study of flavopiridol in relapsed chronic lymphocytic leukemia demonstrating high response rates in genetically high-risk disease. J Clin Oncol. 2009;27:6012–6018. doi: 10.1200/JCO.2009.22.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosato RR, Almenara JA, Kolla SS, Maggio SC, Coe S, Gimenez MS, et al. Mechanism and functional role of XIAP and Mcl-1 downregulation in flavopiridol/vorinostat antileukemic interactions. Mol Cancer Ther. 2007;6:692–702. doi: 10.1158/1535-7163.MCT-06-0562. [DOI] [PubMed] [Google Scholar]
- 17.Parry D, Guzi T, Shanahan F, Davis N, Prabhavalkar D, Wiswell D, et al. Dinaciclib (SCH 727965), a novel and potent cyclin-dependent kinase inhibitor. Mol Cancer Ther. 2010;9:2344–2353. doi: 10.1158/1535-7163.MCT-10-0324. [DOI] [PubMed] [Google Scholar]
- 18.Iordanov MS, Pribnow D, Magun JL, Dinh TH, Pearson JA, Chen SL, et al. Ribotoxic stress response: activation of the stress-activated protein kinase JNK1 by inhibitors of the peptidyl transferase reaction and by sequence-specific RNA damage to the alpha-sarcin/ricin loop in the 28S rRNA. Mol Cell Biol. 1997;17:3373–3381. doi: 10.1128/mcb.17.6.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byrd JC, Peterson BL, Gabrilove J, Odenike OM, Grever MR, Rai K, et al. Treatment of relapsed chronic lymphocytic leukemia by 72-hour continuous infusion or 1-hour bolus infusion of flavopiridol: results from Cancer and Leukemia Group B study 19,805. Clin Cancer Res. 2005;11:4176–4181. doi: 10.1158/1078-0432.CCR-04-2276. [DOI] [PubMed] [Google Scholar]
- 20.Phelps MA, Lin TS, Johnson AJ, Hurh E, Rozewski DM, Farley KL, et al. Clinical response and pharmacokinetics from a phase 1 study of an active dosing schedule of flavopiridol in relapsed chronic lymphocytic leukemia. Blood. 2009;113:2637–2645. doi: 10.1182/blood-2008-07-168583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flynn JM, Johnson AJ, Andritsos LA, Blum KA, Jones JA, Wiley EA, et al. The cyclin dependent kinase inhibitor SCH 727965 demonstrates promising preclinical and early clinical activitiy in chronic lymphocytic leukemia; Abstract 51st ASH Annual Meeting and Exposition 2009. [Google Scholar]
- 22.Garriga J, Grana X. Cellular control of gene expression by T-type cyclin/CDK9 complexes. Gene. 2004;337:15–23. doi: 10.1016/j.gene.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Hussain SR, Lucas DM, Johnson AJ, Lin TS, Bakaletz AP, Dang VX, et al. Flavopiridol causes early mitochondrial damage in chronic lymphocytic leukemia cells with impaired oxygen consumption and mobilization of intracellular calcium. Blood. 2008;111:3190–3199. doi: 10.1182/blood-2007-10-115733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosato RR, Dai Y, Almenara JA, Maggio SC, Grant S. Potent antileukemic interactions between flavopiridol and TRAIL/Apo2L involve flavopiridol-mediated XIAP downregulation. Leukemia. 2004;18:1780–1788. doi: 10.1038/sj.leu.2403491. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto K, Ichijo H, Korsmeyer SJ. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G(2)/M. Mol Cell Biol. 1999;19:8469–8478. doi: 10.1128/mcb.19.12.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan M, Goodwin M, Vu T, Brantley-Finley C, Gaarde WA, Chambers TC. Vinblastine-induced phosphorylation of Bcl-2 and Bcl-XL is mediated by JNK and occurs in parallel with inactivation of the Raf-1/MEK/ERK cascade. J Biol Chem. 2000;275:29980–29985. doi: 10.1074/jbc.M003776200. [DOI] [PubMed] [Google Scholar]
- 27.Fan M, Goodwin ME, Birrer MJ, Chambers TC. The c-Jun NH(2)-terminal protein kinase/AP-1 pathway is required for efficient apoptosis induced by vinblastine. Cancer Res. 2001;61:4450–4458. [PubMed] [Google Scholar]
- 28.Muscarella DE, Bloom SE. The contribution of c-Jun N-terminal kinase activation and subsequent Bcl-2 phosphorylation to apoptosis induction in human B-cells is dependent on the mode of action of specific stresses. Toxicol Appl Pharmacol. 2008;228:93–104. doi: 10.1016/j.taap.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kharbanda S, Saxena S, Yoshida K, Pandey P, Kaneki M, Wang Q, et al. Translocation of SAPK/JNK to mitochondria and interaction with Bcl-x(L) in response to DNA damage. J Biol Chem. 2000;275:322–327. doi: 10.1074/jbc.275.1.322. [DOI] [PubMed] [Google Scholar]
- 30.Inoshita S, Takeda K, Hatai T, Terada Y, Sano M, Hata J, et al. Phosphorylation and inactivation of myeloid cell leukemia 1 by JNK in response to oxidative stress. J Biol Chem. 2002;277:43730–43734. doi: 10.1074/jbc.M207951200. [DOI] [PubMed] [Google Scholar]
- 31.Tsuruta F, Sunayama J, Mori Y, Hattori S, Shimizu S, Tsujimoto Y, et al. JNK promotes Bax translocation to mitochondria through phosphorylation of 14-3-3 proteins. EMBO J. 2004;23:1889–1899. doi: 10.1038/sj.emboj.7600194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sunayama J, Tsuruta F, Masuyama N, Gotoh Y. JNK antagonizes Akt-mediated survival signals by phosphorylating 14-3-3. J Cell Biol. 2005;170:295–304. doi: 10.1083/jcb.200409117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Putcha GV, Le S, Frank S, Besirli CG, Clark K, Chu B, et al. JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron. 2003;38:899–914. doi: 10.1016/S0896-6273(03)00355-6. [DOI] [PubMed] [Google Scholar]
- 34.Rudek MA, Bauer KS, Jr, Lush RM, 3rd, Stinson SF, Senderowicz AM, Headlee DJ, et al. Clinical pharmacology of flavopiridol following a 72-hour continuous infusion. Ann Pharmacother. 2003;37:1369–1374. doi: 10.1345/aph.1C404. [DOI] [PubMed] [Google Scholar]
- 35.Senderowicz AM, Headlee D, Stinson SF, Lush RM, Kalil N, Villalba L, et al. Phase I trial of continuous infusion flavopiridol, a novel cyclin-dependent kinase inhibitor, in patients with refractory neoplasms. J Clin Oncol. 1998;16:2986–2999. doi: 10.1200/JCO.1998.16.9.2986. [DOI] [PubMed] [Google Scholar]
- 36.Senderowicz AM, Sausville EA. Preclinical and clinical development of cyclin-dependent kinase modulators. J Natl Cancer Inst. 2000;92:376–387. doi: 10.1093/jnci/92.5.376. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.