Abstract
We sought to assess the feasibility and reproducibility of performing tissue-based immune characterization of the tumor microenvironment using CT-compatible needle biopsy material. Three independent biopsies were obtained intraoperatively from one metastatic epithelial ovarian cancer lesion of seven consecutive patients undergoing surgical cytoreduction using a 16-gauge core biopsy needle. Core specimens were snap-frozen and subjected to immunohistochemistry (IHC) against human CD3, CD4, CD8 and FoxP3. A portion of the cores was used to isolate RNA for (1) real-time quantitative (q)PCR for CD3, CD4, CD8, FoxP3, IL-10 and TGFβ, (2) multiplexed PCR-based T-cell receptor (TCR) CDR3 V-β region spectratyping and (3) gene expression profiling. Pearson's correlations were examined for immunohistochemistry and PCR gene expression, as well as for gene expression array data obtained from different tumor biopsies. Needle biopsy yielded sufficient tissue for all assays in all patients. IHC was highly reproducible and informative. Significant correlations were seen between the frequency of CD3+, CD8+ and FoxP3+ T cells by IHC with CD3-epsilon, CD8A and FoxP3 gene expression, respectively, by qPCR (r = 0.61, 0.86 and 0.89; all p < 0.05). CDR3 spectratyping was feasible and highly reproducible in each tumor, and indicated a restricted repertoire for specific TCR V-β chains in tumor-infiltrating T cells. Microarray gene expression revealed strong correlation between different biopsies collected from the same tumor. Our results demonstrate a feasible and reproducible method of immune monitoring using CT-compatible needle biopsies from tumor tissue, thereby paving the way for sophisticated translational studies during tumor biological therapy.
Key words: ovarian neoplasms, lymphocytes, tumor-infiltrating, adoptive immunotherapy, gene expression profiling, immunohistochemistry
Introduction
Immune therapy is an emerging modality in the clinical treatment of solid tumors. Recent advances in vaccine technology and adoptive T-cell therapy have been highly promising. Positive results of clinical trials of vaccine therapy for prostate cancer and non-Hodgkin lymphoma, for example, have recently been reported.1,2 Adoptive T-cell therapy, where patients' own lymphocytes are harvested and engineered to specifically target the tumor itself, has had objective response rates of 49–72% for metastatic melanoma.3 These successes, however, have been tempered by low responses in other tumors, and by the highly variable responses seen from patient to patient, including failure in many cases. In spite of generating tangible antitumor cellular immune responses in peripheral blood, tumor vaccines as a whole have proven largely ineffective to date.
The mechanisms underlying these failures remain unclear, but it is becoming evident that tumor microenvironment factors play a key role.4,5 The success of immune therapy depends partly on the ability of effector cells to infiltrate and function in tumors.6 Most immune therapy trials have monitored events in peripheral blood. However, it is becoming progressively clear that peripheral events may not reflect events at the tumor microenvironment. Thus, understanding events at the tumor microenvironment will allow us to interpret clinical result of immune therapy and understand mechanisms of failure. It is expected that this rational approach will enhance troubleshooting, allow us to develop predictive biomarkers, and accelerate the pace to the development of effective immune therapy of cancer.
We hypothesized that sophisticated immune monitoring at the tumor tissue level is feasible on tumor samples obtained through routine needle biopsy procedures performed in the clinic for diagnostic purposes. Epithelial ovarian cancer is an appropriate test case for such monitoring, as recent preclinical and clinical results have shown this disease to be a suitable target for immune therapy.7–9 Our lab has previously demonstrated a significant correlation of CD3+ T cells infiltrating tumor islets (intratumoral or intraepithelial T cells) with longer survival,10 an observation validated by different studies in ovarian cancer11–17 and other tumors such as melanoma, breast, prostate, renal cell, esophageal and colorectal carcinoma.18–24 The association of spontaneous antitumor immune response with improved survival implies that many patients could benefit from strengthening tumor rejection through immunotherapy and that similar mechanisms can be interrogated or monitored during immunotherapy in many solid tumors.
We sought to investigate the feasibility and reproducibility of immune surveillance of the tumor and its microenvironment using tumor tissue obtained via CT-compatible needle biopsy. In this manuscript we examine one single tumor deposit per patient. In a companion manuscript we examine the possible heterogeneity across different metastatic deposits within patients and its impact on assessing immune response. Our findings here support the notion that tumor samples obtained through CT-compatible needle biopsy are suitable material to perform detailed immune monitoring and interrogate the tumor microenvironment during clinical trials and that a high degree of immune cellular and molecular homogeneity exists within a single tumor deposit.
Results
Tissue core characteristics.
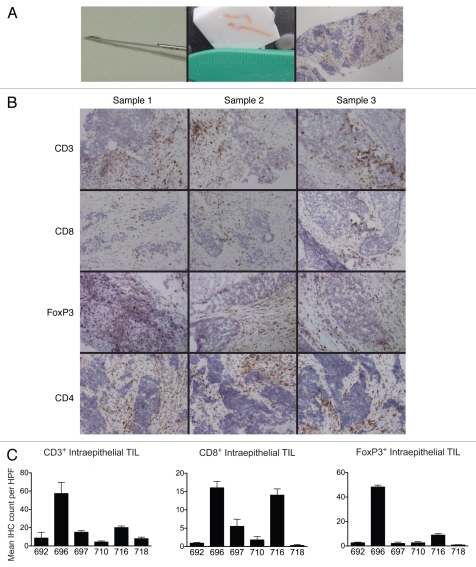
One single metastatic deposit was analyzed per patient. Each solid tumor nodule was biopsied in three separate areas, and from each area we obtained one to three needle cores (Fig. 1A). Adequate tissue was obtained from each tumor area sampled. Of the seven patients sampled, one patient's specimens revealed tissue necrosis, which precluded further analysis in all three samplings. In the remaining six tumors, tissue histology was adequate for morphology via H&E staining and IHC. For each individual tumor, staining for each marker was consistent across the three separate areas (Fig. 1B), for both total and intraepithelial cells. Mean immunohistochemistry cell counts and standard deviations are reported in Table 1. There was small overall within-patient variability, as assessed by the mean squared error (MSE), which reflects the weighted average of the patient-specific variances. Such variability was most pronounced for FoxP3 among all parameters evaluated. Variability was much more pronounced among patients, and the mean cell counts were statistically different among patients (Fig. 1C).
Figure 1.
(A) Tumor biopsy acquisition and histology. Left, 16-gauge Quick-Core* biopsy needle. Center, tumor specimen obtained with two needle passes, embedded in O.C.T. Right, representative immunohistochemical section, anti-CD3-DAB stain, hematoxylin counterstain, 40× original magnification. (B) Representative immunostains of triplicate specimens; anti-CD3, CD8, FoxP3 and CD4 stains, 40× original magnification. (C) Mean intratumoral CD3+, CD8+ and FoxP3+ TILs for each patient, reflecting the mean of 5, 200× high-power fields. Error bars indicate standard deviation of the mean.
Table 1.
Tumor-infiltrating lymphocyte counts as assessed by immunohistochemistry
| Patient | pa | Square root MSEb | ||||||
| 692 | 696 | 697 | 710 | 716 | 718 | |||
| n = 3 | n = 3 | n = 3 | n = 3 | n = 3 | n = 3 | |||
| Stromal and intratumoral TILs | ||||||||
| CD3 | ||||||||
| Mean | 38.0 | 98.7 | 27.7 | 32.2 | 113.5 | 62.8 | 0.002 | |
| SDc | 17.3 | 21.1 | 5.5 | 34.0 | 24.1 | 10.7 | 21.2 | |
| CD8 | ||||||||
| Mean | 21.2 | 48.2 | 22.5 | 19.4 | 45.3 | 1.8 | 0.0001 | |
| SD | 2.3 | 10.3 | 9.3 | 17.7 | 2.9 | 0.7 | 9.7 | |
| FoxP3 | ||||||||
| Mean | 20.3 | 79.0 | 7.5 | 9.3 | 22.5 | 3.9 | 0.0001 | |
| SD | 5.0 | 6.4 | 5.1 | 9.3 | 4.8 | 2.0 | 5.9 | |
| Intratumoral TILs only | ||||||||
| CD3 | ||||||||
| Mean | 2.2 | 57.4 | 15.0 | 4.3 | 20.1 | 8.1 | 0.0002 | |
| SD | 0.8 | 20.9 | 3.4 | 2.1 | 3.0 | 3.0 | 9.2 | |
| CD8 | ||||||||
| Mean | 0.9 | 16.0 | 5.5 | 1.8 | 14.6 | 0.3 | 0.0001 | |
| SD | 0.4 | 3.7 | 3.4 | 1.6 | 3.0 | 0.5 | 2.6 | |
| FoxP3 | ||||||||
| Mean | 2.5 | 48.2 | 2.8 | 2.5 | 8.8 | 0.7 | 0.001 | |
| SD | 1.1 | 2.7 | 2.9 | 1.8 | 2.1 | 0.7 | 2.1 | |
Mean cell counts per 400× high power field are represented for both total cell counts (intraepithelial + stromal) and cells within tumor islets (intraepithelial alone). Each value represents an average of five fields in each of three biopsies, i.e., 15 fields total.
One-way ANOVA test for equality of mean counts among patients.
MSE (mean square error) is a weighted average of the patient-specific variances.
SD = Standard deviation.
RNA isolation and quantitative PCR.
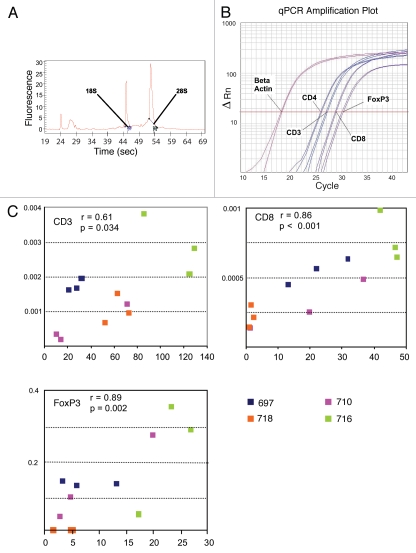
RNA of adequate quantity and quality (Fig. 2A) was obtained from five tumors (14/15 areas) tested. Total RNA yield ranged from 7–63 µg (median 19). Specimens obtained using one, two and three tissue cores yielded mean RNA quantities of 17, 32 and 63 µg, respectively. The only specimen with inadequate RNA (1 µg) was obtained from the same patient whose tumor also gave suboptimal tissue for histology. Real-time qPCR was performed for the genes CD3, CD4, CD8, FoxP3, IL-10 and TGFβ, normalized against β-actin (Fig. 2B). All samples were run in duplicate and normalized expression values were found to be reproducible among the different samples from the same metastatic tumor deposit. The mean normalized expression values and their standard deviations for CD3, CD8, FoxP3, IL-10 and TGFβ were statistically different among patients. Similarly to IHC, the overall within-patient variability was small, except for FoxP3, which exhibited higher patient-specific variances than other genes (Table 2). Correlations of IHC cell counts with qPCR gene expression data were assessed (Fig. 2C). Statistically significant correlations were found between cell counts and normalized qPCR values for CD3, CD8 and FoxP3 with p values of 0.034, <0.001 and 0.002, respectively.
Figure 2.
RNA expression from tumor embedded in cryomolds. (A) Spectrophotometric documentation of high-quality RNA preparation on Agilent Bioanalyzer. (B) Duplicate qPCR samples from one tumor for CD3, CD4, CD8 and FoxP3, with β-actin housekeeping gene. (C) Correlation of qPCR and total (stromal plus intraepithelial) IHC cell counts for CD3, CD8 and FoxP3 from four representative patients. All Pearson correlation coefficients show a linear relationship between the two methods of analysis, with p < 0.05.
Table 2.
qPCR abundance of immune-related markers, normalized to β-actin, representing duplicate determinations from three biopsies per patient
| Patient | ||||||
| 697 | 710 | 716 | 717 | 718 | pa | |
| n | n = 3 | n = 3 | n = 3 | n = 2 | n = 3 | |
| CD3 (×1,000) | ||||||
| Mean | 1.74 | 0.57 | 2.89 | 0.17 | 1.05 | 0.0015 |
| SDb | 0.17 | 0.55 | 0.87 | 0.01 | 0.42 | |
| CD8 (×1,000) | ||||||
| Mean | 0.55 | 0.28 | 0.78 | 0.13 | 0.22 | 0.0021 |
| SD | 0.09 | 0.17 | 0.18 | 0.00 | 0.08 | |
| FoxP3 (×,1000) | ||||||
| Mean | 0.16000 | 0.16000 | 0.27000 | 0.00040 | 0.00010 | <0.0001 |
| SD | 0.01000 | 0.15000 | 0.21000 | 0.00020 | 0.00003 | |
| IL-10 (×1,000) | ||||||
| Mean | 13.52 | 671.87 | 1309.20 | 1083.98 | 1519.40 | <0.0001 |
| SD | 3.82 | 300.46 | 1033.73 | 5218.10 | 585.22 | |
| TGFβ (×1,000) | ||||||
| Mean | 9.42 | 5.34 | 10.23 | 5.58 | 4.93 | 0.0053 |
| SD | 1.13 | 2.15 | 1.22 | 0.73 | 0.83 | |
Means and standard deviations are reported for each patient.
ANOVA test for equality of mean values after log10 transformation among patients.
SD = Standard deviation.
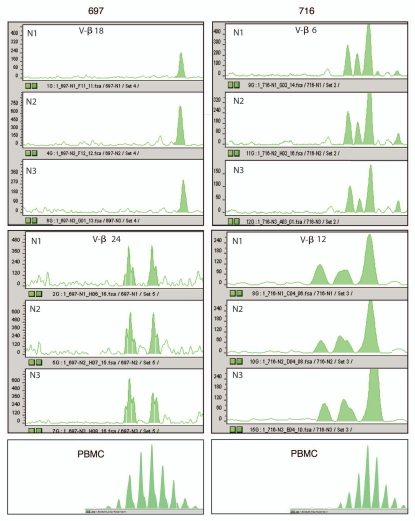
TCR spectratyping.
TCR spectratyping is a PCR based method that allows assessing the T-cell clonal repertoire in a sample. It is based on multiplexed PCR assessment of the entire CDR3 Vβ subfamily. Peaks corresponding to in-frame transcripts are detected at three nucleotide intervals. Since the position of the 5′ and 3′ primers are fixed, fragment size differences within each Vβ subfamily are due to different CDR3 lengths, reflecting VDJ region junctional diversity. In Figure 3, we show representative results obtained in two of the seven Vβ subfamilies analyzed in three areas from the same metastatic tumor deposit and matched PBMC. A highly reproducible TCR pattern is seen among different biopsies from the same tumor, confirming the feasibility and reproducibility of this analytical method. As expected, unstimulated PBMC of patients displayed a polyclonal pattern with a Gaussian-like distribution in all TCR Vβ subfamilies. Tumor tissue, on the contrary, displayed an oligoclonal and highly biased clonal pattern in the majority of the Vβ subfamilies examined.
Figure 3.
Selected oligoclonal V-β T-cell receptor (TCR) spectratyping expansion patterns in triplicate samples from two different patients (697 and 716) with peripheral blood mononuclear cell (PBMC) reference pattern.
Expression profiling.
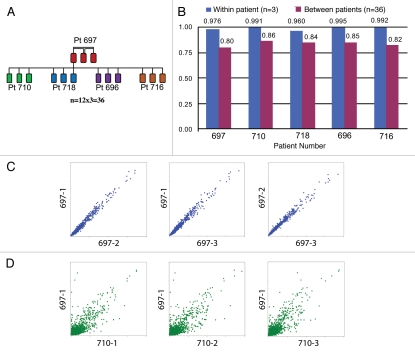
Gene expression profiling was performed in all 15 samples (three areas for each of five patients) for which RNA was isolated (including the lowest yield specimen of 1 µg) to test the feasibility of this approach using core needle biopsy material from solid tumors in general, and to assess the possible heterogeneity of gene expression among different areas of the same metastatic tumor deposit in ovarian cancer. We used 20,950 named genes that were on the Affymetrix array to compute pairwise Pearson's correlation coefficients. First, for each patient, we computed the within-patient correlations of gene expression between two of the three tumor areas (we performed a total of three pairwise correlations). Then, for each patient and for each of the three samples we computed between-patient correlations of gene expression between a given sample from this patient and all the other patients' samples. In other words, for each patient, we compared the three patient's samples to each of the other three samples from each of the other four patients, performing a total of 36 correlations for each patient (12 correlations for each of the three samples per patient) (Fig. 4A). The average correlation coefficient for the within-patient comparison was higher and closer to 1 (range 0.970–0.995) than the between-patient correlations, which ranged from 0.888–0.922 (Fig. 4B). Representative correlations for within-patient and between-patient comparisons are shown in Figure 4C and D, respectively. Thus, gene expression profiling is feasible and reproducible from tumor needle biopsy material and reveals significant homogeneity in within-patient comparisons.
Figure 4.
Expression profiling analysis. Pairwise Pearson correlations were computed using the expression of 20,950 named genes between samples as depicted in (A). There were a total of three within-patient correlations among samples from the same patient and 36 between-sample correlations when comparing each patient area to all the samples of all other patients. (B) A high average correlation was observed between samples from the same patient (within patient, blue) compared with the average correlation observed between different patients' samples (purple). (C and D) Scatter plots describing the correlation of gene expression values between samples: (C) between samples from the same patient and (D) among samples from different patients.
Discussion
This study tested the feasibility of tumor-based immune monitoring and concludes that it can easily be performed using tissue obtained with a CT-compatible biopsy needle. Quick and accurate assessment of tumor immune phenotype can aid in patient selection as well as monitoring of patients undergoing immunologic therapy. Here we demonstrate that adequate tissue for immunohistochemistry, quantitative real time PCR, TCR V-β CDR3 spectratyping and gene expression microarrays can be obtained with as little as one pass of a core biopsy needle.
Our tissue collection and processing technique provided good histology and excellent RNA quality. Immunohistochemistry was feasible in 18/21 specimens, with all failures accounted for by one tumor which demonstrated significant necrosis. The determination of the frequency of tumor infiltrating CD3, CD8 and regulatory T cells was feasible and reproducible within serial specimens. In addition, a large quantity of high-quality RNA was obtained from 14/15 needle biopsies, including from two samples that yielded less-than-optimal histology quality. RNA was sufficient for performing qPCR analysis of several immune-related genes, TCR spectratyping as well as global gene expression analysis, which can provide a detailed analysis of immune infiltrate and the tumor microenvironment. In addition, for most samples a surplus of RNA remained which could be used for additional testing. Analysis of the isolated RNA revealed that expression data from selected immune markers as well as from gene expression microarrays were reproducible when tumor nodules were assayed multiple times. Importantly, these assays revealed relatively limited tissue heterogeneity among different areas within the same tumor nodules.
The focus of immune therapies is to increase immunologic recognition of tumors leading to increased tumor immune rejection. Traditionally, studies evaluating immune-based therapies have measured responses in the periphery by assessing various immunologic parameters in patients' blood or serum. Such assays include profiles of serum cytokine; detailed phenotypic analysis of peripheral blood lymphocytes; flow cytometry quantification of antigen-specific T cells by tetramers; cytokine responses of T cells to antigen ex vivo using Elispot, ELISA and/or intracellular cytokine assays; and chromium release assays.25 These studies have allowed patient classification into cohorts based on presence or absence of immune response, which are then used to test correlation between measured immune response and tumor radiographic response or survival endpoints. However, except for the persistence in circulation of T cells with central memory phenotype after adoptive transfer,26 these studies have fallen short of expectations in terms of predicting clinical response to immune therapy and have proven not particularly helpful in helping interpret failures or successes of immunotherapy. Mechanisms at the tumor microenvironment are critical to control the ability of effector cells to infiltrate tumors and therefore the success of immunotherapy. These mechanisms can be now interrogated with sophisticated molecular tools using tumor needle biopsies.
Previously, our lab reported that T-cell infiltration of tumor islets was associated with significantly improved survival for patients with advanced ovarian cancer.10 Further data from our lab and others4,11,14 have supplemented these findings, documenting that the presence of effector CD8+ T cells predicts improved survival, while the presence of regulatory T cells negatively affects survival. These two cellular biomarkers are straightforward to interpret and can be used on biopsy material as meaningful endpoints in patients as they undergo immune-based therapies. Furthermore, we show that quantitative analysis of mRNA levels of cell lineage markers such as CD3-ε (T cells), CD8A (CD8+ cells), FoxP3 (CD4+ Treg), cytokines as well as genes mediating or regulating the immune response can now be done reproducibly quantified through qPCR or even gene expression arrays. While the analyses performed in this study produced voluminous information about each tumor and its immune microenvironment, this is merely a fraction of the data that could be obtained using tissue obtained in this fashion. In addition to T-cell infiltration, immunologic profiles can be constructed using the array expression data and TCR spectratyping can be used to follow the development and expansion of oligoclonal T-cell populations. With modifications in tissue processing, tumor tissue could also be used for phosphoprotein pathway analysis to identify candidates for other biologic and small molecule therapies.
Some limitations to this work must be acknowledged. Our specimens were collected at surgery, so it remains to be determined if samples of equal quality can be obtained using a radiographic-guided approach for ovarian tumors. One would expect similar results if a similar needle were used. Furthermore, while we provide examples of reproducible results from three separate sites within one metastatic tumor deposit, this study does not address the possible heterogeneity, cellular and/or molecular, among different metastatic sites within the same patient, and how this might affect longitudinal or cross-sectional studies. Our companion manuscript analyzes precisely these parameters and concludes that heterogeneity among metastatic sites exists in some patients but some biomarkers, specifically intraepithelial TILs, can be estimated from a single biopsy with minimal error. Despite limitations and challenges, the present study demonstrates that a large volume of consistent, informative data can be obtained about a tumor's biology and its microenvironment using tissue specimens obtained via needle biopsy.
Materials and Methods
Patients and tissue collection.
This study was approved by Penn's Institutional Review Board. Tumor samples were collected at the University of Pennsylvania between October 2005 and May of 2006 from a total of seven patients ranging in age from 40–71, median age of 54. Patients were prospectively identified and informed consent for tissue collection was obtained preoperatively. Six patients were undergoing primary surgery while another was undergoing secondary debulking for recurrent disease. Of the six with primary diagnosis, two had primary peritoneal and four had epithelial ovarian cancer, all stage IIIC. Just prior to tumor collection, plastic cryomolds were filled half to three-quarters full with Optimal Cutting Temperature (OCT) Compound (Sakura Tissue-Tek®) and placed on dry ice. Immediately upon removal from the patient, fresh tumor nodules 2–5 cm in diameter were subjected to open air needle biopsy in the operating room. A single metastatic deposit was analyzed per patient and each tumor nodule was biopsied in up to three different areas at least 0.5 cm apart (to assess tissue heterogeneity) using a 16-gauge Quick-Core® Biopsy Needle (Cook Medical®); for each area we obtained up to three needle cores (Fig. 1A). Only metastatic tumor was sampled, comprising omental and peritoneal metastases. The tumor tissue cores were immediately transferred into the frozen cryomold prefilled with OCT, covered in OCT and stored at −80°C. A total of three specimens were collected for each patient.
Tissue processing.
Tumor tissue was cut concurrently for RNA isolation and immunohistochemistry. The middle portion of the sample with the largest area was cut in 7 µ thick sections for IHC (Fig. 1A). The remainder of the specimen was cut in 20 µ thick sections and used for RNA isolation.
Immunohistochemistry.
Tissue slides were fixed in ice-cold acetone and peroxidase block. For FoxP3 staining, protein block was performed using horse serum. Primary antibodies used were rabbit polyclonal anti-human CD3 (1:200, Dako); mouse monoclonal anti-human CD8 (1:100, Dako), mouse monoclonal anti-human CD4 (1:50 BD PharMingen) and goat polyclonal anti-human FoxP3 (1:200, Novus Biologicals). Secondary antibody detection system (Dako Envision Plus) with Dako DAB Plus was used for CD3, CD4 and CD8. Biotinylated anti-goat antibody (Vectastain) and Dako streptavidin and DAB were used to develop FoxP3 staining. All stains were performed using the Dako Autostainer and slides were counterstained with hematoxylin. Slides were read in a blinded fashion for each marker. Total cell counts and intraepithelial cell counts were recorded and averaged over five high power fields (200×) and reported for each tumor.
RNA isolation.
RNA was isolated from tumor sections and peripheral blood mononuclear cells (PBMC) using the TRIzol Reagent (Invitrogen®) and reconstituted in RNase-free water. RNA quantity and quality were then measured using the Agilent 2100 Bioanalyzer.
Quantitative PCR.
RNA samples were treated with DNase I (Invitrogen) and cDNA was created for each sample using Superscript II First Strand Synthesis Kit for RT-PCR (Invitrogen). Experiments with real-time quantitative PCR (qPCR) were performed with the use of the ABI Prism 7900 Analyzer and SYBR Green PCR kits (Applied Biosystems). cDNA was normalized against the housekeeping gene β-actin.
TCR-spectratyping.
T-cell receptor (TCR) spectratyping was performed as previously described at the Molecular Pathology Core of the University of Pennsylvania.27 Each CDR3 β chain variable region (Vβ) was amplified with one of the 24 Vβ subfamily-specific primers previously described and a β chain constant region (Cβ) primer recognizing both Cβ1 and Cβ2 regions. PCR amplification of Vβ5 and Vβ13 each required the use of two sets of primers to identify the entire Vβ subfamily. Amplification primers are provided as supplemental information. The Cβ primer was conjugated to fluorescent dye 6-FAM (Applied Biosystems, Inc., Foster City, CA) for CDR3 size analysis. The size distribution of each fluorescent PCR product was determined by electrophoresis on an automated 373 DNA sequencer (Applied Biosystems) using a 5.5% polyacrylamide gel and data was analyzed by ABI Prism software.
Gene expression profiling.
Gene expression studies were performed at the Cancer Biology Branch, NCI, as previously described in reference 28. Two rounds of amplification were used as previously described. Briefly, during first round cDNA synthesis, 100 ng of total RNA was reverse transcribed using the Two-Cycle cDNA Synthesis Kit (Affymetrix, Santa Clara, CA) and oligo-dT24-T7 (5′-GGC CAG TGA ATT GTA ATA CGA CTC ACT ATA GGG AGG CGG-3′) primer according to the manufacturer's instructions. First round amplification was completed using the T7 promoter coupled double stranded cDNA as template and the MEGAscript T7 Kit (Ambion, Inc., Austin, TX). Following cleanup of the cRNA with a GeneChip® Sample Cleanup Module IVT column (Affymetrix, Santa Clara, CA), second round double stranded cDNA was generated and purified using a GeneChip® Sample Cleanup Module cDNA column (Affymetrix, Santa Clara, CA). After amplification and biotinylation with the IVT Labeling Kit (Affymetrix, Santa Clara, CA), a 15.0 µg aliquot of labeled product was fragmented by heat and ion-mediated hydrolysis and hybridized to human U133A 2.0 oligonucleotide GeneChip® arrays (Affymetrix, Santa Clara, CA), which comprise over 500,000 unique oligonucleotide features covering more than 18,400 transcripts and variants on a single chip. The microarrays were subsequently stained for visualization in a Fluidics Station 450 and scanned using the laser confocal GeneChip® Scanner 3000 (Affymetrix, Santa Clara, CA). Affymetrix array CEL files were processed in R using an algorithm that uses genes in the least variant set (LVS) to normalize the expression data.29
Statistical analysis.
Mean values and standard deviations were calculated from the three specimens from each tumor and ANOVA was used to compare means between the various tumors for each of the immunohistochemical cell counts as well as for normalized qPCR values. Pearson's correlations were computed to evaluate the association between immunohistochemistry and PCR results within and between patients. ANOVA was applied to the microarray data with the five different patients defining the groups of measurements (each with three replicates). Pairwise Pearson's correlations were performed between all specimens. The three correlation coefficients for the index patient were then averaged and this average was compared with the average of the correlation coefficients computed using a specimen from the index patient and a specimen from one of the other patients.
Acknowledgments
This work was supported by Ovarian Cancer SPORE NIH P50-CA083638 and the Ovarian Cancer Research Foundation.
References
- 1.Brill TH, Kubler HR, Pohla H, Buchner A, Fend F, Schuster T, et al. Therapeutic vaccination with an interleukin-2-interferon-gamma-secreting allogeneic tumor vaccine in patients with progressive castration-resistant prostate cancer: a phase I/II trial. Hum Gene Ther. 2009;20:1641–1651. doi: 10.1089/hum.2009.101. [DOI] [PubMed] [Google Scholar]
- 2.Redfern CH, Guthrie TH, Bessudo A, Densmore JJ, Holman PR, Janakiraman N, et al. Phase II trial of idiotype vaccination in previously treated patients with indolent non-Hodgkin's lymphoma resulting in durable clinical responses. J Clin Oncol. 2006;24:3107–3112. doi: 10.1200/JCO.2005.04.4289. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 5.Croci DO, Zacarias Fluck MF, Rico MJ, Matar P, Rabinovich GA, Scharovsky OG. Dynamic cross-talk between tumor and immune cells in orchestrating the immunosuppressive network at the tumor microenvironment. Cancer Immunol Immunother. 2007;56:1687–1700. doi: 10.1007/s00262-007-0343-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leffers N, Lambeck AJ, Gooden MJ, Hoogeboom BN, Wolf R, Hamming IE, et al. Immunization with a p53 synthetic long peptide vaccine induces P53-specific immune responses in ovarian cancer patients, a phase II trial. Int J Cancer. 2009;125:2104–2113. doi: 10.1002/ijc.24597. [DOI] [PubMed] [Google Scholar]
- 8.Chianese-Bullock KA, Irvin WP, Jr, Petroni GR, Murphy C, Smolkin M, Olson WC, et al. A multipeptide vaccine is safe and elicits T-cell responses in participants with advanced stage ovarian cancer. J Immunother. 2008;31:420–430. doi: 10.1097/CJI.0b013e31816dad10. [DOI] [PubMed] [Google Scholar]
- 9.Wei S, Kryczek I, Edwards RP, Zou L, Szeliga W, Banerjee M, et al. Interleukin-2 administration alters the CD4+FOXP3+ T-cell pool and tumor trafficking in patients with ovarian carcinoma. Cancer Res. 2007;67:7487–7494. doi: 10.1158/0008-5472.CAN-07-0565. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 11.Adams SF, Levine DA, Cadungog MG, Hammond R, Facciabene A, Olvera N, et al. Intraepithelial T cells and tumor proliferation: impact on the benefit from surgical cytoreduction in advanced serous ovarian cancer. Cancer. 2009;115:2891–2902. doi: 10.1002/cncr.24317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarke B, Tinker AV, Lee CH, Subramanian S, van de Rijn M, Turbin D, et al. Intraepithelial T cells and prognosis in ovarian carcinoma: novel associations with stage, tumor type and BRCA1 loss. Mod Pathol. 2009;22:393–402. doi: 10.1038/modpathol.2008.191. [DOI] [PubMed] [Google Scholar]
- 13.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah CA, Allison KH, Garcia RL, Gray HJ, Goff BA, Swisher EM. Intratumoral T cells, tumor-associated macrophages and regulatory T cells: Association with p53 mutations, circulating tumor DNA and survival in women with ovarian cancer. Gynecol Oncol. 2008;109:215–219. doi: 10.1016/j.ygyno.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Tomšová M, Melichar B, Sedlakova I, Steiner I. Prognostic significance of CD3+ tumor-infiltrating lymphocytes in ovarian carcinoma. Gynecol Oncol. 2008;108:415–420. doi: 10.1016/j.ygyno.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Stumpf M, Hasenburg A, Riener MO, Jutting U, Wang C, Shen Y, et al. Intraepithelial CD8-positive T lymphocytes predict survival for patients with serous stage III ovarian carcinomas: relevance of clonal selection of T lymphocytes. Br J Cancer. 2009;101:1513–1521. doi: 10.1038/sj.bjc.6605274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, et al. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res. 2001;61:5132–5136. [PubMed] [Google Scholar]
- 19.Schumacher K, Haensch W, Roefzaad C, Schlag PM. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res. 2001;61:3932–3936. [PubMed] [Google Scholar]
- 20.Vesalainen S, Lipponen P, Talja M, Syrjanen K. Histological grade, perineural infiltration, tumour-infiltrating lymphocytes and apoptosis as determinants of long-term prognosis in prostatic adenocarcinoma. Eur J Cancer. 1994;30:1797–1803. doi: 10.1016/0959-8049(94)E0159-2. [DOI] [PubMed] [Google Scholar]
- 21.Marrogi AJ, Munshi A, Merogi AJ, Ohadike Y, El-Habashi A, Marrogi OL, et al. Study of tumor infiltrating lymphocytes and transforming growth factor-beta as prognostic factors in breast carcinoma. Int J Cancer. 1997;74:492–501. doi: 10.1002/(sici)1097-0215(19971021)74:5<492::aid-ijc3>3.0.co;2-z. DOI:10.1002/(SICI)1097-0215(19971021)74:5<492::AID-IJC3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 22.Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–3494. [PubMed] [Google Scholar]
- 23.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 24.Pagís F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 25.Clay TM, Hobeika AC, Mosca PJ, Lyerly HK, Morse MA. Assays for monitoring cellular immune responses to active immunotherapy of cancer. Clin Cancer Res. 2001;7:1127–1135. [PubMed] [Google Scholar]
- 26.Dudley ME, Rosenberg SA. Adoptive cell transfer therapy. Semin Oncol. 2007;34:524–531. doi: 10.1053/j.seminoncol.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maus MV, Thomas AK, Leonard DG, Allman D, Addya K, Schlienger K, et al. Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4-1BB. Nat Biotechnol. 2002;20:143–148. doi: 10.1038/nbt0202-143. [DOI] [PubMed] [Google Scholar]
- 28.Donninger H, Bonome T, Radonovich M, Pise-Masison CA, Brady J, Shih JH, et al. Whole genome expression profiling of advance stage papillary serous ovarian cancer reveals activated pathways. Oncogene. 2004;23:8065–8077. doi: 10.1038/sj.onc.1207959. [DOI] [PubMed] [Google Scholar]
- 29.Calza S, Valentini D, Pawitan Y. Normalization of oligonucleotide arrays based on the least-variant set of genes. BMC Bioinformatics. 2008;9:140. doi: 10.1186/1471-2105-9-140. [DOI] [PMC free article] [PubMed] [Google Scholar]