Abstract
Background
Natriuretic peptides (NP’s) have prognostic value across a wide spectrum of cardiovascular diseases and may predict cognitive dysfunction in patients with cardiovascular disease even in the absence of prior stroke. Little is known about the association of NP’s with cognitive function in community-dwelling adults. We assessed the association between NT-proBNP levels and cognitive function in community-dwelling ambulatory older adults in the Rancho Bernardo Study.
Methods
We studied 950 men and women, aged 60 years and older, who attended a research clinic visit where a medical history and examination were performed, and blood for cardiovascular disease risk factors and NT-proBNP levels were obtained. Three cognitive function tests were administered: Mini Mental State Exam (MMSE), Trail-Making Test B (Trails B), and Category Fluency.
Results
Participants with high NT-proBNP levels (≥450 pg/mL, n=198) were older and had a higher prevalence of coronary heart disease (12% vs. 30%), and stroke (5% vs. 11%) (both p’s≤0.001). In unadjusted analyses, all three cognitive function test scores were significantly associated with NT-proBNP levels (p<0.001). After adjusting for age, sex, education, hypertension, body mass index, exercise, alcohol use, smoking, low density lipoprotein cholesterol, creatinine clearance, and prior cardiovascular disease, elevated NT-proBNP levels remained independently associated with poor cognitive performance on MMSE (odds ratio [95% confidence interval] 2.0 [1.1–3.6], p=0.02) and Trails B (1.7 [1.2–2.7], p=0.01), but not Category Fluency (1.4 [0.9–2.2], p=0.19). Results were unchanged after excluding the 6% of participants with a history of stroke.
Conclusions
NT-proBNP levels were strongly and independently associated with poor cognitive function in community-dwelling older adults.
Keywords: natriuretic peptides, cerebrovascular disorders, cardiovascular diseases, community, elderly, cognitive function
INTRODUCTION
Cardiovascular disease is associated with cognitive deficits in patients without a history of stroke.1 Coronary heart disease, congestive heart failure, and hypertension (even when controlled) have been associated with reduced cognitive function.1–5 However, by the time cognitive dysfunction becomes clinically apparent, significant cerebral damage may have already occurred.6,7 Because of this, there is an urgent need for early markers of cognitive dysfunction.
Natriuretic peptides (NP’s), neurohormones released from cardiomyocytes in response to stretch, have prognostic value across a wide spectrum of cardiovascular disease, and are predictive of future cardiovascular morbidity and mortality in apparently healthy individuals.8–11 A small study of 56 older adults reported that NP’s can predict cognitive function in patients with cardiovascular disease in the absence of a prior stroke.12 However, little is known about the association of NP’s with cognitive function in community-dwelling individuals.
The release of NP’s by the heart reflects the cumulative effects of a number of pathophysiologic pathways which, in concert, result in cardiomyocyte strain. Because previous studies have shown that elevated NP levels are triggered not just by overt cardiovascular disease and heart failure but also by subclinical disease processes and even by cardiovascular risk factors,13–15 and because of the increasingly recognized link between cardiovascular disease and cognitive dysfunction,1,4,16 we hypothesized that NP’s may be associated with cognitive impairment early in the disease process. The purpose of this study was to assess the cross-sectional association between N-terminal pro B-type NP (NT-proBNP) levels and cognitive function in a population-based sample of ambulatory community-dwelling older adults in the Rancho Bernardo Study.
METHODS
Study Population
The Rancho Bernardo Study is a prospective, population-based study of the epidemiology of chronic disease. Between 1972–1974, all community-dwelling residents (ages 30 to 79 years) of Rancho Bernardo, a community in Southern California, were invited to participate, and 82% (n=5,052) enrolled. Nearly all were Caucasian, middle to upper-middle class, and relatively well-educated. The present analysis consists of the 950 of 1,096 surviving, locally resident participants who underwent cognitive function testing at a follow-up visit between 1997–1999, and who had frozen plasma available for NT-proBNP analysis. The study protocol was approved by the human research protection program at the University of California at San Diego (La Jolla, California); all participants gave written informed consent prior to participation.
Data Collection
At the 1997–1999 research visit, information was collected on demographics, medical history, and lifestyle. Information on physical activity (exercise 3+ times/week, yes/no), alcohol consumption (3+drinks/week, yes/no), and current smoking (yes/no) were obtained using standard Rancho Bernardo Study questionnaires. Current medication use was validated by examination of pills and prescriptions. Blood pressure was measured in seated, resting subjects;17 the mean of two readings was used. Body mass index (BMI) was derived from height and weight measured with participants wearing light clothing and no shoes. Diabetes was defined by a fasting plasma glucose level ≥126mg/dl, reported physician diagnosis, or use of diabetes medications. Hypertension was defined by use of anti-hypertensive medication, or resting blood pressure >140mmHg systolic or 90mmHg diastolic. Estimated creatinine clearance (CrCl) was calculated using the Cockroft-Gault formula.18,19 Prevalent coronary heart disease was defined as a history of physician-diagnosed myocardial infarction or coronary revascularization; prevalent cardiovascular disease was defined as a history of coronary heart disease or stroke, as previously described.20 Self-reported cardiovascular disease was validated by review of medical records in 85% of a random 30% sample.
Laboratory Methods
Blood samples were obtained after an overnight fast; serum and plasma were separated and stored at −70°C. Lipid and fasting plasma glucose levels were measured as previously described.9 In 2006, the Elecsys® 2010 system (Roche Diagnostics, Indianapolis, Indiana) was used for analysis of NT-proBNP using the Elecsys® proBNP sandwich immunoassay (measurable range 5–35,000pg/ml)21,22 in plasma from the 1997–1999 research clinic visit, that had been stored at −70°C. Intra-assay and inter-assay coefficients of variation at various concentrations of NT-proBNP have been described,21 and NT-proBNP has previously been shown to have excellent stability and correlation with fresh samples (r=0.99) after long-term storage even at −20°C.23
Cognitive Function Testing
Mini Mental State Exam (MMSE) (n=950), Trail-Making Test B (Trails B) (n=950), and Category Fluency (n=939) tests were administered to study participants at the 1997–1999 research visit by specially trained staff who were blinded to the participant’s medical history. The MMSE is designed to assess orientation, registration, attention, calculation, language, and recall; this test of global cognitive function is used as a screening test for possible dementia. Scores range from 0 to 30 with poor performance characterized by a score ≤24.24 The Trails B is a test of visuomotor tracking and attention plus executive function, whereby participants scan a page continuously to identify numbers and letters in a specified sequence while shifting from number to letter sets. Participants are scored based on the time it takes to complete the task, with a maximum of 300 seconds allowed; higher scores reflect poorer performance, with categorical poor performance defined as ≥132.25 In the Category Fluency test, an assessment of cognitive flexibility and executive functioning, participants name as many animals as possible in 1 minute, with higher scores reflecting more items named correctly; a score ≤12 is the cut-point for poor performance.26
Statistical Analysis
Continuous variables are presented as means ± standard deviation, and dichotomous variables as percentages. Participants were classified by NT-proBNP level into quartiles and also into “Low” (<450pg/ml, n=752) and “High” (≥450pg/ml, n=198) groups. This cut-point corresponds to the recommended decision threshold for the general age group represented in the Rancho Bernardo Study,21 and has been used previously for stratification of community-based populations.9 Comparisons of risk factors and clinical characteristics between participants with low versus high NT-proBNP levels were performed with t tests for continuous variables and Chi-squared tests for categorical variables. HDL, triglycerides, and NT-proBNP were not normally distributed and were log transformed for analyses; geometric means are reported.
LogNT-proBNP levels were compared for groups based on quartiles of cognitive function test score using analysis of variance, with means expressed as the antilogs of transformed means. Multiple regression analysis was used to examine the association of low vs. high NT-proBNP with cognitive function test scores before and after adjustment for age, BMI, and low density lipoprotein (LDL) cholesterol as continuous variables, and for sex, education, hypertension, exercise, alcohol use, smoking status, and estrogen use (for women) as categorical variables. The influence of prevalent cardiovascular disease was tested by further adjusting for a history of cardiovascular disease.
Poor performance on each test was defined using the pre-validated binary cut-points described above. Logistic regression analysis was used to examine the association between high NT-proBNP levels and poor performance on each cognitive function test before and after adjustment for covariates. Again, the influence of prevalent cardiovascular disease was tested by additionally adjusting for a history of cardiovascular disease, and also by repeating analyses after excluding those with prior stroke and prior cardiovascular disease. All statistical tests were two-tailed; p<0.05 was considered statistically significant. Data were analyzed using SPSS 12.0 (Chicago, IL).
RESULTS
Baseline Characteristics
Demographic, clinical, and laboratory characteristics of this study population by NT-proBNP level are shown in Table 1. The mean age of participants was 77 (range 60–97) years; 39% were men. Overall, 12% of the population had diabetes, 66% had hypertension, and 19% had prevalent cardiovascular disease, including 6% with a prior stroke. Participants with high NT-proBNP levels (n=198, 21%) were older, and had higher systolic blood pressure, poorer renal function, and a higher prevalence of hypertension, myocardial infarction and stroke than participants with low NT-proBNP levels. They also had lower BMI, lower total and LDL-cholesterol levels, and were less likely to exercise regularly or be taking statins.
Table 1.
Baseline characteristics of the study population
| NT-proBNP* |
|||
|---|---|---|---|
| Variable | Low (n=752) |
High (n=198) |
p |
| Age, yrs | 74.9 ± 8.2 | 82.8 ± 6.6 | <0.001 |
| % Male | 39.8 | 36.4 | 0.38 |
| College graduate, % | 40.1 | 42.0 | 0.66 |
| High School Graduate, % | 98.2 | 98.8 | 0.99 |
| Cardiovascular Risk Factors | |||
| Hypertension, % | 61.2 | 84.8 | <0.001 |
| Heart rate, bpm | 65.8 ± 9.2 | 65.5 ± 11.6 | 0.76 |
| Systolic BP, mm Hg | 135.7 ± 18.7 | 147.1 ± 24.6 | <0.001 |
| Diastolic BP, mm Hg | 73.9 ± 8.7 | 72.2 ± 11.0 | 0.046 |
| Current smoking, % | 4.0 | 4.0 | 0.97 |
| Ever smoked, % | 53.7 | 56.1 | 0.56 |
| Diabetes, % | 11.2 | 13.6 | 0.34 |
| Pre-Existent Disease | |||
| MI, % | 8.0 | 25.8 | <0.001 |
| CHD, % | 12.1 | 30.3 | <0.001 |
| Stroke, % | 4.8 | 11.1 | 0.001 |
| CVD, % | 14.6 | 36.4 | <0.001 |
| Medication Use | |||
| Aspirin, % | 41.9 | 37.1 | 0.22 |
| Lipid-Lowering, % | 19.6 | 12.7 | 0.025 |
| Nutrition and Activity | |||
| Body mass index, kg/m2 | 25.6 ± 4.1 | 24.4 ± 4.1 | <0.001 |
| Exercise ≥3x/wk, % | 76.7 | 62.6 | <0.001 |
| Alcohol ≥3x/wk, % | 49.6 | 48.0 | 0.69 |
| Laboratory Values | |||
| Fasting glucose, mg/dl | 103.7 ± 20.6 | 103.9 ± 21.0 | 0.86 |
| CrCl, ml/min | 66.1 ± 23.1 | 47.9 ± 17.9 | <0.001 |
| Triglycerides†, mg/ld | 110.2 | 102.2 | 0.093 |
| HDL cholesterol†, mg/dl | 57.5 | 58.3 | 0.65 |
| LDL cholesterol, mg/dl | 122.9 ± 31.8 | 113.4 ± 34.0 | <0.001 |
| NT-proBNP†, pg/ml | 112.0 | 973.0 | <0.001 |
| Poor Performance on: | |||
| MMSE, % | 5.1 | 16.7 | <0.001 |
| Trails B, % | 23.4 | 53.6 | <0.001 |
| Category Fluency, % | 11.6 | 26.3 | <0.001 |
For NT-proBNP groups, "Low " is <450 pg/ml; "High" is ≥450 pg/ml.
Geometric mean
BP=blood pressure; BPM=beats per minute; CHD=coronary heart disease; CrCl=creatinine clearance; CVD=cardiovascular disease; HDL=high-density lipoprotein; LDL=low -density lipoprotein; MI=myocardial infarction; MMSE=Mini Mental State Exam; NT-proBNP=N-terminal pro B-type natriuretic peptide.
NT-proBNP Levels and Cognitive Function Scores
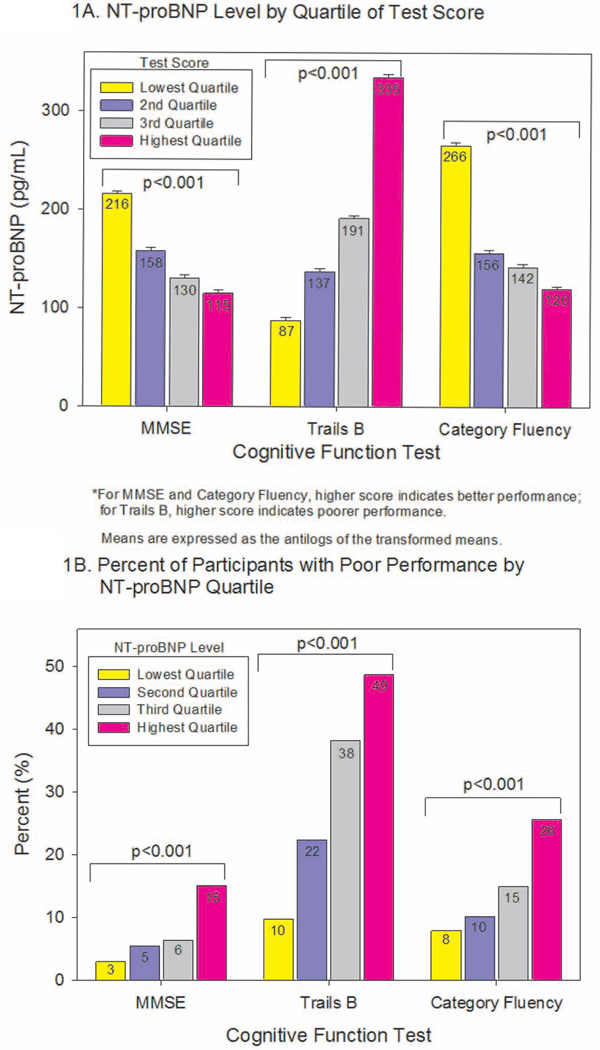
We first analyzed cognitive function based on quartiles of test scores, and found that NT-proBNP levels increased with worsening scores (Figure 1A). Comparisons of scores between participants with categorically-defined low versus high NT-proBNP levels are shown in Table 2. Before and after adjusting for age, sex, and education, participants with elevated NT-proBNP had worse scores on each of the three tests. After further adjusting for hypertension, BMI, exercise, alcohol use, smoking, and LDL-cholesterol, elevated NT-proBNP levels were still independently associated with worse MMSE and Trails B scores, and with a similar but nonsignificant trend toward worse scores on Category Fluency testing. Results were essentially unchanged after additionally adjusting for a history of cardiovascular disease. Stratification by sex yielded similar results for men and for women, except NT-proBNP levels were more strongly associated with MMSE scores in women than in men.
Figure 1.
A. Mean NT-proBNP level by quartile of cognitive function test performance. Means and error bars are expressed as the antilog of the transformed means and standard deviations, respectively. Cut-points for quartile of MMSE scores are: ≤27, 28, 29, and 30. Cut-points for Trails B are: ≤75, ≤100, ≤139, and >139. Cut-points for Category Fluency are: ≤15, ≤18, ≤21, and >21.
B. Percent of participants with poor performance on each cognitive function test, by quartile of NT-proBNP. Cut-points for quartiles of NT-proBNP are: ≤74.8 pg/mL, ≤175.1 pg/mL, ≤364.7 pg/mL, and >364.7 pg/mL.
MMSE = Mini Mental State Exam, Trails B = Trail-Making Test B.
Table 2.
Cognitive function scores by NT-proBNP level (mean ± SD) in men and women
| All Participants NT-proBNP* |
Men NT-proBNP* |
Women NT-proBNP* |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | Low (n=752) |
High (n=198) |
p | Low (n=299) |
High (n=72) |
p | Low (n=453) |
High (n=126) |
p |
| MMSE Score | |||||||||
| Unadjusted | 27.5 ± 1.8 | 26.4 ± 2.9 | <0.001 | 27.4 ± 1.7 | 26.3 ± 2.9 | 0.004 | 27.6 ± 1.9 | 26.4 ± 2.8 | <0.001 |
| Age, sex, education† | 27.3 ± 2.0 | 26.9 ± 2.1 | 0.012 | 27.2 ± 1.9 | 27.0 ± 2.0 | 0.58 | 27.4 ± 2.0 | 26.9 ± 2.1 | 0.010 |
| All covariates†‡ | 27.3 ± 2.0 | 26.9 ± 2.1 | 0.013 | 27.2 ± 1.9 | 27.0 ± 2.0 | 0.56 | 27.4 ± 2.0 | 26.9 ± 2.2 | 0.014 |
| All covariates + prior CVD | 27.3 ± 2.0 | 26.9 ± 2.1 | 0.021 | 27.2 ± 1.9 | 27.0 ± 2.0 | 0.60 | 27.4 ± 2.0 | 26.9 ± 2.2 | 0.022 |
| Trails B Score§ | |||||||||
| Unadjusted | 114 ± 59 | 162 ± 77 | <0.001 | 106 ± 54 | 157 ± 78 | <0.001 | 120 ± 62 | 165 ± 76 | <0.001 |
| Age, sex, education† | 120 ± 57 | 141 ± 60 | <0.001 | 111 ± 54 | 134 ± 57 | 0.003 | 125 ± 59 | 145 ± 61 | 0.005 |
| All covariates†‡ | 121 ± 57 | 139 ± 60 | <0.001 | 111 ± 54 | 133 ± 58 | 0.007 | 127 ± 59 | 142 ± 62 | 0.015 |
| All covariates + prior CVD | 121 ± 57 | 137 ± 60 | 0.001 | 111 ± 54 | 132 ± 58 | 0.012 | 127 ± 59 | 140 ± 63 | 0.042 |
| Category Fluency Score | |||||||||
| Unadjusted | 18.1 ± 4.9 | 15.7 ± 4.9 | <0.001 | 18.8 ± 5.0 | 16.8 ± 5.3 | 0.002 | 17.7 ± 4.8 | 15.1 ± 4.5 | <0.001 |
| Age, sex, education† | 17.8 ± 4.6 | 17.0 ± 4.8 | 0.031 | 18.5 ± 4.9 | 18.1 ± 5.2 | 0.53 | 17.4 ± 4.4 | 16.3 ± 4.6 | 0.016 |
| All covariates†‡ | 17.8 ± 4.6 | 17.0 ± 4.9 | 0.056 | 18.5 ± 4.9 | 18.2 ± 5.3 | 0.63 | 17.3 ± 4.4 | 16.4 ± 4.7 | 0.062 |
| All covariates + prior CVD | 17.8 ± 4.7 | 17.1 ± 5.0 | 0.078 | 18.5 ± 4.9 | 18.1 ± 5.3 | 0.60 | 17.3 ± 4.4 | 16.5 ± 4.8 | 0.10 |
For NT-proBNP groups, "Low" is <450 pg/ml; "High" is ≥450 pg/ml.
CVD=cardiovascular disease; MMSE = Mini Mental State Exam
Not adjusted for sex in sex-stratified analyses.
All covariates = age, sex, education, hypertension, BMI, exercise, alcohol use, current smoking, LDL cholesterol, creatinine clearance, and estrogen use (for women only).
For Trails B, lower score is better; n=939 (747 with low NT-proBNP, 192 with high NT-proBNP).
NT-proBNP Levels and Poor Cognitive Performance
Based on established cut-points, 7% of participants performed poorly on the MMSE, 30% on Trails B, and 15% on Category Fluency testing. When stratified by NT-proBNP level, poor performance was more common among participants with increasing quartile of NT-proBNP for each of the three tests (Figure 1B).
Logistic regression analysis was used to determine the independent association of NT-proBNP with poor performance for each of the cognitive function tests. In unadjusted analyses, participants with elevated NT-proBNP levels were almost four times more likely to have poor MMSE and Trails B scores, and almost three times more likely to have poor Category Fluency scores compared to those with low NT-proBNP levels (Table 3). After adjusting for age, sex, and education, high NT-proBNP was still associated with an increased likelihood of poor cognitive performance on all three tests. Further adjusting for hypertension, BMI, exercise, alcohol use, smoking, and LDL-cholesterol only moderately attenuated the risk estimates, although the increased risk was no longer statistically significant for the Category Fluency test. Further adjustment for use of aspirin or lipid lowering agents did not materially change the results (data not shown).
Table 3.
Association of high NT-proBNP with poor performance on cognitive function tests.
| Model | All Participants (n=950) OR (95% CI)* |
Participants Without Prior Stroke (n=892) OR (95% CI)* |
Participants Without Prior CVD (n=768) OR (95% CI)* |
|---|---|---|---|
| MMSE | |||
| Unadjusted | 3.76 (2.29–6.17) | 4.12 (2.44–6.95) | 3.81 (2.12–6.87) |
| Age, sex, education | 2.04 (1.18–3.52) | 2.18 (1.22–3.90) | 1.90 (0.98–3.72) |
| All covariates† | 1.96 (1.09–3.52) | 2.03 (1.10–3.78) | 1.62 (0.80–3.29) |
| All covariates + prior CVD | 1.98 (1.09–3.57) | 2.13 (1.14–3.98) | N/A |
| Trails B‡ | |||
| Unadjusted | 3.78 (2.72–5.26) | 3.62 (2.56–5.13) | 3.48 (2.33–5.20) |
| Age, sex, education | 1.84 (1.26–2.67) | 1.67 (1.12–2.49) | 1.47 (0.93–2.34) |
| All covariates† | 1.80 (1.21–2.67) | 1.69 (1.11–2.57) | 1.49 (0.92–2.43) |
| All covariates + prior CVD | 1.66 (1.12–2.48) | 1.64 (1.07–2.51) | N/A |
| Category Fluency | |||
| Unadjusted | 2.72 (1.85–4.01) | 2.90 (1.93–4.35) | 2.47 (1.54–3.95) |
| Age, sex, education | 1.54 (1.004–2.36) | 1.62 (1.03–2.55) | 1.26 (0.74–2.13) |
| All covariates† | 1.36 (0.86–2.13) | 1.43 (0.89–2.31) | 1.09 (0.62–1.90) |
| All covariates + prior CVD | 1.36 (0.86–2.15) | 1.46 (0.90–2.36) | N/A |
Odds ratio is for risk of poor performance in participants with High vs. Low NT-proBNP
All covariates = age, sex, education, hypertension, BMI, exercise, alcohol use, current smoking, LDL cholesterol, creatinine clearance
CVD = cardiovascular disease; MMSE = Mini Mental State Exam; OR = odds ratio
For Trails B testing, n=939 (883 without prior stroke; 761 without prior CVD).
Association of NT-proBNP and Cognitive Function in Participants without Prior Cardiovascular Disease
The influence of prevalent cardiovascular disease on the association between NT-proBNP levels and poor cognitive function was tested by additionally adjusting for a history of cardiovascular disease (which includes stroke), and also by repeating the logistic regression analyses after excluding participants with (1) a history of stroke, or (2) a history of cardiovascular disease. As shown in Table 3, neither additionally adjusting for a history of cardiovascular disease, nor excluding participants with prior stroke materially altered the results. In each case, fully adjusted results were similar to those obtained for the group as a whole, with a significantly greater likelihood of poor performance on the MMSE and Trails B tests, and a trend toward a greater likelihood of poor performance on the Category Fluency test, among participants with elevated NT-proBNP levels.
After excluding participants with a history of cardiovascular disease (i.e. stroke or coronary heart disease), those with elevated NT-proBNP levels still had an increased likelihood of poor performance on all three tests in unadjusted analyses, although the association was attenuated in adjusted models.
DISCUSSION
In this cohort of older community-dwelling adults, NT-proBNP levels were strongly and independently associated with poor cognitive function. This association was independent of stroke history, signifying that the association is not merely reflecting prior overt neurovascular disease.
In our study, the association between NT-proBNP levels and cognitive function was strongest for the MMSE and Trails B tests, tests of global cognitive function and executive function respectively, and less so for the Category Fluency test, a test of cognitive flexibility and executive function which may be more sensitive to education level. The 30-item MMSE, designed as a screening test for possible dementia, is a relatively insensitive test. The fact that the association of NP levels with MMSE was significant at all shows this is a robust association.
To our knowledge, the association between NP levels and cognitive function in community-dwelling individuals has not been described previously. The only prior study evaluating NP’s and cognitive function in non-demented individuals was limited to a small number of cardiovascular disease patients.12 In that study, BNP levels were predictive of cognitive function in 56 older adults with a history of cardiovascular disease. A second study found that BNP levels were associated with vascular dementia but not Alzheimer’s disease,27 though more recently Buerger et al. found that NP levels were associated with progression from mild cognitive impairment to clinical Alzheimer’s disease.28 Our results are the first to show that NP levels are associated with poor cognitive function even among community-dwelling individuals unselected for cardiovascular disease.
Although data linking NP’s and cognitive dysfunction are sparse, a number of smaller studies have linked cardiovascular disease with poor cognitive function. Cohen et al. reported that 35 outpatients with a history of coronary heart disease but without a history of stroke or other neurologic brain disease had worse performance on tasks of executive function such as verbal fluency, compared with controls.1 Among 62 outpatients with heart failure, Vogels et al. found that cognitive dysfunction was common, with the most prominent deficits in the domains of executive function, memory, language, and mental speed.4 Hoth et al. found that heart failure patients performed worse on tests of executive function and psychomotor speed than patients without heart failure.5 This link between cardiovascular disease and cognitive dysfunction, even in the absence of stroke history, is consistent with our present findings.
Dementia in the elderly can result from at least three distinct biologic processes: Alzheimer’s disease, Lewy body disease, and vascular brain injury.29 Of these three entities, only vascular disease is preventable to any significant degree with current medical knowledge and practice. Identifying individuals at risk for vascular dementia at an early stage is therefore desirable. While the present study does not identify the mechanism for the association between NP levels and cognitive dysfunction, it does lend support to several hypotheses. Based on what is known about NP’s, the association between NT-proBNP levels and poor cognitive function may reflect the presence of underlying cardiovascular disease (including subclinical neurovascular or cardiac disease) or a milieu conducive to the development of cardiovascular disease, although neurodegenerative disease may also be playing a role.
Our results suggest that a portion of the association between NT-proBNP and cognitive dysfunction is due to subclinical rather than overt cardiovascular disease. Others have shown that elevated levels of NP’s can be found in individuals with subclinical cardiovascular disease or in those at risk.10 In the Framingham population, Wang et al. found that small elevations in NP levels predicted future cardiovascular events, including new atrial fibrillation,8 providing one possible mechanism for our observed association between cognitive dysfunction and NP levels even after adjusting for a history of cardiovascular disease.
In addition, recent studies provide data linking subclinical cardiovascular disease to cognitive dysfunction. A study of community-dwelling adults found that a lower cardiac index, even at levels still within the normal range, was associated with signs of brain aging on magnetic resonance imaging.30 Left ventricular dysfunction even in the absence of overt heart failure, or silent cardiac dysrhythmias, could each increase the risk of cardiogenic stroke and of impaired cerebral perfusion. Either of these entities could thus lead to both cognitive impairment and to elevated NP levels. Other factors that may contribute to both cognitive deficits and elevated NP levels include neurodegenerative disease,28 sleep apnea,31–33 neurohormonal activation,34 endothelial or other vascular abnormalities,35,36 or a direct neurotoxic effect of NP’s (although we are aware of no evidence to support this last hypothesis).
Study Strengths and Limitations
A strength of this study is its large, population-based sample, well-characterized for many potentially confounding covariates. Also, we included three standard cognitive function tests which measure different domains of cognitive function, and used predefined clinical cut-points for NP’s and for test scores in order to increase the clinical relevance of the associations, as well as the likelihood that we are identifying cognitive dysfunction that may progress to dementia. This study also has several limitations. First, this is not a prospective analysis, so causality cannot be assumed. It seems unlikely, however, that modestly impaired cognitive function leads to elevation of cardiovascular disease biomarkers. Although not prospective, causality is more likely when there is a graded response, as reported here linking higher NP quartile and increased likelihood of poor cognitive performance. Second, most of the cohort is fairly well-educated and is largely of European ancestry; results may not be generalizable to other populations. However, this homogeneity is also an advantage in the present study because there is less confounding of poor cognitive test performance due to low socioeconomic status or education.
Although not all domains of cognitive function were tested by our battery of tests, our focus was on vascular cognitive impairment, where executive function is lost earlier than other domains such as memory; the cognitive function tests we used (which were recommended by the Alzheimer’s Disease Research Center) are appropriate to our hypothesis. Finally, prevalent cardiovascular disease was based on self-report of a physician diagnosis or a revascularization procedure, which could have resulted in misclassification. As noted in the Methods section, the cardiovascular disease medical history was validated by medical record review in a subset of participants. The relatively high education level of participants, their long term participation, and the interest of older individuals in their health, are all likely to improve health literacy and the reliability of self-reports.
Conclusions
In this study, NT-proBNP levels were strongly and independently associated with poor cognitive function in ambulatory community-dwelling older individuals. Individuals with elevations of NT-proBNP are at increased risk of impaired cognitive performance. Further studies are needed to elucidate the mechanisms behind this association, which likely reflects a combination of neurovascular, cardiac, and neurodegenerative etiologies.
Acknowledgments
FUNDING SOURCES:
This work was supported by the National Institutes of Health [AG07181, AG028507, and DK31801]; the American Heart Association [LBD, GAL]; and by the American College of Cardiology/Guidant Foundation [LBD].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST:
Roche Diagnostics, Inc. supplied the reagents for analyzing the biomarkers for this report, but had no input into data acquisition, data analysis, manuscript preparation, or any other aspect of the study. ASM and LBD have received research grants from Roche Diagnostics; there are no other conflicts of interest to report.
All authors had access to the data and a role in writing the manuscript.
REFERENCES
- 1.Cohen RA, Moser DJ, Clark MM, et al. Neurocognitive functioning and improvement in quality of life following participation in cardiac rehabilitation. Am J Cardiol. 1999;83(9):1374–1378. doi: 10.1016/s0002-9149(99)00103-4. [DOI] [PubMed] [Google Scholar]
- 2.Cacciatore F, Abete P, Ferrara N, et al. Congestive heart failure and cognitive impairment in an older population. Osservatorio Geriatrico Campano Study Group. J Am Geriatr Soc. 1998;46(11):1343–1348. doi: 10.1111/j.1532-5415.1998.tb05999.x. [DOI] [PubMed] [Google Scholar]
- 3.Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: vulnerability of the prefrontal regions and executive functions. Behav Neurosci. 2003;117(6):1169–1180. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- 4.Vogels RL, Oosterman JM, van Harten B, et al. Profile of cognitive impairment in chronic heart failure. J Am Geriatr Soc. 2007;55(11):1764–1770. doi: 10.1111/j.1532-5415.2007.01395.x. [DOI] [PubMed] [Google Scholar]
- 5.Hoth KF, Poppas A, Moser DJ, et al. Cardiac dysfunction and cognition in older adults with heart failure. Cogn Behav Neurol. 2008;21(2):65–72. doi: 10.1097/WNN.0b013e3181799dc8. [DOI] [PubMed] [Google Scholar]
- 6.Bell-McGinty S, Lopez OL, Meltzer CC, et al. Differential cortical atrophy in subgroups of mild cognitive impairment. Arch Neurol. 2005;62(9):1393–1397. doi: 10.1001/archneur.62.9.1393. [DOI] [PubMed] [Google Scholar]
- 7.Saykin AJ, Wishart HA, Rabin LA, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67(5):834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350(7):655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 9.Daniels LB, Laughlin GA, Clopton P, et al. Minimally elevated cardiac troponin T and elevated N-terminal pro-B-type natriuretic peptide predict mortality in older adults: results from the Rancho Bernardo Study. J Am Coll Cardiol. 2008;52(6):450–459. doi: 10.1016/j.jacc.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50(25):2357–2368. doi: 10.1016/j.jacc.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 11.Wallen T, Landahl S, Hedner T, et al. Brain natriuretic peptide predicts mortality in the elderly. Heart. 1997;77(3):264–267. doi: 10.1136/hrt.77.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunstad J, Poppas A, Smeal S, et al. Relation of brain natriuretic peptide levels to cognitive dysfunction in adults > 55 years of age with cardiovascular disease. Am J Cardiol. 2006;98(4):538–540. doi: 10.1016/j.amjcard.2006.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daniels LB, Clopton P, Jiang K, et al. Prognosis of stage A or B heart failure patients with elevated B-type natriuretic peptide levels. J Card Fail. 2010;16(2):93–98. doi: 10.1016/j.cardfail.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 14.McDonagh TA, Robb SD, Murdoch DR, et al. Biochemical detection of left-ventricular systolic dysfunction. Lancet. 1998;351(9095):9–13. doi: 10.1016/s0140-6736(97)03034-1. [DOI] [PubMed] [Google Scholar]
- 15.Galasko GI, Lahiri A, Barnes SC, et al. What is the normal range for N-terminal pro-brain natriuretic peptide? How well does this normal range screen for cardiovascular disease? Eur Heart J. 2005;26(21):2269–2276. doi: 10.1093/eurheartj/ehi410. [DOI] [PubMed] [Google Scholar]
- 16.Bennett SJ, Sauve MJ. Cognitive deficits in patients with heart failure: a review of the literature. J Cardiovasc Nurs. 2003;18(3):219–242. doi: 10.1097/00005082-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 17.The hypertension detection and follow-up program: Hypertension detection and follow-up program cooperative group. Prev Med. 1976;5(2):207–215. doi: 10.1016/0091-7435(76)90039-6. [DOI] [PubMed] [Google Scholar]
- 18.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 19.Vervoort G, Willems HL, Wetzels JF. Assessment of glomerular filtration rate in healthy subjects and normoalbuminuric diabetic patients: validity of a new (MDRD) prediction equation. Nephrol Dial Transplant. 2002;17(11):1909–1913. doi: 10.1093/ndt/17.11.1909. [DOI] [PubMed] [Google Scholar]
- 20.Krentz AJ, von Muhlen D, Barrett-Connor E. Searching for polycystic ovary syndrome in postmenopausal women: evidence of a dose-effect association with prevalent cardiovascular disease. Menopause. 2007;14(2):284–292. doi: 10.1097/GME.0b013e31802cc7ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elecsys proBNP package insert. Indianapolis: Roche Diagnostics. IN2003. [Google Scholar]
- 22.Yeo KT, Wu AH, Apple FS, et al. Multicenter evaluation of the Roche NT-proBNP assay and comparison to the Biosite Triage BNP assay. Clin Chim Acta. 2003;338(1–2):107–115. doi: 10.1016/j.cccn.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 23.Cauliez B, Guignery J, Marinier S, et al. Two-year stability of NT-proBNP in frozen samples using the Roche Elecsys system. Ann Clin Biochem. 2008;45(Pt 3):318–319. doi: 10.1258/acb.2007.007187. [DOI] [PubMed] [Google Scholar]
- 24.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 25.Reitan R. Validity of the Trail-Making Test as an indicator of organic brain disease. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 26.Borkowski JB, Benton AL, Spreen O. Word fluency and brain damage. Neuropsychologia. 1967;5:135–140. [Google Scholar]
- 27.Kondziella D, Gothlin M, Fu M, et al. B-type natriuretic peptide plasma levels are elevated in subcortical vascular dementia. Neuroreport. 2009;20(9):825–827. doi: 10.1097/WNR.0b013e328326f82f. [DOI] [PubMed] [Google Scholar]
- 28.Buerger K, Uspenskaya O, Hartmann O, et al. Prediction of Alzheimer's disease using midregional proadrenomedullin and midregional proatrial natriuretic peptide: a retrospective analysis of 134 patients with mild cognitive impairment. J Clin Psychiatry. 2010 doi: 10.4088/JCP.09m05872oli. [DOI] [PubMed] [Google Scholar]
- 29.Montine TJ, Larson EB. Late-life dementias: does this unyielding global challenge require a broader view? JAMA. 2009;302(23):2593–2594. doi: 10.1001/jama.2009.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jefferson AL, Himali JJ, Beiser AS, et al. Cardiac index is associated with brain aging: the Framingham Heart Study. Circulation. 2010;122(7):690–697. doi: 10.1161/CIRCULATIONAHA.109.905091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simmons MS, Clark GT. The potentially harmful medical consequences of untreated sleep-disordered breathing: the evidence supporting brain damage. J Am Dent Assoc. 2009;140(5):536–542. doi: 10.14219/jada.archive.2009.0221. [DOI] [PubMed] [Google Scholar]
- 32.Staniforth AD, Kinnear WJ, Cowley AJ. Cognitive impairment in heart failure with Cheyne-Stokes respiration. Heart. 2001;85(1):18–22. doi: 10.1136/heart.85.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Usui Y, Tomiyama H, Hashimoto H, et al. Plasma B-type natriuretic peptide level is associated with left ventricular hypertrophy among obstructive sleep apnoea patients. J Hypertens. 2008;26(1):117–123. doi: 10.1097/HJH.0b013e3282f06eb4. [DOI] [PubMed] [Google Scholar]
- 34.Bruunsgaard H, Andersen-Ranberg K, Jeune B, et al. A high plasma concentration of TNF-alpha is associated with dementia in centenarians. J Gerontol A Biol Sci Med Sci. 1999;54(7):M357–M364. doi: 10.1093/gerona/54.7.m357. [DOI] [PubMed] [Google Scholar]
- 35.Ferrari R, Bachetti T, Agnoletti L, et al. Endothelial function and dysfunction in heart failure. Eur Heart J. 1998;19(Suppl G):G41–G47. [PubMed] [Google Scholar]
- 36.Ninomiya Y, Hamasaki S, Ishida S, et al. Elevated levels of brain natriuretic peptide as a predictor of impaired coronary endothelial function in patients with left ventricular remodeling. J Cardiol. 2006;48(3):125–132. [PubMed] [Google Scholar]