Abstract
Pink disease (infantile acrodynia) was especially prevalent in the first half of the 20th century. Primarily attributed to exposure to mercury (Hg) commonly found in teething powders, the condition was developed by approximately 1 in 500 exposed children. The differential risk factor was identified as an idiosyncratic sensitivity to Hg. Autism spectrum disorders (ASD) have also been postulated to be produced by Hg. Analogous to the pink disease experience, Hg exposure is widespread yet only a fraction of exposed children develop an ASD, suggesting sensitivity to Hg may also be present in children with an ASD. The objective of this study was to test the hypothesis that individuals with a known hypersensitivity to Hg (pink disease survivors) may be more likely to have descendants with an ASD. Five hundred and twenty-two participants who had previously been diagnosed with pink disease completed a survey on the health outcomes of their descendants. The prevalence rates of ASD and a variety of other clinical conditions diagnosed in childhood (attention deficit hyperactivity disorder, epilepsy, Fragile X syndrome, and Down syndrome) were compared to well-established general population prevalence rates. The results showed the prevalence rate of ASD among the grandchildren of pink disease survivors (1 in 25) to be significantly higher than the comparable general population prevalence rate (1 in 160). The results support the hypothesis that Hg sensitivity may be a heritable/genetic risk factor for ASD.
Pink disease, or infantile acrodynia as it was also known (primarily in Europe and America), was an especially prevalent condition in Australia, North America, and Central Europe in the first half of the 20th century (Rocaz 1933). The first description of pink disease in the literature dates back to 1903 by Selter, a German physician, although cases in Australia predate this time by at least two decades (Selter 1903; Wood and Wood 1935). Pink disease remained in relative obscurity in the greater medical community until 1914, when it was again described, this time by Swift, an Australian-born physician, at an Australasian medical congress in New Zealand (Swift 1914).
Case studies provided a comprehensive clinical picture of pink disease long before its etiology was established. The most commonly reported symptoms included: irritability, neurosis, photophobia (light sensitivity), hyperhidrosis (excessive sweating), hypotonia (low muscle tone), ataxia (lack of coordination), digestive problems (including loss of weight, loss of appetite, vomiting, and constipation), anemia, excessive salivation, respiratory problems, lethargy, extreme misery, slurring/lossof speech, loosening/loss of teeth, swollen extremities, and perhaps most famously (and from which the name “pink disease” was derived), marked reddening of the extremities, particularly the hands and feet (Rocaz 1933; Wood and Wood 1935; Leys 1950). Fatality was reasonably high, with death resulting in 10–33% of cases (Rocaz 1933). For the survivors, recovery was considered to be complete, although research conducted in recent decades revealed medical sequelae to be high in this group, including Young's syndrome (infertility in men) and bronchiectasis (Hendry et al. 1993; Williams and O'Reilly 1959).
In 1945, a critical study became the catalyst that led to the discovery of the etiology of pink disease. Upon noticing a similarity of pink disease symptomatology to arsenic and thallium poisoning, American physicians Warkany and Hubbard (1948) undertook a quantitative metal determination of 14 children diagnosed with pink disease and found that 12 of the 14 children had elevated levels of urinary mercury (Hg). A replication study by Warkany and Hubbard (1951) consolidated the finding with Hg levels elevated in 92% of 41 children diagnosed with pink disease in comparison to 15% of controls. Although Hg was used in a wide range of medicinal products at the time, the primary culprit was determined to be teething powders containing calomel (mercurous chloride). Following the removal of calomel from most teething powders in 1954, pink disease essentially disappeared (Curtis et al. 1987). Interestingly, however, while millions of teething powders were sold (some 7 million annually in North England alone of one of the most famous brands, Steedman's Teething Powder), only 1 in 500 exposed children developed pink disease (Emsley 2005). The differential risk factor was identified as an idiosyncratic sensitivity to Hg (Warkany and Hubbard 1948; Bivings 1949; Bivings and Lewis 1948).
It was postulated that autism spectrum disorders (ASD), which are primarily comprised of autistic disorder and Asperger's disorder, also have a pathogenesis stemming from Hg exposure (Austin 2008; Bernard et al. 2001; Mutter et al. 2005). The mounting evidence is compelling. Behavioral changes, hyperactivity, and alterations in spontaneous and learned behaviors have been observed in animals exposed to Hg during the prenatal or early postnatal period (Agency for Toxic Substances and Disease Registry 1999). Studies also showed that there were significant elevations of Hg in the urine of children with autism in comparison to controls, as well as in urinary markers of Hg damage (Austin and Shandley 2008; Bradstreet et al. 2003; Geier and Geier 2007; Nataf et al. 2006). Other investigations found the severity of autism to positively correlate with the child's body burden of toxic metals (Adams et al. 2009; Geier et al. 2009; Holmes et al. 2003). In addition, some epidemiological studies demonstrated an association between Hg exposure and ASD prevalence (Gallagher and Goodman 2010; Geier and Geier 2006a; Palmer et al. 2006; 2009; Windham et al. 2006), but not all studies confirmed this correlation (Hviid et al. 2003; Verstraeten et al. 2003).
Mercury contained in vaccines (as a preservative under the tradename Merthiolate, but more commonly known as thiomersal/ thimerosal), dental amalgams (silver fillings), seafood, and the atmosphere is argued to be the primary set of sources of Hg exposure for infants both in utero and in their early years (Austin 2008). However, not all children exposed to such sources of Hg develop an ASD, suggesting, as was the case with pink disease, that a hypersensitivity to the adverse effects of Hg needs to be present in addition to the Hg exposure for the condition to manifest. Therefore, the Hg–autism hypothesis is, in reality, a two-part hypothesis that states that Hg exposure combined with a genetic/physiological sensitivity to Hg or a predisposition to impaired Hg excretion capacity leads to a chronic elevation of Hg in the brain and body (Bernard et al. 2001).
The purpose of the present study was to test the Hg–autism hypothesis. If the hypothesis is indeed correct, and a sensitivity to Hg is heritable (genetic), the prevalence of ASD among the descendants of a cohort confirmed as having a hypersensitivity to Hg (pink disease survivors) should be higher than a comparable general population prevalence.
MATERIALS AND METHODS
Participants and Study Design
Ethical approval to conduct the present study was received from the Swinburne University Human Research Ethics Committee. Individuals who had previously been diagnosed with pink disease were invited to participate in this study by completing a survey either online, by mail, or via telephone interview. In cases where the pink disease survivor was incapacitated or deceased, family members were able to complete the survey as a proxy. The Australian Pink Disease Support Group (PDSG) estimated that approximately 5000 survivors were still living at the time of study commencement in July 2009 (D Farnsworth personal communication 19 November 2009).
Participants were recruited via the PDSG, an Australian not-for-profit group dedicated to providing support and information to pink disease survivors and their families—the only such group in the world. The PDSG maintains a membership database and sent out the survey to all of its past and present members, in addition to advertising the study on its website (www.pinkdisease.org). In order to minimize response bias, the true purpose of the study was not included on recruitment materials sent out to potential participants; instead, recruitment materials indicated that the purpose of the study was to investigate the general health outcomes of the descendants of pink disease survivors. In total, 2600 surveys were sent, 2000 by mail and 600 by e-mail, with an anticipated overlap of approximately 300 members receiving the survey by both mail and e-mail. A chance to win one of 10 shopping vouchers to the value of $75 was offered as an incentive to participate.
The survey included sociodemographic questions regarding the pink disease survivor (gender, date of birth, current place of residence), the relationship of the respondent to the survivor (if the survey was completed by a proxy), and information pertaining to the pink disease survivor's descendants (children and grandchildren). With respect to the descendants, survey respondents were asked to provide details regarding the number of children and grandchildren, the gender and age of each, and whether they had been diagnosed with any of the following conditions prior to the age of 16 years: autism, Asperger's disorder, attention deficit hyperactivity disorder (ADHD), epilepsy, Fragile X syndrome, mental retardation, and/or Down syndrome.
The survey was commenced or returned by 531 people (a response rate of 23.1%); however, 9 surveys were removed from analysis, as 6 were repeated entries, and 3 surveys were incomplete. This left a total of 522 surveys that were included in the analysis. The characteristics of the pink disease survivor cohort are provided in Table 1.
TABLE 1.
Characteristics of the Pink Disease Survivor Cohort
| Parameter | Totala(n = 522) | Male (n = 105) | Female (n = 409) |
|---|---|---|---|
| Age | |||
| Mean | 64.07 | 63.78 | 64.07 |
| SD | 6.63 | 6.06 | 6.77 |
| Range | 44–86 | 54–81 | 44–86 |
| Survey completion type | |||
| Online | 92 | 30 | 62 |
| Postal | 424 | 75 | 341 |
| Telephone | 6 | 0 | 6 |
| Survey respondent status | |||
| PD survivor | 509 | 100 | 401 |
| Otherb | 13 | 5 | 8 |
| Current place of residencec | |||
| New South Wales | 218 | 42 | 173 |
| Queensland | 88 | 15 | 70 |
| Victoria | 68 | 11 | 56 |
| South Australia | 33 | 6 | 27 |
| Western Australia | 31 | 7 | 24 |
| Tasmania | 13 | 2 | 11 |
| Australian Capital Territory | 7 | 2 | 4 |
| Northern Territory | 2 | 0 | 2 |
| Overseasd | 61 | 20 | 41 |
Note. PD = pink disease.
aGender was not provided by eight survey respondents; therefore, the total is not the sum of male and female in all cases.
bWhere the survey was completed by proxy the most common person was the mother (n = 5), followed by the daughter (n = 4), wife/widow (n = 3), and granddaughter (n = 1).
cPlace of residence was not provided by one survey respondent.
adWhere the survivor wasn't located in Australia, the next most common location was the United Kingdom (n = 38) followed by New Zealand (n = 14) and Canada (n = 5).
Descendants of Pink Disease Survivors
Children
Pink disease survivors had a cumulative total of 1103 children. Only live births, biological children, and children surviving to at least 5 yr were included in the analysis; therefore, 17 children were not included for the following reasons: 7 children were adopted, 4 stillborn, and 6 died at an early age (cot death: n = 2, prematurity: n = 1, congenital heart disease: n = 1, immature lungs: n = 1, unspecified: n = 1). Additionally, three of the survey respondents stated that they had children but failed to provide details and were therefore not included in the analysis. This left a total of 1086 children that were included. Of the 522 survey respondents, 79.5% (n = 415) stated that they had at least one child, with the average being 2.2 children (range: 1–8). The mean age of the children was 37.1 yr (SD = 8.81), ranging from 3 to 61 yr.
Grandchildren
Pink disease survivors had a cumulative total of 1380 grandchildren. As was the case for children, only live births, biological grandchildren, and grandchildren surviving to at least 5 yr were included in the analysis. Therefore, 14 grandchildren were not included, as 8 grandchildren were stillborn and 6 died at an early age (Potter's syndrome: n = 1, Werdnig Hoffmann's disease: n = 1, stroke in the womb: n = 1, congenital heart defect: n = 1, unspecified: n = 1). This left a total of 1366 grandchildren that were included in the analysis. Of the 522 survey respondents, 60.7% (n = 317) stated that the pink disease survivor had at least one biological grandchild, with an average of 4.3 grandchildren (range: 1–15). The mean age of the grandchildren was 11.3 yr (SD = 7.65), ranging from <1 to 38 yr.
RESULTS
The numbers of children and grandchildren diagnosed with autism, Asperger's disorder, ADHD, epilepsy, fragile X syndrome, mental retardation or Down syndrome are presented in Table 2.
TABLE 2.
Frequency of Self-Reported Clinical Conditions Among the Descendants of Pink Disease Survivors
| Children |
Grandchildren |
|||||
|---|---|---|---|---|---|---|
| Condition | Totala (n = 1086) | Male (n = 545) | Female (n = 513) | Total3 (n = 1366) | Male (n = 638) | Female (n = 567) |
| Autism | 3 | 3 | 0 | 12 | 10 | 2 |
| Asperger's disorder | 8 | 6 | 2 | 22 | 17 | 5 |
| ADHD | 38 | 30 | 8 | 29 | 19 | 8 |
| Epilepsy | 18 | 10 | 7 | 9 | 6 | 2 |
| Fragile X syndrome | 1 | 1 | 0 | 1 | 0 | 1 |
| Mental retardation | 2 | 1 | 1 | 2 | 1 | 1 |
| Down syndrome | 1 | 1 | 0 | 1 | 0 | 1 |
Note. ADHD = attention deficit hyperactivity disorder.
aGender was not provided for all children and grandchildren, therefore the total is not the sum of male and female in all cases.
ASD Prevalence
The general population ASD prevalence rates used as the comparison group for this study were taken from a report commissioned by the Australian Advisory Board on Autism Spectrum Disorders (MacDermott et al. 2007). ASD prevalence is well understood to be difficult to measure, due, in part, to changing diagnostic criteria and databases of variable quality (Charman et al. 2009). The Australian Advisory Board data, however, were largely immune from these confounding variables as they were gathered over a period of time when diagnostic criteria for autism and Asperger's disorder did not change. Data were collected from multiple sources across health, disability, and education sectors in addition to Australian state and territory autism associations, allowing for the vastmajority of Australian ASD cases to be captured. Furthermore, the reported rates were highly consistent with other ASD prevalence studies conducted internationally around that same time (Centers for Disease Control and Prevention 2007).
This study presents the comparative rates for two age groups used in the Australian Advisory Board report (6–12 and 13–16 yr) for the most recent year for which data is available, 2005. The Australian Advisory Board calculated its ASD prevalence rates from Centrelink data. Centrelink is an Australian federal government social service agency and is considered the most comprehensive single source of Australian ASD data. Figures 1 and 2 provide a graphical comparison of the prevalence rates of autism and Asperger's disorder, respectively, among the general population reported by the Australian Advisory Board and the age-matched grandchildren of pink disease survivors. Only data for grandchildren are reported here, as the children of the pink disease cohort were mainly born in the 30-year period from 1950 to 1980, a period for which no reliable Australian ASD population prevalence rates are available. The most recent Australian ASD prevalence rate for children 6–12 years is 1 in 160, 13–16 years 1 in 272, and for 6–16 years, 1 in 189 (MacDermott et al. 2007). This figure is a combination of the autism and Asperger's disorder rates in 2005. The comparative ASD prevalence rate for the grandchildren of pink disease survivors aged 6–12 years is 1 in 25 (n = 398), 13–16 years 1 in 35 (n = 141), and for 6–16 years, 1 in 27 (n = 539).
FIGURE 1.
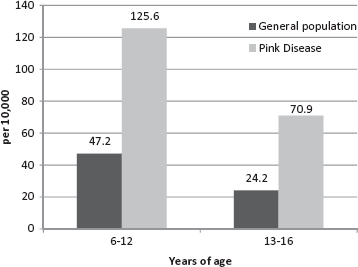
Autism prevalence rates for children aged 6–12 and 13–16 years among the Australian population and the grandchildren of pink disease survivors.
FIGURE 2.
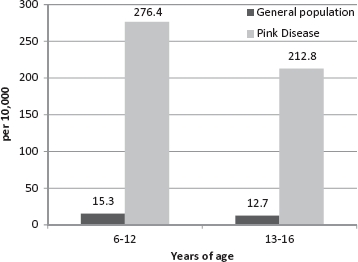
Asperger's disorder prevalence rates for children aged 6–12 and 13–16 years among the Australian population and the grandchildren of pink disease survivors.
To determine whether the difference in ASD prevalence rate between the pink disease grandchildren and the general population is significant and, furthermore, whether there is an elevated risk for disease generally among pink disease descendants (for the clinical conditions captured in our survey: ADHD, epilepsy, Fragile X syndrome, and Down Syndrome), one-sided Poisson probabilities were calculated, the results of which are presented in Table 3. Ninety-five percent confidence intervals were calculated using Byar's approximation. Well-established population prevalence rates were used as a comparison for fragile X syndrome (Fragile X Association of Australia 2009) and Down syndrome (Down's Syndrome Association of Victoria 2009). For ADHD, there is no definitive agreement regarding an accepted prevalence rate, with figures ranging from 1.7 to 6%; consequently, the midpoint (3.85%) was used as the comparison rate (Buckmeister 2004). In Australia, there have been no apparent studies undertaken to determine the prevalence of epilepsy in childhood. Consequently, for this study, the Australian adult epilepsy prevalence rate was used for comparison (0.68%) (Australian Bureau of Statistics 1998). This figure is broadly equivalent to international studies reporting both childhood and adult prevalence rates for epilepsy (Sridharan 2002; Oka et al. 2006). Mental retardation was not included in this analysis as an accepted prevalence rate is not available due to complications in measuring intellectual disability among school-aged children (Wen 1997). As is shown in Table 3, the elevated risk for an ASD among pink disease grandchildren was significant. There were no significant elevations in prevalence among the grandchildren on any of the non-ASD conditions.
TABLE 3.
Comparison of Observed and Expected Cases of Clinical Conditions Among the Grandchildren of Pink Disease Survivors Aged 6–16 Years at 2005 (n = 539)
| Condition | Observed cases | Expected cases | SIR | 95% confidence interval | 1-Sided Poisson Probability (P) |
|---|---|---|---|---|---|
| ASD | 20 | 2.85 | 7.02 | 4.28–10.84 | <0.0001 |
| ADHD | 13 | 20.75 | 0.63 | 0.33–1.07 | 0.97 |
| Epilepsy | 3 | 3.67 | 0.82 | 0.16–2.39 | 0.71 |
| Fragile X syndrome | |||||
| Males | 0 | 0.14 | 0.00 | ∗-26.20 | 1.00 |
| Females | 1 | 0.13 | 7.69 | 0.10–42.80 | 0.12 |
| Down syndrome | 0 | 0.67 | 0.00 | ∗-5.47 | 1.00 |
Note. SIR = Standardized incidence ratios; ASD = autism spectrum disorder; ADHD = attention deficit hyperactivity disorder; Asterisk indicates lower limit for fragile X males and down syndrome not calculable; Data for males and females were pooled unless otherwise stated.
DISCUSSION
The prevalence of ASD was found to be significantly higher among the grandchildren of pink disease survivors in comparison to the general population, providing support for the hypothesis that Hg sensitivity may be a heritable/genetic risk factor for ASD. Furthermore, an examination of the prevalence rates of a group of non-ASD clinical conditions (ADHD, epilepsy, Fragile X syndrome, and Down syndrome) among the pink disease descendants and the general population indicates there is not a general elevated risk for disease among this cohort, but rather a specific risk for ASD. An alternative explanation for thefindings may relate to the higher body burden of Hg in a parent being passed to their offspring. It is difficult to conceptualize how this may occur in terms of paternal transfer, but the phenomenon of Hg preferentially distributing to the developing fetus via the umbilical cord in Hg-exposed mothers is well documented (Sakamoto et al. 2010).
As identified earlier, numerous studies demonstrated a relationship between ASD and Hg (Agency for Toxic Substances and Disease Registry 1999; Austin and Shandley 2008; Bradstreet et al. 2003; Geier and Geier 2006a; 2007; Nataf et al. 2007; Adams et al. 2009; Geier et al. 2009; Holmes et al. 2003; Gallagher and Goodman 2010; Palmer et al. 2006; 2009; Windham et al. 2006), and our results add further compelling evidence in support of this relationship. The unique contribution of this study is that, to our knowledge, it is the first to examine the “individual susceptibility” variable inherent in the Hg-autism hypothesis. Our results suggest that this variable may have a heritable component and therefore, of course, a genetic basis. What our results do not do, however, is enable an understanding of the degree to which the susceptibility is inheritable and the mechanism by which this may occur. This is clearly an important focus for future research.
A possible mechanism for Hg-induced autism was proposed by James et al. (2004) and Geier and Geier (2006b), who demonstrated that oxidative stress is high and glutathione (GSH) levels are low in children with autism. This is entirely consistent with an etiology of autism based on a gene–Hg interaction, as low GSH predisposes an individual to damage from Hg exposure by limiting the body's capacity to both minimize oxidative damage produced by the metal and excrete the toxin.
Of additional note is the gender ratio in which the descendants of the pink disease cohort were diagnosed with ASD. ASD is reported to occur more commonly in males; typically the quoted ratio is four males to one female (MacDermott et al. 2007). Interestingly, the present study replicates this pattern, with male descendants approximately fourfold more likely to be reported as diagnosed with an ASD in comparison to female descendants. Unfortunately, data are not available to confirm (or disconfirm) whether males were more likely to be stricken with pink disease, as the condition was never mandated as a reportable medical condition.
The Hg–autism hypothesis engenders passionate debate on both sides; however, the cumulative science in this field is now of such depth and breadth that it is difficult, if not impossible, to be completely dismissive of the Hg–autism link. Furthermore, our findings clearly suggest that individuals with a family history of pink disease are at significantly greater risk of having a grandchild with an ASD than the general population. Irrespective of the Hg–autism hypothesis, this has implications for public and environmental health, fields of genetic counseling and family planning, and autism research generally.
The present study design had several notable strengths. In order to minimize response biases and maximize the validity of the data, the true purpose of the study was not included on recruitment materials sent out to potential participants (i.e., information statement); instead, recruitment materials merely stated that the researchers were investigating the health outcomes of their descendants. Recruitment was maximized by obtaining the assistance of the Australian Pink Disease Support Group, which sent out survey packs to its members, both past and present, and publicized the study online. In addition, pink disease survivors were provided with a variety of response format options (online, telephone, mail). This is especially important as we were largely dealing with an elderly cohort. A further strength was that our comparison groups (grandchildren and general population) were matched for age and birth year.
A challenge encountered with this study was finding an appropriate comparative Australian population prevalence rate for ADHD. As mentioned earlier, no agreement has been reached regarding a definitive prevalence rate for ADHD with estimates rangingfrom 1.7 to 6%. Therefore, the difference between the observed and expected cases displayed in Table 3 for ADHD needs to be viewed with caution. Weaknesses of this study include the fact that independent assessments of the pink disease survivors’ descendents were not conducted in order to validate the accuracy of the self-reported diagnoses. Information on other variables hypothesized to play a role in the development of ASD, such as birth weight, breastfeeding history, or maternal and paternal age, was not collected. Nevertheless, the results shed significant light on the gene–environment interaction hypothesized to underpin the etiology of ASD. The role that accessory factors such as birth weight and breastfeeding history may play in the etiology warrants further research.
CONCLUSIONS
Given that Hg is well established as a potent neurotoxin (especially to developing fetuses and young children), and that the findings from this study show that individuals with a confirmed hypersensitivity to Hg are at significantly greater risk of having a descendant with an ASD, it would appear a matter of fundamental ethics for health professionals and health policy makers to minimize Hg exposure (particularly for pregnant and nursing mothers and their children). Furthermore, there is an urgent need to fund research programs designed to build upon our understanding of the gene–environment pathogenesis (and possibly pathogeneses) of ASD. This research effort needs to be multidisciplinary in nature so as to facilitate research into multivariate models of ASD etiology, with the primary focus on environmental triggers (i.e., Hg) and heritable (genetic) risk factors.
Acknowledgments
This study was funded by donations generously made to the Swinburne Autism Bio-Research Initiative (www.sabri.org.au). We thank Di Farnsworth, the facilitator of the Pink Disease Support Group (www.pinkdisease.org), for her support of the study and role in the recruitment of Pink Disease survivors. We also thank Sam Critchley for her assistance in entering the survey data and Dr. Denny Meyer and Dr. Jahar Bhowmik for their statistical assistance.
REFERENCES
- Adams J. B., Baral M., Geis E., Mitchell J., Ingram J., Hensley A., Zappia I., Newmark S., Gehn E., Rubin R. A., Mitchell K., Bradstreet J., el-Dahr J. M. The severity of autism is associated with toxic metal body burden and red blood cell glutathione levels. 2009. p. 7. J. Toxicol.Article ID 532640. [DOI] [PMC free article] [PubMed]
- Agency for Toxic Substances and Disease Registry. Toxicological profile for mercury. 1999. Atlanta, GA. http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=115&tid=24.
- Austin D. W., Shandley K. An Investigation of porphyrinuria in Australian children with autism. J. Toxicol. Environ. Health A. 2008;71:1403–5. doi: 10.1080/15287390802271723. [DOI] [PubMed] [Google Scholar]
- Austin D. W. An epidemiological analysis of the ‘autism as mercury poisoning’ hypothesis. Int. Risk. Safety Med. 2008;20:135–40. [Google Scholar]
- Australian Bureau of Statistics. 4102.0— Australian Social Trends, 1998. 1998. http://www.abs.gov.au/ausstats/abs@.NSF/2f762f95845417aeca25706c00834efa/1f6bee4052f3fb49ca2570ec00192aa1!OpenDocument.
- Bernard S., Enayati A., Redwood H., Binstock T. Autism: A novel form of mercury poisoning. Med. Hypoth. 2001;56:463–71. doi: 10.1054/mehy.2000.1281. [DOI] [PubMed] [Google Scholar]
- Bivings L., Lewis G. Acrodynia: A new treatment with BAL. J. Pediatr. 1949;32:63–65. doi: 10.1016/s0022-3476(48)80131-9. [DOI] [PubMed] [Google Scholar]
- Bivings L. Acrodynia: A summary of BAL therapy reports and a case report of calomel disease. J. Pediatr. 1949;34:322–24. [Google Scholar]
- Bradstreet J., Geier D. A., Kartzinel J. J., Adams J. B., Geier M. R. A case-control study of mercury burden in children with autistic spectrum disorders. J. Am. Phys. Surg. 2003;8:76–79. [Google Scholar]
- Buckmeister L. Medication for attention deficit/hyperactivity disorder (ADHD): An analysis by Federal Electorate (200103) 2004. http://www.aph.gov.au/library/pubs/RB/2004-05/05rb02.htm.
- Centers for Disease Control and Prevention. Surveillance Summaries. Feb. 9, 56 (no. SS-1) 2007. Morbidity and Mortality Weekly Report. http://www.cdc.gov/mmwr/pdf/ss/ss5601.pdf.
- Charman T., Pickles A., Chandler S., Wing L., Bryson S., Simonoff E., Loucas T., Baird G. Commentary: Effects of diagnostic thresholds and research vs service and administrative diagnosis on autism prevalence. Int. J. Epidemiol. 2009;38:1234–38. doi: 10.1093/ije/dyp256. [DOI] [PubMed] [Google Scholar]
- Curtis H. A., Ferguson S. D., Kell R. L., Samuel A. H. Mercury as a health hazard. Arch. Dis. Child. 1987;62:293–95. doi: 10.1136/adc.62.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Down Syndrome Association of Victoria. About Down syndrome. 2009. http://www.dsav.asn.au/AboutDs/aboutDS.html.
- Emsley J. The elements of murder: A history of poisoning. Oxford: Oxford University Press; 2005. [Google Scholar]
- Fragile X Association of Australia. Today is fragile X awareness day. 2009. http://www.fragilex.org.au/2009/awareness-day-fragilex.
- Gallagher C. M., Goodman M. S. Hepatitis B vaccination of male neonates and autism diagnosis, NHIS 1997–2002. J. Toxicol. Environ. Health A. 2010;73:1665–77. doi: 10.1080/15287394.2010.519317. [DOI] [PubMed] [Google Scholar]
- Geier D. A., Geier M. R. A prospective study of mercury toxicity biomarkers in autistic spectrum disorders. J. Toxicol. Environ. Health A. 2007;70:1723–30. doi: 10.1080/15287390701457712. [DOI] [PubMed] [Google Scholar]
- Geier D. A., Geier M. R. An evaluation of the effects of thimerosal on neurodevelopmental disorders reported following DTP and Hib vaccine in comparison to DTPH vaccine in the United States. J. Toxicol. Environ. Health A. 2006a;69:1481–95. doi: 10.1080/15287390500364556. [DOI] [PubMed] [Google Scholar]
- Geier D. A., Geier M. R. A clinical and laboratory evaluation of methionine cycle—Transsulfuration and androgen pathway markers in children with autistic disorders. Horm. Res. 2006b;66:182–188. doi: 10.1159/000094467. [DOI] [PubMed] [Google Scholar]
- Geier D. A., Kern J. K., Garver C. R., Adams J. B., Audhya T., Nataf R., Geier M. R. Biomarkers of environmental toxicity and susceptibility in autism. J. Neurol. Sci. 2009;280:101–8. doi: 10.1016/j.jns.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Hendry W. F., A'Hern R. P., Cole P. J. Was Young's syndrome caused by exposure to mercury in childhood? Br. Med. J. 1993;307:1579–82. doi: 10.1136/bmj.307.6919.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A. S., Blaxill M. F., Haley B. E. Reduced levels of mercury in first baby haircuts of autistic children. Int. J. Toxicol. 2003;22:277–85. doi: 10.1080/10915810305120. [DOI] [PubMed] [Google Scholar]
- Hviid A., Stellfeld M., Wohlfahrt J., Melbye M. Association between thimerosal-containing vaccine and autism. J. Am. Med. Assoc. 2003;290:1763–66. doi: 10.1001/jama.290.13.1763. [DOI] [PubMed] [Google Scholar]
- James S. J., Cutler P., Melnyk S., Jernigan S., Janak l., Gaylor D. W., Neubrander J. A. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am. J. Clin. Nutr. 2004;80:1611–1617. doi: 10.1093/ajcn/80.6.1611. [DOI] [PubMed] [Google Scholar]
- Leys D. A review of infantile acrodynia. Arch. Dis. Child. 1950;25:302–310. doi: 10.1136/adc.25.123.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott M. A., Williams K., Ridley G., Glasson E., Wray J. The prevalence of autism in Australia. Can it be established from existing data? 2007. http://autismaus.com.au/uploads/pdfs/PrevalenceReport.pdf. [DOI] [PubMed]
- Mutter J., Naumann J., Schneider R., Walach H., Haley B. Mercury and autism: Accelerating evidence? Neuro. Endocrinol. Lett. 2005;26:439–446. [PubMed] [Google Scholar]
- Nataf R., Skorupka C., Amet L., Lam A., Springbett A., Lathe R. Porphyrinuria in childhood autistic disorder: Implications for environmental toxicity. Toxicol. Appl. Pharmacol. 2006;214:99–108. doi: 10.1016/j.taap.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Oka E., Ohtsuka Y., Yoshinaga H., Murakami N., Kobayashi K., Ogino T. Prevalence of childhood epilepsy and distribution of epileptic syndromes: A population-based survey in Okayama, Japan. Epilepsia. 2006;47:626–30. doi: 10.1111/j.1528-1167.2006.00477.x. [DOI] [PubMed] [Google Scholar]
- Palmer R. F., Blanchard S., Stein Z., Mandell D., Miller C. Environmental mercury release, special education rates, and autism disorder: An ecological study of Texas. Health Place. 2006;12:203–9. doi: 10.1016/j.healthplace.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Palmer R. F., Blanchard S., Wood R. Proximity to point sources of environmental mercury release as a predictor of autism prevalence. Health Place. 2009;15:18–24. doi: 10.1016/j.healthplace.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Rocaz C. In: Pink disease (infantile acrodynia) Wood I. J., editor. London: Martin Hopkinson Ltd.; 1933. from L'Acrodynie Infantile. [Google Scholar]
- Sakamoto M., Murata K., Kubota M., Nakai K., Satoh H. Mercury and heavy metal profiles of maternal and umbilical cord RBCs in Japanese population. Ecotoxicol. Environ. Safety. 2010;73:1–6. doi: 10.1016/j.ecoenv.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Selter P. Über Trophodermatoneurose. Verh. Ges. Kinderheilkd. (Cassel) 1903;20:45–50. [Google Scholar]
- Sridharan R. Epidemiology of epilepsy. Curr. Sci. 2002;82:664–70. [Google Scholar]
- Swift H. Transactions. Paper presented at the Tenth Session Australasian Medical Congress, Auckland, New Zealand, 1914.
- Verstraeten T., Davis R. L., DeStefano F., Lieu T. A., Rhodes P. H., Black S. B., Shinefield H., Chen R. T. Safety of thimerosal-containing vaccines: A twophased study of computerized health maintenance organization databases. Pediatrics. 2003;112:1039–48. [PubMed] [Google Scholar]
- Warkany J., Hubbard D. M. Adverse mercurial reactions in the form of acrodynia and related conditions. Am. J. Dis. Child. 1951;81:335–73. doi: 10.1001/archpedi.1951.02040030345004. [DOI] [PubMed] [Google Scholar]
- Warkany J., Hubbard D. M. Mercury in the urine of children with acrodynia. Lancet. 1948;1:829–38. doi: 10.1016/s0140-6736(48)91924-2. [DOI] [PubMed] [Google Scholar]
- Wen X. The definition and prevalence of intellectual disability in Australia. 1997. Australian Institute of Health and Welfare (AIHW) Catalogue no. DIS 2. Canberra: AIHW. http://www.aihw.gov.au/publications/index.cfm/title/94.
- Williams H., O'Reilly R. N. Bronchiectasis in children: Its multiple clinical and pathological aspects. Arch. Dis. Child. 1959;34:192–201. doi: 10.1136/adc.34.175.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windham G. C., Zhang L., Gunier R., Croen L. A., Grether J. Autism spectrum disorders in relation to distribution of hazardous air pollutants in the San Francisco Bay air. Environ. Health Perspect. 2006;114:1438–44. doi: 10.1289/ehp.9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood A. J., Wood I. Pink disease. Br. Med. J. 1935;2:527–31. doi: 10.1136/bmj.2.3898.527. [DOI] [PMC free article] [PubMed] [Google Scholar]


