Abstract
Wnt signalling is an evolutionarily conserved pathway that directs cell-fate determination and morphogenesis during metazoan development. Wnt ligands are secreted glycoproteins that act at a distance causing a wide range of cellular responses from stem cell maintenance to cell death and cell proliferation. How Wnt ligands cause such disparate responses is not known, but one possibility is that different outcomes are due to different receptors. Here, we examine PTK7/Otk, a transmembrane receptor that controls a variety of developmental and physiological processes including the regulation of cell polarity, cell migration and invasion. PTK7/Otk co-precipitates canonical Wnt3a and Wnt8, indicating a role in Wnt signalling, but PTK7 inhibits rather than activates canonical Wnt activity in Xenopus, Drosophila and luciferase reporter assays. Loss of PTK7 function activates canonical Wnt signalling and epistasis experiments place PTK7 at the level of the Frizzled receptor. In Drosophila, Otk interacts with Wnt4 and opposes canonical Wnt signalling in embryonic patterning. We propose a model where PTK7/Otk functions in non-canonical Wnt signalling by turning off the canonical signalling branch.
Keywords: Dsh, Otk, PTK7, Wingless, Wnt4
Introduction
Protein tyrosine kinase-7 (PTK7, also known as colon carcinoma kinase-4, CCK-4) is an evolutionarily conserved transmembrane protein that regulates diverse developmental and disease-related processes that require coordinated cellular movements. The expression of PTK7 is frequently deregulated in cancers (Easty et al, 1997; Endoh et al, 2004; Muller-Tidow et al, 2004), and PTK7 was recently shown to promote cell migration and cell survival of leukaemia cells (Prebet et al, 2010). Orthologues of PTK7 have been characterized in mouse, Xenopus, chick (KLG), Drosophila (Off-track, Otk) and in Hydra (Lemon) and have been implicated in the control of a diverse range of morphogenetic cell movements, including neural tube closure, neural crest migration and endothelial cell migration (Miller and Steele, 2000; Winberg et al, 2001; Lu et al, 2004; Toyofuku et al, 2004; Shnitsar and Borchers, 2008).
PTK7 has a role in the regulation of non-canonical Wnt (planar cell polarity, PCP) signalling, but the mechanism remains largely unknown. In vertebrates, loss of PTK7 leads to characteristic PCP phenotypes such as defects in convergent extension and inner ear hair cell polarity (Lu et al, 2004; Yen et al, 2009; Wehner et al, 2011). The Drosophila orthologue of PTK7, Off-track, has not been reported to participate in PCP with its role restricted to neural connectivity and axon guidance (Pulido et al, 1992; Whitford and Ghosh, 2001; Winberg et al, 2001; Cafferty et al, 2004). PTK7 is a transmembrane protein with extracellular immunoglobulin domains and an intracellular kinase homology domain. Although PTK7 resembles a receptor tyrosine kinase, neither interaction partners of the extracellular domain nor possible PTK7 ligands have so far been identified. The kinase homology domain of PTK7 is evolutionarily conserved, but it lacks important amino-acid motifs required for catalytic activity, and kinase function has not been demonstrated (Miller and Steele, 2000; Kroiher et al, 2001). Recently, we showed that the tyrosine kinase homology domain is required for membrane recruitment of Dishevelled (Dsh) (Shnitsar and Borchers, 2008), which is a prerequisite for PCP signalling (Park et al, 2005). Our data show that PTK7 interacts with RACK1 (receptor of activated protein kinase C1) and PKCδ1 to recruit Dsh (Wehner et al, 2011). Interestingly, RACK1 antagonizes canonical Wnt signalling (Li et al, 2011), suggesting that PTK7/RACK1 signalling could also affect canonical Wnt signalling.
In the canonical Wnt pathway, the reception of Wnt occurs through the tripartite binding of Wingless (Wg) and the receptors Frizzled (Fz) and Arrow (Arr, LRP 5/6) (Logan and Nusse, 2004), but other non-canonical receptors such as Derailed/Ryk and Ror have also been reported (van Amerongen et al, 2008). PTK7 could join this list of non-canonical receptors, since it is a part of a Fz/Dsh complex (Shnitsar and Borchers, 2008). The current hypothesis put forward to explain how different Wnts lead to different cellular outcomes posits that different co-receptors provide pathway specificity either by selecting between different Wnt molecules or by recruiting different intracellular components. We put this hypothesis to the test by examining the role of PTK7/Otk in the Wnt pathway in Drosophila and Xenopus model systems.
Results
Wnt4/PTK7 regulates embryonic patterning in Drosophila
The Drosophila embryonic epidermis develops in repeating segments where specialized cells secrete either denticle covered or naked cuticle. Naked cuticle cells are specified by Wg at the anterior of segments. Posterior cells express engrailed and secrete denticles. The basic segment polarity is established by these competing signals, but further signals are used in elaborating the pattern during later stages (Hatini and DiNardo, 2001; Sanson, 2001). A second Wnt (Wnt4) molecule influences the pattern by opposing Wg and eliciting denticle cell fates when overexpressed and loss of denticles when eliminated (Gieseler et al, 1995, 1999). The mechanism of this opposing function has not been described, but Wnt4 may function through a different co-receptor than Wg (van Amerongen et al, 2008).
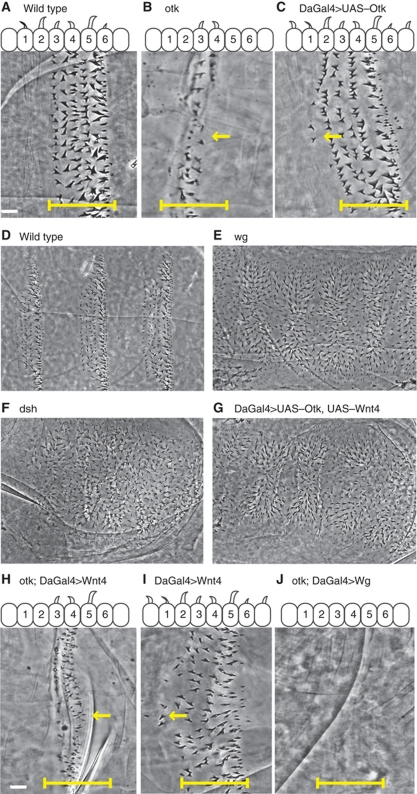
To investigate the epithelial role of PTK7/Otk in Drosophila, we assessed the loss-of-function phenotype in Drosophila embryos. We found that otk3 mutants affect patterning. Compared with a wild-type denticle band, several rows of denticles disappeared from the ventral epidermis of otk3 mutant embryos (compare Figure 1A with B). Similarly, otkRNAi, led to a loss of denticles (Supplementary Figure S1B). In contrast to these loss-of-function phenotypes, Otk overexpression led to ectopic denticles (Figure 1C, arrow). These two phenotypes are very similar to those previously reported for wnt4 mutants (Gieseler et al, 1995, 1999), and led us to investigate a potential link between the two genes. Both Wnt4 and Otk are expressed in stripes localized to the posterior of the segment, suggesting a mechanism that restricts their activity to the denticle making cells (Pulido et al, 1992; Gieseler et al, 1999; Winberg et al, 2001). If Otk is a receptor for Wnt4, then the limited effect of uniform Wnt4 expression may be due to the absence of its receptor in non-responding cells. To test this possibility, we co-expressed Otk and Wnt4 uniformly in embryos. We observed embryos where all epidermal cells secreted denticles (compare Figure 1D with G). This patterning phenotype is very similar to the loss of Wg or Dsh throughout the embryo (Figure 1E and F), or the basic segment polarity phenotype (Nusslein-Volhard and Wieschaus, 1980). Since uniform expression of ligand and receptor led to synergistic cell-fate transformation throughout the epidermis, we investigated whether loss of the receptor could block the cell-fate transforming activity of Wnt4. To test this, we expressed Wnt4 uniformly in otk3 mutant embryos. Loss of Otk blocked the activity of Wnt4 as shown by the narrow denticle bands in otk3 mutant embryos in contrast to the ectopic denticles in embryos expressing only Wnt4 (compare Figure 1H with I). Loss of Otk had no effect on the uniform expression of Wg (Figure 1J), suggesting that Otk is necessary for Wnt4 reception but not for Wg reception.
Figure 1.
Otk opposes Wg-dependent cell-fate determination. (A) Close up of a wild-type segment showing naked cuticle and the six basic rows of denticles. The bar marks the band of the denticle expressing cells. For comparison, the same area is indicated in the following images (B, C, H–J). (B) Close up of an otk3 mutant segment showing 1–3 rows of denticles where fewer cells make denticles and more adopt a naked cell fate (arrow). (C) Overexpression of Otk leads to ectopic denticles appearing in rows 7 and 8 (arrow). (D) Wild-type embryo showing three full segments of repeating pattern. (E) Loss of wg (wgIG22) showing loss of patterning where all cells made denticles. (F) Similarly to wg, in maternal and zygotic dsh mutant embryos, all cells secrete denticles. (G) Expression of both Otk and Wnt4 led to a phenotype indistinguishable from loss of wg. (H) Close up of an otk3 mutant expressing Wnt4 uniformly showing a loss of denticle rows similar to (B) (arrow marks the cells that should be making denticles). (I) Overexpression of Wnt4 in an otherwise wild-type embryo led to ectopic denticles in rows 7 and 8 (arrow). (J) Overexpression of Wg in otk3 mutants showing no denticles rows. Scale bar, 10 μm.
Otk expression affects Arm and engrailed levels
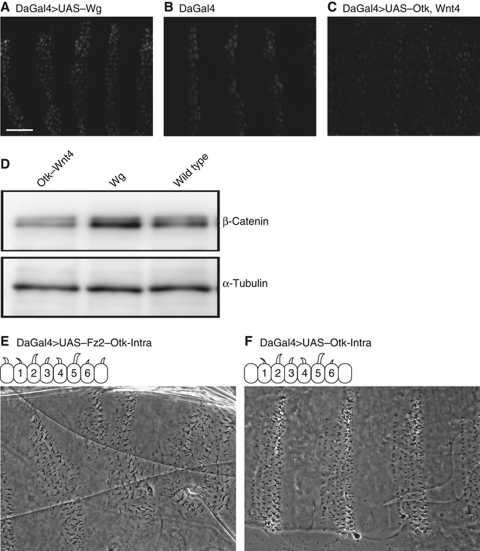
During Drosophila embryogenesis, Wg has two roles. First, early Wg signalling maintains engrailed and Hedgehog expression in adjacent cells ensuring proper segment polarity along the anterior–posterior body axis. Second, Wg signalling assigns specific cell fates within segments (DiNardo et al, 1994; Sanson, 2001). This second role of Wnt signalling was addressed above by observing the denticle versus naked cell fates in cuticle preparations; however, to address Otk's function in the first role necessitated the removal of maternal mRNA from embryos. Unfortunately, we were unable to generate maternal effect mutants for otk as oogenesis appeared to be affected, and no embryos were laid. This was not surprising as Wnt4 has been reported to have a role in oogenesis (Cohen et al, 2002), but it prevented us from being able to examine engrailed expression in otk3 mutants. Instead, we looked at Otk and Wnt4 co-expression and observed a loss of engrailed in these embryos as opposed to engrailed expansion in Wg expressing embryos (compare Figure 2A–C). A second approach was to look at the effect of Otk on β-catenin protein levels in embryos. Canonical Wnt signalling regulates β-catenin protein levels by blocking proteasome-dependent degradation (Tolwinski and Wieschaus, 2004). The levels of β-catenin were lowered by co-expression of Wnt4 and Otk, as compared with the higher levels of β-catenin in wild-type embryos and those expressing Wg (Figure 2D). Overall, Otk phenotypes appear to be indistinguishable from Wnt4 and point to a role for Wnt4 and Otk in blocking canonical Wnt signalling.
Figure 2.
Otk regulates β-catenin and engrailed levels. (A) Uniform expression of Wg leads to expansion of En stripes. (B) Comparable stage wild-type embryo showing normal engrailed expression. (C) Uniform expression of Otk and Wnt4 leads to a loss of engrailed staining in epithelial cells. (D) β-Catenin levels as assayed by western blot from embryos expressing Wg as opposed to Wnt4 and Otk. (E) Expression of Fz2–Otk intracellular domain fusion protein leads to ectopic denticles, whereas expression of (F) Otk intracellular alone does not. Scale bar, 20 μm.
Drosophila Wnt4 interacts with Otk
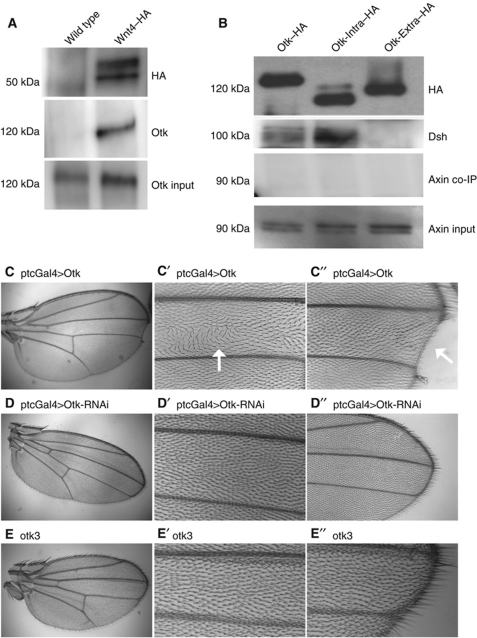
Wnt ligands bind directly to receptors of the Frizzled family, but for signalling to proceed co-receptors are also engaged (Wu and Nusse, 2002; van Amerongen et al, 2008). In canonical Wg signalling, binding to the co-receptor Arr brings Dsh and Axin to the cell surface, thereby inactivating the destruction complex and allowing β-catenin signalling. Wnt4 binds most tightly to Fz2, but its preference in co-receptors is not known (Wu and Nusse, 2002). To test the possibility that Wnt4 and Otk interact, we expressed HA-tagged Wnt4 in embryos and showed that it could pull down endogenous Otk protein in a co-immunoprecipitation assay (Figure 3A). In contrast, Wnt5–HA failed to pull down endogenous Otk (Supplementary Figure S1C). As Wnt4 binds to Fz2 strongly (Wu and Nusse, 2002) and associates with Otk, we asked whether a similar mechanism was at work here as in the Wg–Fz2–Arr complex (Tolwinski et al, 2003). Previously, we had shown this to be the case by fusing the intracellular domain of Arr to the C-terminus of Fz2. This fusion activated signalling (Tolwinski et al, 2003). Here, we fused the intracellular domain of Otk to Fz2 and expressed it in embryos. This appeared to block canonical signalling (Figure 2E) more strongly than the intracellular domain of Otk alone (Figure 2F) or Fz2 alone (Moline et al, 2000), suggesting that the combination of Fz2 and Otk act in blocking signalling in contrast to Fz2 and Arr.
Figure 3.
Otk binds Wnt4 and Dsh. (A) Co-IP experiment where HA antibody was used to pull down Wnt4 from wild-type and Wnt4 expressing embryos and probed for endogenous Otk. (B) Three forms of Otk were expressed in embryos, full-length, intracellular domain, extracellular domain and these pulled down with the HA antibody and probed for the presence of endogenous Dsh and Axin. Dsh was precipitated by the full-length Otk or its intracellular domain. Axin was not co-precipitated with Otk, although it was present in extracts. (C) Overexpression, but not loss of Otk, led to polarity and cell-fate phenotypes. Expression of Otk along the central portion of the wing leads to bristle arrangement defects, arrow (C′), and loss of wing margin (C′′), arrow. (D) Expression of Otk-RNAi does not show an effect on either bristle arrangement (D′) or wing margin specification (D′′). (E–E′′) No phenotypes were observed in wing clones of otk3.
Otk binds Dsh but not Axin
The Dsh protein takes the role of pathway activator directly downstream of the Wnt receptors in all Wnt pathways (Logan and Nusse, 2004). As Dsh functions downstream of PTK7 and Fz7 in Xenopus neural crest cell migration (Shnitsar and Borchers, 2008), we tested for an interaction between Otk and Dsh. We generated HA-tagged Otk constructs comprising the whole protein, the extracellular domain alone or the intracellular domain. We then expressed these in embryos, immunoprecipitated with αHA antibody and probed for the endogenous Dsh protein. Full-length Otk and the intracellular domain alone pulled down Dsh well, but the extracellular domain alone did not (Figure 3B). When the same three Otk isoforms were used in pull-down experiments for endogenous Axin, we found that Axin did not co-precipitate with Otk (Figure 3B), but could be pulled down by APC2 a known pathway component (Supplementary Figure S1D). Therefore, the cytoplasmic domain of Otk binds Dsh but not Axin. Localization of Dsh to the membrane is important for its activation (Cliffe et al, 2003), and a key function of PTK7 (Shnitsar and Borchers, 2008).
The interaction of Otk with Dsh, but not Axin, suggested a role for Otk in non-canonical signalling rather than in canonical Wnt signalling. To test this, we looked at the fly wing where canonical Wg patterns the wing margin (Couso et al, 1994) and non-canonical PCP signalling regulates the alignment of wing hairs (Veeman et al, 2003). Expression of Otk led to a loss of wing margin, the expected result for loss of Wg signal (Figure 3C). Additionally, we observed PCP defects where wing hairs were not properly aligned (Figure 3C). Importantly, this effect was only observed when Otk was overexpressed and not in RNAi knockdowns (Figure 3D) or mutant clones (Figure 3E). These findings, however, correspond with the reported function of Wnt4, which does not cause PCP phenotypes when lost (Lawrence et al, 2002; Chen et al, 2008), but can induce PCP defects when overexpressed (Lim et al, 2005). In summary, Drosophila Otk interacts with Wnt4 and antagonizes canonical Wnt signalling, but are these functions conserved in vertebrates?
Xenopus PTK7 interacts with Wnt3a and Wnt8
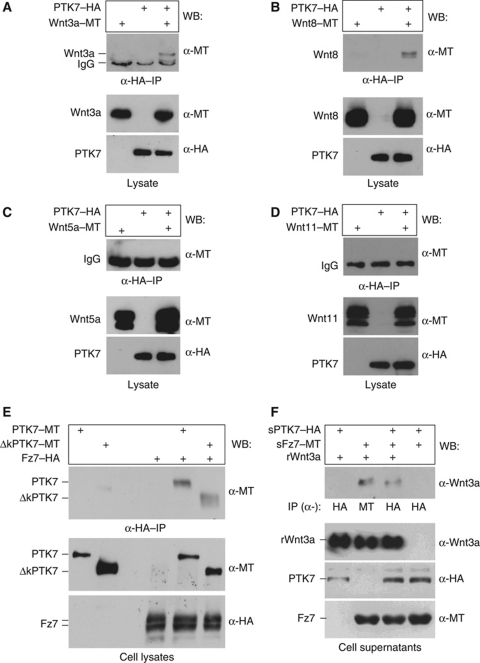
We examined PTK7's function by co-immunoprecipitation and tested for the interaction of Xenopus PTK7 with Wnt3a, Wnt5a, Wnt8 and Wnt11. HA-tagged PTK7 co-precipitated myc-tagged Wnt3a and Wnt8 (Figure 4A and B) in Xenopus lysates. Confirming this finding, Wnt3a and Wnt8 co-precipitated a deletion construct consisting only of the extracellular domain of PTK7 (Supplementary Figure S2), but myc-tagged Wnt5a and Wnt11, which are known effectors of non-canonical Wnt signalling (Heisenberg et al, 2000; Tada and Smith, 2000; Westfall et al, 2003) did not co-precipitate with PTK7 (Figure 4C and D). Therefore, the co-immunoprecipitation data indicate that PTK7 selectively interacts with Wnt3a and Wnt8, but not with Wnt5a and Wnt11.
Figure 4.
PTK7 co-precipitates members of the canonical Wnt family. (A–D) Immunoprecipitation experiments showing co-precipitated Wnt proteins in the upper panel and Xenopus lysates used for immunoprecipitation in the lower panels. Injected constructs are indicated at the top, antibodies used for western blotting on the right. HA-tagged PTK7 co-precipitates myc-tagged Wnt3a (A) or myc-tagged Wnt8 (B). HA-tagged PTK7 does not co-precipitate myc-tagged Wnt5a (C) or myc-tagged Wnt11 (D). Protein bands for Wnt3a and Wnt8 were detected after short exposure times, while longer exposure times did not reveal a signal for Wnt5 and Wnt11 (exposure times are also apparent in the intensity of the IgG signal). (E) Fz7 co-precipitates full-length PTK7 or its kinase deletion mutant (ΔkPTK7) in HEK293 cell lysates. Upper panel shows co-precipitated PTK7 protein and lower panel shows PTK7 and Fz7 expression in cell lysates used for immunoprecipitation. (F) Co-precipitation experiments using cell supernatants of HEK293 cells secreting HA-tagged extracellular PTK7 (sPTK7) or myc-tagged extracellular Fz7 (sFz7) in combination with recombinant Wnt3a (rWnt3a). Supernatants containing PTK7 were precipitated with anti-HA antibodies, the supernatant containing sFz7 and rWnt3a was precipitated using anti-MT antibodies.
As we have previously shown that PTK7 is part of a Fz7/Dsh complex required for membrane recruitment of Dsh (Shnitsar and Borchers, 2008), it is possible that the PTK7–Wnt interaction is mediated by Frizzled receptors. To test this hypothesis, we first characterized the interaction of PTK7 with Fz7 using co-immunoprecipitation in HEK293 cells. Indeed, Fz7 co-precipitates full-length PTK7 and a PTK7-deletion mutant lacking the kinase domain, which is required for Dsh binding (Figure 4E). These data indicate that PTK7 and Fz7 form a receptor complex, independent of Dsh interaction. Thus, we asked if the PTK7–Wnt interaction requires the presence of Fz7. Cell supernatants of HEK293 cells secreting an HA-tagged construct consisting of the extracellular domain of PTK7 (sPTK7), or a myc-tagged construct comprising the extracellular Fz7 domain (sFz7) (Winklbauer et al, 2001), were incubated in combination with recombinant Wnt3a protein. The ability of HA-tagged PTK7 to co-precipitate recombinant Wnt3a was tested in the presence or absence of myc-tagged Fz7. Interestingly, sPTK7 could only precipitate Wnt3a in the presence of sFz7 (Figure 4F). In contrast, sFz7 co-precipitated Wnt3a in the absence of PTK7. Thus, these data indicate that PTK7 forms a receptor complex with Fz7 and that PTK7 requires the presence of Fz7 to interact with Wnt
PTK7 inhibits vertebrate canonical Wnt signalling
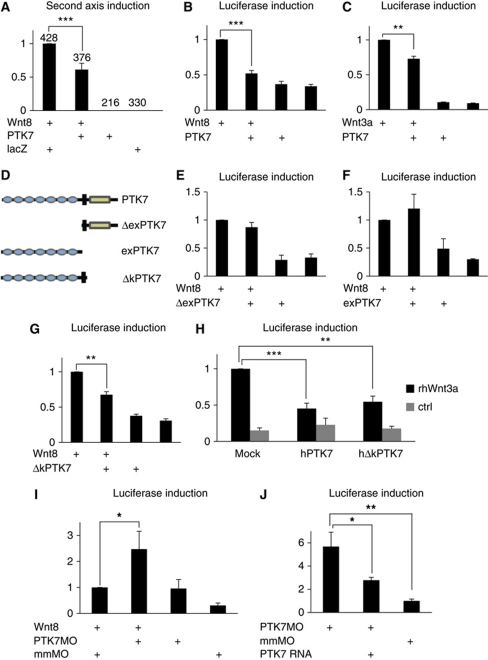
The interaction of PTK7 with Wnt3a and Wnt8 in immunoprecipitation assays suggests that PTK7 may interfere with canonical Wnt signalling. Wnt3a and Wnt8 are known activators of canonical Wnt signalling and can induce a second axis in Xenopus (Smith and Harland, 1991; Sokol et al, 1991; Wolda et al, 1993), which is a hallmark of activation of β-catenin-dependent Wnt signalling (Kuhl and Pandur, 2008). Therefore, we used Xenopus second axis assays to analyse if PTK7 affects the ability of Wnt to activate β-catenin-dependent Wnt signalling. Ectopic expression of Wnt8 on the ventral side of a Xenopus embryo leads to the development of a second axis, thereby generating in the most extreme cases two-headed embryos. Co-expression of PTK7 inhibited the second axis-inducing activity of Wnt8 (Figure 5A). Similar results were seen for Wnt3a-induced second axes (data not shown). These results were confirmed using luciferase reporter assays to measure β-catenin-mediated transcriptional activation. Co-injection of PTK7 inhibited the Wnt8- and Wnt3a-mediated activation of a siamois luciferase reporter (Brannon et al, 1997) in Xenopus embryos (Figure 5B and C).
Figure 5.
PTK7 regulates canonical Wnt signalling. (A) PTK7 significantly inhibits the second axis-inducing activity of Wnt8. Xenopus embryos were injected with equal concentrations of PTK7 or lacZ RNA as a control. The graph summarizes five independent experiments normalized to the second axis-inducing activity of Wnt8, which ranges between 50 and 60% in individual experiments. Numbers of injected embryos and s.e.m. are indicated for each column. (B, C) PTK7 inhibits Wnt8- and Wnt3a-induced siamois reporter activity. Injected RNA concentrations were 100 pg Wnt3a, 100 pg Wnt8 and 500 pg PTK7. Graphs summarize three independent experiments, normalized by the Wnt activity. (D) PTK7 constructs used for luciferase assays. (E–G) Xenopus luciferase reporter assays using a siamois reporter construct; PTK7 constructs were injected at a concentration of 500 pg RNA. Graphs summarize three independent experiments normalized to the Wnt activity. (H) Human PTK7 (hPTK7) and its kinase deletion mutant (hΔkPTK7) inhibit Wnt3a-induced canonical Wnt activity in HEK293 cells. To activate canonical Wnt signalling, cells were treated with recombinant Wnt3a protein (rhWnt3a). The graph summarizes the TOPFLASH reporter activity of three independent experiments. The luciferase activity of Wnt3a-treated mock-transfected cells was set to 1; s.e.m. are shown. (I, J) PTK7 loss of function activates canonical Wnt signalling in Xenopus ectodermal explants. (I) Injection of 20 ng PTK7 MO significantly increased Wnt8-induced siamois reporter activity in ectodermal explants compared with explants injected with 20 ng mismatch control MO (mmMO). The graph summarizes three independent experiments, normalized to the reporter activation of Wnt8 in combination with the mismatch control MO, s.e.m. are shown. (J) Injection of 20 ng PTK7 MO significantly increased the endogenous canonical Wnt activity in ectodermal explants. The graph summarizes five independent experiments, s.e.m. are shown. Siamois reporter activity was normalized to the endogenous Wnt activity of ectodermal explants injected with 20 ng mismatch control MO. Co-injection of 700 pg PTK7 RNA rescues the activation of canonical Wnt signalling induced by the PTK7 MO. ***P-values in a Student's t-test <0.001, **P<0.01, *P<0.05.
To determine which domains of the PTK7 protein are required for the inhibition of canonical Wnt signalling, the function of different deletion mutants (Figure 5D) was analysed using luciferase reporter assays. As the extracellular domain of PTK7 (exPTK7) was precipitated by Wnt3a and Wnt8 (immunoprecipitation; Supplementary Figure S2), it is sufficient for interaction with Wnt and, therefore, likely required for the PTK7-mediated inhibition of Wnt signalling. Indeed, deletion of the extracellular domain of PTK7 (ΔexPTK7) abolished inhibition of canonical Wnt signalling; ΔexPTK7 did not affect the Wnt8-mediated activation of canonical Wnt signalling in siamois reporter assays (Figure 5E). However, the extracellular domain of PTK7 was not sufficient for the inhibition of canonical Wnt signalling. Co-expression of a deletion construct of PTK7 consisting of only its extracellular domain (exPTK7) does not inhibit canonical Wnt signalling (Figure 5F), indicating that other domains or membrane anchorage of the PTK7 protein are required for this function.
The kinase domain of PTK7 is critical for function and could have a role in the inhibition of canonical Wnt signalling. Although this domain seems to be catalytically inactive, it is required for the recruitment of Dsh (Shnitsar and Borchers, 2008). A PTK7-deletion mutant that lacks the kinase domain (ΔkPTK7) inhibits Wnt8 activity (Figure 5G), although not as strongly as full-length PTK7 (Figure 5B). Thus, these data indicate that the extracellular domain, but not the kinase domain, is necessary for inhibition of canonical Wnt signalling.
The function of PTK7 in the inhibition of canonical Wnt signalling is not limited to the Xenopus system, but is also observed in TOPFLASH luciferase reporter assays (Korinek et al, 1997) using HEK293 cells. Transfection of the full-length human PTK7 or its kinase deletion mutant both inhibited the activation of canonical Wnt signalling by Wnt3a (Figure 5H). Furthermore, as recombinant Wnt3a protein was used to activate Wnt signalling, these experiments demonstrate that PTK7 inhibits canonical Wnt signalling in the responding cell. Thus, PTK7 and its kinase deletion mutant inhibit Wnt signalling in Xenopus as well as in human cells, suggesting that PTK7's ability to inhibit canonical Wnt signalling is conserved in vertebrates.
Loss of function of PTK7 activates canonical Wnt signalling
As overexpression of PTK7 inhibits canonical Wnt signalling, we asked if loss of function of PTK7 could activate this signalling pathway. In Xenopus PTK7 is maternally expressed in the animal hemisphere and zygotic expression is detected in the neural ectoderm and the cranial neural crest (Supplementary Figure S3; Lu et al, 2004; Shnitsar and Borchers, 2008). As PTK7 is strongly expressed in the ectoderm, we dissected ectodermal explants from the animal pole of blastula stage embryos to analyse if loss of PTK7 can affect canonical Wnt signalling in this tissue. Xenopus embryos were injected with RNA coding for Wnt8 in combination with PTK7 or control morpholino-oligonucleotides (MO) (Wehner et al, 2011). Ectodermal explants were dissected and cultured up to neurula stages to efficiently knockdown PTK7 function. Wnt8-induced activation of canonical Wnt signalling was significantly enhanced by co-injection of PTK7 MO (Figure 5I), while co-injection of a mismatch control MO did not activate canonical Wnt signalling. Similar effects are seen if PTK7 MO-injected ectodermal explants are compared with ones injected with the mismatch control MO (Figure 5J), indicating that PTK7 loss of function stimulates the endogenous canonical Wnt activity. Furthermore, injection of wild-type PTK7 RNA lacking the MO-binding site rescues the induction of canonical Wnt signalling caused by the PTK7 MO, suggesting that PTK7 loss of function activates canonical Wnt signalling. If this is the case, then loss of PTK7 should at least partially be rescued by inhibition of canonical Wnt signalling. Previously, we have shown that PTK7 is required for neural tube closure (Lu et al, 2004; Wehner et al, 2011). Here, we note that co-injection of RNA coding for a dominant negative Wnt8, which blocks the activity of canonical Wnts (Hoppler et al, 1996), improved the PTK7 loss-of-function neural tube closure defects (Supplementary Figure S4). Taken together, these data support an in vivo role of PTK7 in the inhibition of canonical Wnt signalling.
PTK7 inhibits Wnt signalling upstream of β-catenin and Dsh by interfering with Fz receptors
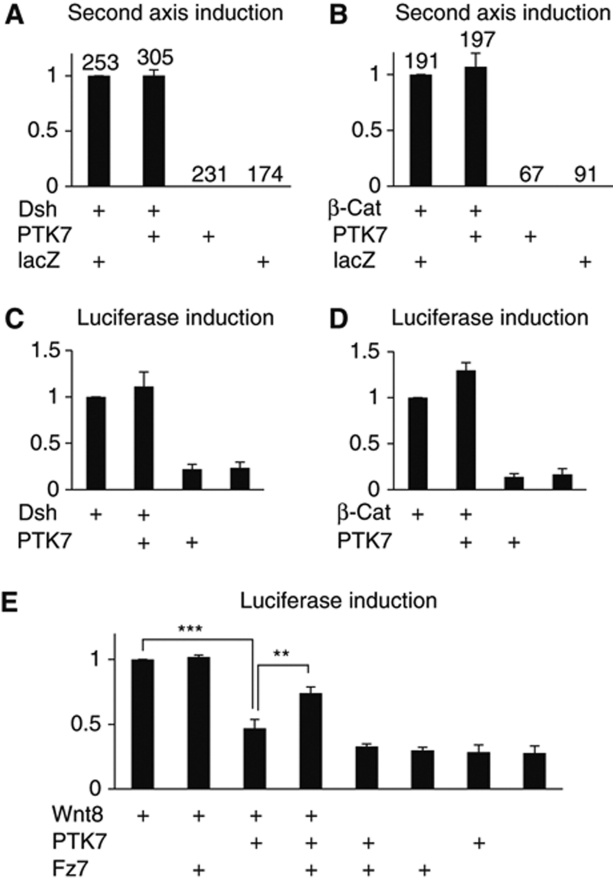
PTK7 inhibits canonical Wnt activity in second axis and luciferase assays; however, it is unclear at which level PTK7 intersects with the canonical Wnt signalling cascade. Second axis assays and luciferase reporter assays suggest that PTK7 inhibits canonical Wnt signalling upstream of β-catenin and Dsh. Ectopic expression of Dsh generates second axes, and the axis-inducing ability of Dsh is not affected by co-injection of PTK7 (Figure 6A). Similar results are obtained if second axes are induced by β-catenin injection (Figure 6B). Luciferase reporter assays confirm these results (Figure 6C and D), showing that neither Dsh nor β-catenin-induced canonical Wnt activity are inhibited by PTK7.
Figure 6.
PTK7 inhibits the activation of canonical Wnt signalling at the level of the Frizzled receptor. (A–D) PTK7 does not inhibit the activation of canonical Wnt signalling by Dsh or β-catenin in Xenopus second axis (A, B) or siamois luciferase reporter assays (C, D). (A, B) Each graph summarizes three independent experiments, normalized to the second axis-inducing activity of Dsh (60–90% axis induction in individual experiments) or β-catenin (50–60% axis induction in individual experiments). The number of injected embryos and s.e.m. are indicated for each column. (C, D) Graphs summarize three independent experiments, normalized to the activation of the siamois luciferase reporter by Dsh (C) or β-catenin (D). (E) Fz7 rescues the inhibition of Wnt8 by PTK7 in siamois luciferase reporter assays. Injected RNA concentration were 100 pg Wnt8, 250 pg PTK7 and 500 pg Fz7. ***P-values in a Student's t-test <0.001, **P<0.01.
PTK7 is part of a Fz/Dsh complex required for Dsh membrane localization (Shnitsar and Borchers, 2008), suggesting that PTK7 may affect canonical Wnt signalling at the Frizzled receptor level. Co-precipitation experiments indicate that PTK7 is a Fz7 co-receptor and requires Fz7 to bind canonical Wnt (Figure 4E and F). Here, we find that Fz7 can rescue the PTK7-mediated inhibition of canonical Wnt signalling (Figure 6E), suggesting that PTK7 functions as a Fz7 co-receptor that favours non-canonical over canonical Wnt signalling.
PTK7 activates non-canonical Wnt signalling
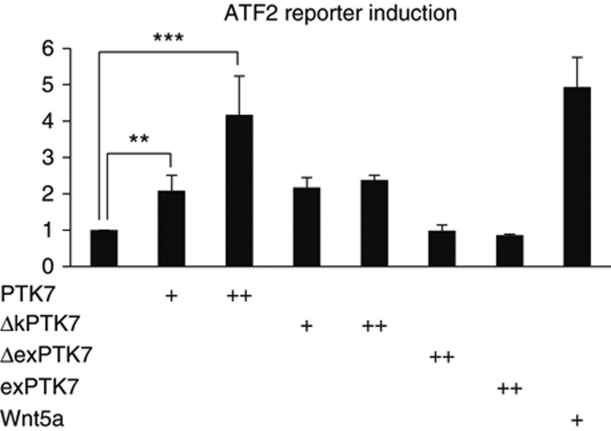
If PTK7 is a non-canonical Fz co-receptor, then it should activate PCP signalling. According to its mouse and Xenopus phenotypes, PTK7 is required for vertebrate PCP (Lu et al, 2004; Shnitsar and Borchers, 2008). Further, PTK7 recruits Dsh to the plasma membrane (Shnitsar and Borchers, 2008; Wehner et al, 2011), a phenomenon that has been associated with activation of non-canonical Wnt signalling (Park et al, 2005). To confirm that PTK7 activates PCP signalling, we used an ATF2-based luciferase reporter (Ohkawara and Niehrs, 2011) to monitor activation of non-canonical Wnt signalling in Xenopus lysates. PTK7 did activate the ATF2 reporter (Figure 7). The kinase deletion mutant of PTK7 (ΔkPTK7) also activated, although this activation was weaker compared with full-length PTK7. In contrast, neither the extracellular deletion mutant of PTK7 (ΔexPTK7) nor the extracellular domain of PTK7 alone (exPTK7) activated non-canonical Wnt signalling. Interestingly, activation of the ATF2 reporter by these different PTK7 constructs correlates well with their inhibitory effect on canonical Wnt signalling as monitored using the siamois luciferase reporter (Figure 5B, E–G). Thus, these data suggest that PTK7 activates non-canonical Wnt signalling at the expense of canonical Wnt signalling.
Figure 7.
PTK7 activates non-canonical Wnt signalling. PTK7 activates an ATF2-based non-canonical Wnt reporter in a concentration-dependent manner. The kinase deletion mutant of PTK7 (ΔkPTK7) shows only mild activation, while an extracellular deletion construct (ΔexPTK7) or the extracellular domain of PTK7 (exPTK7) do not activate the ATF2-based luciferase reporter. Wnt5a serves as a positive control. RNA coding for the different PTK7 constructs were injected at concentrations of 500 pg (+) or 1 ng (++); 500 pg Wnt5a RNA were injected. The graph summarizes three independent experiments, s.e.m. are shown.
Discussion
Extracellular signals control a variety of cellular behaviours. Often, the same signal can elicit different behaviours depending on the cellular context. In one such example, the Wnt ligands activate canonical and a variety of non-canonical Wnt signalling pathways (Logan and Nusse, 2004; Schlessinger et al, 2009), but the mechanisms that exist to control which pathway is turned on remain poorly defined. One possible model posits that specificity could be determined by receptor context. For example, the non-canonical Wnt5a can activate β-catenin signalling in the presence of Fz5 or Fz4 and the co-receptor Lrp5 (He et al, 1997; Mikels and Nusse, 2006), suggesting that the receptor cohort present on the membrane, rather than ligand properties, define signalling outcome.
We propose that PTK7 and Otk are Frizzled co-receptors that activate non-canonical signalling at the expense of canonical signalling by forming a tripartite Wnt/Fz/PTK7 or Wnt/Fz/Otk complex. Binding assays confirm a PTK7/Fz7 co-receptor complex and demonstrate that PTK7 and Otk interact selectively with different Wnts. Functional assays show that PTK7 and Otk co-receptors activate non-canonical Wnt signalling as assayed by Dsh localization to the membrane, and increased ATF2-mediated transcription. Conversely, we find that PTK7/Otk inhibits canonical Wnt signalling. PTK7 overexpression inhibits secondary axis formation in Xenopus and loss of function activates β-catenin-mediated transcription. Thus, PTK7 and Otk behave as Wnt co-receptors that activate non-canonical Wnt signalling, but at the same time turn off canonical Wnt signalling.
The opposing roles of PTK7/Otk in blocking canonical and activating non-canonical Wnt signalling could be explained by the observation that PTK7/Otk binds Dsh, but not Axin. In the canonical pathway, Axin is brought to the membrane by Arr/LRP5/6 and this step is required to inactivate the destruction complex (Tolwinski et al, 2003; Tolwinski and Wieschaus, 2001, 2004). Therefore, when PTK7/Otk rather than Arr/LRP5/6 is present at the membrane, Axin is left in the cytoplasm blocking canonical signalling, while Dsh is brought to the plasma membrane where it can affect non-canonical pathways. Another possible mechanism by which PTK7 may inhibit canonical Wnt signalling is by binding and sequestering canonical members of the Wnt protein family. Binding assays show that PTK7 interacts selectively with Wnt3a and Wnt8, which activate canonical Wnt signalling in Xenopus (Smith and Harland, 1991; Sokol et al, 1991; Wolda et al, 1993). Binding of canonical Wnts to the Fz7/PTK7 receptor complex may prevent their interaction with receptor complexes that activate canonical Wnt signalling. We find this interpretation less likely, as the intracellular domain of PTK7/Otk showed some activity, whereas in the sequestration model one would expect it to be dispensable. Interestingly, the intracellular domain of PTK7 was shown to interact with β-catenin (Puppo et al, 2011), which could prevent β-catenin from entering the nucleus thereby inhibiting canonical Wnt signalling. Contrary to our findings, the authors suggest that PTK7 is required for activating canonical Wnt signalling. Future experiments will thus be needed to clarify the molecular mechanism(s) by which PTK7/Otk modulates canonical Wnt signalling. Another caveat of the interpretation of PTK7 function in Xenopus and Otk function in Drosophila, is that Drosophila Wnts are not thought to be involved in PCP signalling (Simons and Mlodzik, 2008); therefore, the distinction of canonical Wnt versus Wnt-PCP signalling does not apply directly in flies. As there are various non-canonical Wnt pathways known, future experiments will focus on what lies downstream of PTK7/Otk.
Materials and methods
Drosophila crosses and expression of UAS constructs
Oregon R was used as the wild-type strain. Please see Flybase (flybase.bio.indiana.edu) for details on mutants used: wgIG22 (Nusslein-Volhard and Wieschaus, 1980), dshV26 (Klingensmith et al, 1994), otk3, otkEP2017(Winberg et al, 2001), wnt4C1, wnt4EMS23 (Cohen et al, 2002). For overexpression experiments, the daughterless-GAL4 and patched-GAL4 drivers were used (Brand and Perrimon, 1993). Expression constructs used were the UAS–Wnt4 (Gieseler et al, 1999), UAS–APC2–FLAG (Tolwinski, 2009), UAS–Otk (Winberg et al, 2001), UAS–OtkRNAi-TRiP.JF01796, HA-tagged Otk, Wnt5 and Wnt4 were made by Gateway recombination (pUASg–3XHA kindly provided by Bischof et al, 2007). Otk-deletion constructs were made by PCR of the intracellular domain for Otk-Intra (corresponding to amino acids 607–1034 and adding a Src myristolation site to the amino terminus (Zecca et al, 1996; Tolwinski, 2009)) and the extracellular and transmembrane domains for Otk-Extra (corresponding to amino acids 1–687) followed by Gateway recombination into pUASg–3XHA. Fz2–Otk intracellular fusion was made by fusing full-length Fz2 to amino acids 607–1034 of Otk and recombining into pUASg–3XHA.
Crosses:
(1) otk3/CyO, twi-GFP X otk3/Cyo, twi-GFP
(2) otk3/CyO, twi-GFP; daGAL4 X otk3/Cyo, twi-GFP; UAS–Wg
(3) otk3/CyO, twi-GFP; daGAL4 X otk3/Cyo, twi-GFP; UAS–Wnt4
(4) dshV26 FRT18/ovoD2 FRT18
(5) daGal4 X UAS–Wg, Wnt4, Otk
(6) ptcGal4 X UAS–Otk RNAi, Otk
Immunofluorescence and western blotting using Drosophila embryos
Embryos were fixed with heat-methanol treatment (Muller and Wieschaus, 1996) or with heptane/4% formaldehyde in phosphate buffer (0.1 M NaPO4 pH 7.4) (Tolwinski and Wieschaus, 2001). The antibodies used were anti-Arm (mAb N2 7A1, Developmental Studies Hybridoma Bank (DSHB) developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA), anti-En (mouse DSHB), rabbit anti-Armadillo (Peifer et al, 1994b), rabbit and goat anti-Axin (Willert et al, 1999) and anti-HA (12CA5, Roche). New affinity-purified rabbit polyclonal antibodies were generated to Axin, Dsh and Otk through genomic antibody production by Strategic Diagnostic Systems (SDIX, Newark, DE).
Staining, detection and image processing were performed as described (Colosimo and Tolwinski, 2006; Colosimo et al, 2010; Kaplan and Tolwinski, 2010; Kaplan et al, 2009, 2011). Images were acquired on a Zeiss Axioimager with Apotome microscope with a Plan-Apochromat 63 × /1.40 oil immersion objective. Z-stacks of 6 or 20 slices at 0.4 μm distance were acquired.
For western blotting and immunoprecipitation experiments, embryos were lysed in extract buffer (50 mM Tris pH 7.5, 150 mM NaCl, 1% NP-40, 1 mM EDTA, 10% Glycerol, Complete Mini Protease, Sigma) (Colosimo et al, 2010; Kaplan et al, 2011). The extracts were separated by 7.5% SDS–PAGE, and blotted as described in Peifer et al (1994a). Extracts were normalized using the BCA assay (Novagen). Overnight embryo collections were used to make extracts for western blots.
Xenopus injection, second axis assay and animal cap explants
Xenopus embryo microinjections were performed as described (Borchers et al, 2001). Ectodermal explants (animal caps) were prepared as described (Wallingford and Harland, 2001). Capped sense RNA for microinjections was synthesized using the mMessage mMachine kit (Ambion) according to the manufacturer's instructions. The following published plasmids were used for sense mRNA synthesis: PTK7–MT, ΔkPTK7–MT, PTK7–HA (Shnitsar and Borchers, 2008), myc-tagged Xenopus Wnt constructs (Damianitsch et al, 2009), Xwnt3a (Wolda et al, 1993), dnXwnt8 (Hoppler et al, 1996), Dsh–MT (Sokol, 1996), Fz7–MT (Winklbauer et al, 2001) and lacZ (Smith and Harland, 1991). The extracellular domain of Xenopus PTK7 (exPTK7–HA) was cloned into pCS2+ by amplifying PTK7–MT using the following primers: forward 5′-AGTGAGAGGATCCGGGGCAGGAAC-3′, reverse 5′-CGATCGATGACCGCAGCAACCACAGAGAGAACAATG-3′. The PCR product was cut with BamHI and ClaI and ligated into the same restriction sites of pCS2+/HA. The myc-tagged extracellular deletion mutant of PTK7, ΔexPTK7–MT, was introduced by PCR amplification of PTK7-MT using 5′-TGCATTGCGGGAAATGCC-3′ (forward) and 5′-CTGGAAGGTTCCACCATCTT-3′ (reverse) primers. HA-tagged Xenopus Fz7 was generated by amplifying, Fz–MT using the following primers: 5′-TTGAATTCATGTCCTCTACAGTCTCGCTGC-3′ (forward) and 5′-TTCTCGAGCACCGCAGTCTCCCCTTTGCTGCC-3′ (reverse). The PCR product was ligated into the EcoRI/XhoI restriction site of pCS2+/HA.
For axis duplication assays, four-cell stage embryos were injected marginally in one ventral blastomere and lacZ RNA was used to equalize the amounts of injected RNA. Second axis induction was scored at early tadpole stage. Axis duplication was induced by injection of 3 pg Wnt3a, 10 pg Wnt8, 250 pg Dsh or 50 pg β-catenin RNA. In all, 500 pg of PTK7 RNA or lacZ RNA were co-injected, as indicated. Each experiment was performed at least three times, averages and standard errors of the mean are presented.
Co-immunoprecipitation
Xenopus embryos were injected with 80 pg myc-tagged Wnt RNA and 800 pg PTK7 RNA into both blastomeres at the two-cell stage and cultured until stage 10.5–11, when 50 embryos were lysed in co-IP lysis buffer (10 mM Tris–HCl, pH 7.5, 100 mM NaCl, 2 mM EDTA, 1 mM EGTA, 0,5% (v/v) NP-40, 10 % (v/v) glycerol) supplemented with Complete EDTA-free protease inhibitor cocktail tablet (Roche). Lysates were centrifuged for 15 min at 16000 g and the supernatants were incubated for 30 min with Protein A Sepharose (Amersham) at 4°C for pre-clearing. After centrifugation, supernatants were incubated with anti-HA.11 (Covance) or anti-MT 9E10 (Sigma) for 2 h at 4°C, followed by a 2-h incubation with Protein A Sepharose (Amersham) at 4°C. Sepharose beads were washed five times for 5 min with co-IP lysis buffer, boiled for 3 min in Laemmli loading buffer and analysed by western blotting.
For PTK7–Fz7 co-immunoprecipitation experiments, HEK293 cells were seeded in six-well plates at a density of 3.5 × 105 cells per well and cultured for 24 h. Transfection was performed using Lipofectamine™ 2000 (Invitrogen Corporation). At 48 h after transfection, cells were washed and lysed using co-IP lysis buffer (50 mM Tris–HCl pH 7.5, 150 mM NaCl, 0.5 % (v/v) NP-40 supplemented with Complete EDTA-free protease inhibitor cocktail tablet (Roche) and 1 mM NaF, 1 mM β-Glycerolphosphat and 1 mM sodium-orthovanadate). Immunoprecipitation was performed using anti-HA.11 antibodies (Covance).
For immunoprecipitation using secreted proteins, HEK293 cells were transfected with NFz7 (Winklbauer et al, 2001), referred to as sFz7 in the text, or sPTK7. The secreted human PTK7 (sPTK7), consisting of the extracellular domains of PTK7, was amplified by PCR using primers containing a 5′ ClaI restriction site and 3′ EcoRI restriction site: forward primer 5′-CACGTGATCGATGCCCTCAGCTCCTTTTCCTGA-3′, reverse primer 5′-GACTGGAATTCAACCCAATGGTCTGGATCATCTTG-3′. The PCR product was cut with ClaI, EcoRI and ligated into the respective sites of pCS2+/HA. Mock-transfected cells served as a control. Three days after transfection, cell supernatants were collected and sterile filtered and mixed at a 1:1 ratio to a total volume of 1 ml. For immunoprecipitation experiments with Wnt3a, 400 ng of human recombinant Wnt3a protein (R&D Systems) was added. Immunoprecipitation was performed using anti-MT 9E10 (Sigma) or anti-HA.11 (Covance) antibodies.
Luciferase assay
To analyse Wnt activity using reporter assays, two-cell stage embryos were injected with 50 pg Siamois luciferase reporter (S0 1234; Brannon et al, 1997) or 100 pg ATF2 luciferase reporter (Ohkawara and Niehrs, 2011) and 10 pg Renilla (Promega) luciferase reporter DNA in combination with the respective Wnt and PTK7 mRNAs at concentrations indicated in the Figures 5, 6, 7. For luciferase assay, either six samples containing lysates from six embryos, lysed at stage 11, or three samples containing lysates from 20 animal caps, lysed at stage 15, were used for each measurement with the Dual Luciferase Assay Kit (Promega) according to the manufacturer's instructions.
For luciferase assays using HEK293 cells, the TOPFLASH reporter (Korinek et al, 1997) was used and the cells were transfected with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. At 24 h after transfection, the cells were stimulated with 200 ng/ml recombinant human Wnt3a protein (R&D Systems) for 24 h. Cell lysates were prepared and luciferase activity was measured using the Dual Luciferase Assay Kit (Promega). The human full-length (hPTK7) and kinase deletion mutant (hΔkPTK7) were amplified by PCR using primers containing a 5′ ClaI restriction site and 3′ EcoRI restriction site. Primer sequences were as follows: forward primer for both constructs 5′-CACGTGATCGATGCCCTCAGCTCCTTTTCCTGA-3′ reverse for full-length PTK7 5′-GACGTGGAATTCCGGCTTGCTGTCCACGGT-3′, reverse for ΔkPTK7 5′-CCGTGAATTCTCGGGCTGCTTCTGCAGC-3′. PCR products were ligated into the ClaI/EcoRI site of pCS2+/HA.
Supplementary Material
Acknowledgments
We thank Eric Wieschaus and Mary Baylies for comments on the manuscript, and Yong Rao, Marta Llimargas, Travis Bischof, Christof Niehrs, Konrad Basler and Tomas Pieler for sharing reagents. We thank Ilona Wunderlich for technical assistance and Tomas Pieler for providing the infrastructure. This project was supported by grants to AB by the Deutsche Forschungsgemeinschaft (DFG), the DFG research group FOR942, the DFG Center for Molecular Physiology of the Brain (CMPB), and the State of Lower-Saxony and the Volkswagen Foundation, Hannover, Germany. HP and PW are students of the Göttingen Graduate School for Neurosciences and Molecular Biosciences (GGNB).
Author contributions: NK, XL and NT performed the Drosophila experiments in Figures 1, 2, 3. HP performed the main Xenopus experiments with the exception of Supplementary Figure S4, which was done by PW. MP performed the experiments in Figures 4E, F and 5H. AB and HP designed and analysed the experiments in Figures 4, 5, 6, 7. AB and NT wrote the article.
Footnotes
The authors declare that they have no conflict of interest.
References
- Bischof J, Maeda RK, Hediger M, Karch F, Basler K (2007) An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci USA 104: 3312–3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchers A, David R, Wedlich D (2001) Xenopus cadherin-11 restrains cranial neural crest migration and influences neural crest specification. Development 128: 3049–3060 [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415 [DOI] [PubMed] [Google Scholar]
- Brannon M, Gomperts M, Sumoy L, Moon RT, Kimelman D (1997) A beta-catenin/XTcf-3 complex binds to the siamois promoter to regulate dorsal axis specification in Xenopus. Genes Dev 11: 2359–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferty P, Yu L, Rao Y (2004) The receptor tyrosine kinase Off-track is required for layer-specific neuronal connectivity in Drosophila. Development 131: 5287–5295 [DOI] [PubMed] [Google Scholar]
- Chen WS, Antic D, Matis M, Logan CY, Povelones M, Anderson GA, Nusse R, Axelrod JD (2008) Asymmetric homotypic interactions of the atypical cadherin flamingo mediate intercellular polarity signaling. Cell 133: 1093–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliffe A, Hamada F, Bienz M (2003) A role of Dishevelled in relocating Axin to the plasma membrane during wingless signaling. Curr Biol 13: 960–966 [DOI] [PubMed] [Google Scholar]
- Cohen ED, Mariol MC, Wallace RM, Weyers J, Kamberov YG, Pradel J, Wilder EL (2002) DWnt4 regulates cell movement and focal adhesion kinase during Drosophila ovarian morphogenesis. Dev Cell 2: 437–448 [DOI] [PubMed] [Google Scholar]
- Colosimo PF, Liu X, Kaplan NA, Tolwinski NS (2010) GSK3beta affects apical-basal polarity and cell-cell adhesion by regulating aPKC levels. Dev Dyn 239: 115–125 [DOI] [PubMed] [Google Scholar]
- Colosimo PF, Tolwinski NS (2006) Wnt, Hedgehog and junctional Armadillo/beta-catenin establish planar polarity in the Drosophila embryo. PLoS ONE 1: e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couso JP, Bishop SA, Martinez Arias A (1994) The wingless signalling pathway and the patterning of the wing margin in Drosophila. Development 120: 621–636 [DOI] [PubMed] [Google Scholar]
- Damianitsch K, Melchert J, Pieler T (2009) XsFRP5 modulates endodermal organogenesis in Xenopus laevis. Dev Biol 329: 327–337 [DOI] [PubMed] [Google Scholar]
- DiNardo S, Heemskerk J, Dougan S, O'Farrell PH (1994) The making of a maggot: patterning the Drosophila embryonic epidermis. Curr Opin Genet Dev 4: 529–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easty DJ, Mitchell PJ, Patel K, Florenes VA, Spritz RA, Bennett DC (1997) Loss of expression of receptor tyrosine kinase family genes PTK7 and SEK in metastatic melanoma. Int J Cancer 71: 1061–1065 [DOI] [PubMed] [Google Scholar]
- Endoh H, Tomida S, Yatabe Y, Konishi H, Osada H, Tajima K, Kuwano H, Takahashi T, Mitsudomi T (2004) Prognostic model of pulmonary adenocarcinoma by expression profiling of eight genes as determined by quantitative real-time reverse transcriptase polymerase chain reaction. J Clin Oncol 22: 811–819 [DOI] [PubMed] [Google Scholar]
- Gieseler K, Graba Y, Mariol MC, Wilder EL, Martinez-Arias A, Lemaire P, Pradel J (1999) Antagonist activity of DWnt-4 and wingless in the Drosophila embryonic ventral ectoderm and in heterologous Xenopus assays. Mech Dev 85: 123–131 [DOI] [PubMed] [Google Scholar]
- Gieseler K, Mariol MC, Sagnier T, Graba Y, Pradel J (1995) Wingless and DWnt4, 2 Drosophila Wnt genes, have related expression, regulation and function during the embryonic development. C R Acad Sci III 318: 1101–1110 [PubMed] [Google Scholar]
- Hatini V, DiNardo S (2001) Divide and conquer: pattern formation in Drosophila embryonic epidermis. Trends Genet 17: 574–579 [DOI] [PubMed] [Google Scholar]
- He X, Saint-Jeannet JP, Wang Y, Nathans J, Dawid I, Varmus H (1997) A member of the Frizzled protein family mediating axis induction by Wnt-5A. Science 275: 1652–1654 [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW (2000) Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature 405: 76–81 [DOI] [PubMed] [Google Scholar]
- Hoppler S, Brown JD, Moon RT (1996) Expression of a dominant-negative Wnt blocks induction of MyoD in Xenopus embryos. Genes Dev 10: 2805–2817 [DOI] [PubMed] [Google Scholar]
- Kaplan NA, Colosimo PF, Liu X, Tolwinski NS (2011) Complex interactions between GSK3 and aPKC in Drosophila embryonic epithelial morphogenesis. PLoS One 6: e18616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan NA, Liu X, Tolwinski NS (2009) Epithelial polarity: interactions between junctions and apical-basal machinery. Genetics 183: 897–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan NA, Tolwinski NS (2010) Spatially defined Dsh-Lgl interaction contributes to directional tissue morphogenesis. J Cell Sci 123: 3157–3165 [DOI] [PubMed] [Google Scholar]
- Klingensmith J, Nusse R, Perrimon N (1994) The Drosophila segment polarity gene dishevelled encodes a novel protein required for response to the wingless signal. Genes Dev 8: 118–130 [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H (1997) Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science 275: 1784–1787 [DOI] [PubMed] [Google Scholar]
- Kroiher M, Miller MA, Steele RE (2001) Deceiving appearances: signaling by “dead” and “fractured” receptor protein-tyrosine kinases. Bioessays 23: 69–76 [DOI] [PubMed] [Google Scholar]
- Kuhl M, Pandur P (2008) Dorsal axis duplication as a functional readout for Wnt activity. Methods Mol Biol 469: 467–476 [DOI] [PubMed] [Google Scholar]
- Lawrence PA, Casal J, Struhl G (2002) Towards a model of the organisation of planar polarity and pattern in the Drosophila abdomen. Development 129: 2749–2760 [DOI] [PubMed] [Google Scholar]
- Li S, Esterberg R, Lachance V, Ren D, Radde-Gallwitz K, Chi F, Parent JL, Fritz A, Chen P (2011) Rack1 is required for Vangl2 membrane localization and planar cell polarity signaling while attenuating canonical Wnt activity. Proc Natl Acad Sci USA 108: 2264–2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Norga KK, Chen Z, Choi KW (2005) Control of planar cell polarity by interaction of DWnt4 and four-jointed. Genesis 42: 150–161 [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R (2004) The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20: 781–810 [DOI] [PubMed] [Google Scholar]
- Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M (2004) PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature 430: 93–98 [DOI] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R (2006) Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol 4: e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Steele RE (2000) Lemon encodes an unusual receptor protein-tyrosine kinase expressed during gametogenesis in Hydra. Dev Biol 224: 286–298 [DOI] [PubMed] [Google Scholar]
- Moline MM, Dierick HA, Southern C, Bejsovec A (2000) Non-equivalent roles of Drosophila Frizzled and Dfrizzled2 in embryonic wingless signal transduction. Curr Biol 10: 1127–1130 [DOI] [PubMed] [Google Scholar]
- Muller HA, Wieschaus E (1996) armadillo, bazooka, and stardust are critical for early stages in formation of the zonula adherens and maintenance of the polarized blastoderm epithelium in Drosophila. J Cell Biol 134: 149–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Tidow C, Schwable J, Steffen B, Tidow N, Brandt B, Becker K, Schulze-Bahr E, Halfter H, Vogt U, Metzger R, Schneider PM, Buchner T, Brandts C, Berdel WE, Serve H (2004) High-throughput analysis of genome-wide receptor tyrosine kinase expression in human cancers identifies potential novel drug targets. Clin Cancer Res 10: 1241–1249 [DOI] [PubMed] [Google Scholar]
- Nusslein-Volhard C, Wieschaus E (1980) Mutations affecting segment number and polarity in Drosophila. Nature 287: 795–801 [DOI] [PubMed] [Google Scholar]
- Ohkawara B, Niehrs C (2011) An ATF2-based luciferase reporter to monitor non-canonical Wnt signaling in Xenopus embryos. Dev Dyn 240: 188–194 [DOI] [PubMed] [Google Scholar]
- Park TJ, Gray RS, Sato A, Habas R, Wallingford JB (2005) Subcellular localization and signaling properties of dishevelled in developing vertebrate embryos. Curr Biol 15: 1039–1044 [DOI] [PubMed] [Google Scholar]
- Peifer M, Pai LM, Casey M (1994a) Phosphorylation of the Drosophila adherens junction protein Armadillo: roles for wingless signal and zeste-white 3 kinase. Dev Biol 166: 543–556 [DOI] [PubMed] [Google Scholar]
- Peifer M, Sweeton D, Casey M, Wieschaus E (1994b) wingless signal and Zeste-white 3 kinase trigger opposing changes in the intracellular distribution of Armadillo. Development 120: 369–380 [DOI] [PubMed] [Google Scholar]
- Prebet T, Lhoumeau AC, Arnoulet C, Aulas A, Marchetto S, Audebert S, Puppo F, Chabannon C, Sainty D, Santoni MJ, Sebbagh M, Summerour V, Huon Y, Shin WS, Lee ST, Esterni B, Vey N, Borg JP (2010) The cell polarity PTK7 receptor acts as a modulator of the chemotherapeutic response in acute myeloid leukemia and impairs clinical outcome. Blood 116: 2315–2323 [DOI] [PubMed] [Google Scholar]
- Pulido D, Campuzano S, Koda T, Modolell J, Barbacid M (1992) Dtrk, a Drosophila gene related to the trk family of neurotrophin receptors, encodes a novel class of neural cell adhesion molecule. EMBO J 11: 391–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puppo F, Thomé V, Lhoumeau AC, Cibois M, Gangar A, Lembo F, Belotti E, Marchetto S, Lécine P, Prébet T, Sebbagh M, Shin WS, Lee ST, Kodjabachian L, Borg JP (2011) Protein tyrosine kinase 7 has a conserved role in Wnt/β-catenin canonical signalling. EMBO Rep 12: 43–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanson B (2001) Generating patterns from fields of cells. Examples from Drosophila segmentation. EMBO Rep 2: 1083–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger K, Hall A, Tolwinski N (2009) Wnt signaling pathways meet Rho GTPases. Genes Dev 23: 265–277 [DOI] [PubMed] [Google Scholar]
- Shnitsar I, Borchers A (2008) PTK7 recruits dsh to regulate neural crest migration. Development 135: 4015–4024 [DOI] [PubMed] [Google Scholar]
- Simons M, Mlodzik M (2008) Planar cell polarity signaling: from fly development to human disease. Annu Rev Genet 42: 517–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WC, Harland RM (1991) Injected Xwnt-8 RNA acts early in Xenopus embryos to promote formation of a vegetal dorsalizing center. Cell 67: 753–765 [DOI] [PubMed] [Google Scholar]
- Sokol S, Christian JL, Moon RT, Melton DA (1991) Injected Wnt RNA induces a complete body axis in Xenopus embryos. Cell 67: 741–752 [DOI] [PubMed] [Google Scholar]
- Sokol SY (1996) Analysis of Dishevelled signalling pathways during Xenopus development. Curr Biol 6: 1456–1467 [DOI] [PubMed] [Google Scholar]
- Tada M, Smith J (2000) Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development 127: 2227–2238 [DOI] [PubMed] [Google Scholar]
- Tolwinski NS (2009) Membrane bound axin is sufficient for wingless signaling in Drosophila embryos. Genetics 181: 1169–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolwinski NS, Wehrli M, Rives A, Erdeniz N, DiNardo S, Wieschaus E (2003) Wg/Wnt signal can be transmitted through arrow/LRP5,6 and Axin independently of Zw3/Gsk3beta activity. Dev Cell 4: 407–418 [DOI] [PubMed] [Google Scholar]
- Tolwinski NS, Wieschaus E (2001) Armadillo nuclear import is regulated by cytoplasmic anchor Axin and nuclear anchor dTCF/Pan. Development 128: 2107–2117 [DOI] [PubMed] [Google Scholar]
- Tolwinski NS, Wieschaus E (2004) Rethinking WNT signaling. Trends Genet 20: 177–181 [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Zhang H, Kumanogoh A, Takegahara N, Suto F, Kamei J, Aoki K, Yabuki M, Hori M, Fujisawa H, Kikutani H (2004) Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev 18: 435–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amerongen R, Mikels A, Nusse R (2008) Alternative wnt signaling is initiated by distinct receptors. Sci Signal 1: re9. [DOI] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT (2003) A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell 5: 367–377 [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Harland RM (2001) Xenopus Dishevelled signaling regulates both neural and mesodermal convergent extension: parallel forces elongating the body axis. Development 128: 2581–2592 [DOI] [PubMed] [Google Scholar]
- Wehner P, Shnitsar I, Urlaub H, Borchers A (2011) RACK1 is a novel interaction partner of PTK7 that is required for neural tube closure. Development 138: 1321–1327 [DOI] [PubMed] [Google Scholar]
- Westfall TA, Brimeyer R, Twedt J, Gladon J, Olberding A, Furutani-Seiki M, Slusarski DC (2003) Wnt-5/pipetail functions in vertebrate axis formation as a negative regulator of Wnt/beta-catenin activity. J Cell Biol 162: 889–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford KL, Ghosh A (2001) Plexin signaling via off-track and rho family GTPases. Neuron 32: 1–3 [DOI] [PubMed] [Google Scholar]
- Willert K, Shibamoto S, Nusse R (1999) Wnt-induced dephosphorylation of axin releases beta-catenin from the axin complex. Genes Dev 13: 1768–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winberg ML, Tamagnone L, Bai J, Comoglio PM, Montell D, Goodman CS (2001) The transmembrane protein Off-track associates with Plexins and functions downstream of Semaphorin signaling during axon guidance. Neuron 32: 53–62 [DOI] [PubMed] [Google Scholar]
- Winklbauer R, Medina A, Swain RK, Steinbeisser H (2001) Frizzled-7 signalling controls tissue separation during Xenopus gastrulation. Nature 413: 856–860 [DOI] [PubMed] [Google Scholar]
- Wolda SL, Moody CJ, Moon RT (1993) Overlapping expression of Xwnt-3A and Xwnt-1 in neural tissue of Xenopus laevis embryos. Dev Biol 155: 46–57 [DOI] [PubMed] [Google Scholar]
- Wu CH, Nusse R (2002) Ligand receptor interactions in the Wnt signaling pathway in Drosophila. J Biol Chem 277: 41762–41769 [DOI] [PubMed] [Google Scholar]
- Yen WW, Williams M, Periasamy A, Conaway M, Burdsal C, Keller R, Lu X, Sutherland A (2009) PTK7 is essential for polarized cell motility and convergent extension during mouse gastrulation. Development 136: 2039–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca M, Basler K, Struhl G (1996) Direct and long-range action of a wingless morphogen gradient. Cell 87: 833–844 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.