Abstract
Apoptosis, the major form of programmed cell death in metazoan organisms, plays critical roles in normal development, tissue homeostasis and immunity, and its disturbed regulation contributes to many pathological states, including cancer, autoimmunity, infection and degenerative disorders. In vertebrates, it can be triggered either by engagement of ‘death receptors’ of the tumour necrosis factor receptor family on the cell surface or by diverse intracellular signals that act upon the Bcl-2 protein family, which controls the integrity of the mitochondrial outer membrane through the complex interactions of family members. Both pathways lead to cellular demolition by dedicated proteases termed caspases. This review discusses the groundbreaking experiments from many laboratories that have clarified cell death regulation and galvanised efforts to translate this knowledge into novel therapeutic strategies for the treatment of malignant and perhaps certain autoimmune and infectious diseases.
Keywords: apoptosis, Bcl-2 protein family, cancer therapy, mitochondria, tumourigenesis
Introduction
Cell death programmes have evolved to meet many needs. While certain unicellular organisms can invoke cell death to match cell numbers to the nutrient supply, multicellular organisms utilise ‘altruistic’ cell suicide for diverse essential purposes (Golstein, 1998). During embryogenesis, cell death sculpts the tissues by removing superfluous cells—hollowing out ducts, for example, or deleting interdigital cells during limb formation. During neuronal development, cell death is critical for matching neuron numbers with their targets (e.g. other neurons or muscle cells). In adults, cell death counterbalances cell proliferation to maintain homeostasis in rapidly renewing tissues (e.g. the intestinal epithelium or haemopoietic tissues), and drives mammary gland involution at weaning and thymus atrophy on ageing (Kerr et al, 1972). Cell death also eliminates irreparably damaged or potentially dangerous cells, such as those undergoing neoplastic transformation or lymphocytes that target self-tissues (Strasser et al, 2000). Finally, to limit pathogen spread, both the innate and adaptive immune systems target infected cells (Trambas and Griffiths, 2003)—provoking an arms race in which pathogens evolve new ways to prevent host cell suicide while their hosts refine their weaponry for killing infected cells (Vaux et al, 1994).
The major mode of programmed cell death, apoptosis, is prominent in diverse animal species and has been intensely investigated in mammals, the fruit fly Drosophila, and the worm Caenorhabditis elegans. Although evidence for additional types of programmed cell death processes (e.g. necroptosis) is growing, they have been reviewed recently (Hotchkiss et al, 2009; Yuan and Kroemer, 2010) and will only be briefly addressed here.
Morphologically, apoptosis is characterised by chromatin condensation and shrinkage of the nucleus and cytoplasm, followed by fragmentation of the cell into plasma membrane-bound ‘apoptotic bodies’, which are quickly engulfed by nearby phagocytic cells and ultimately digested in lysosomes (Kerr et al, 1972). Removal of the cellular corpse, triggered by ‘eat me’ signals on the dying cells, is so rapid that apoptosis is not readily visible histologically, even in tissues with massive cell turnover, such as the thymus (Egerton et al, 1990). In contrast to necrosis, apoptosis does not rupture the plasma membrane, thereby minimising release of inflammatory cellular contents and the risk of inducing autoimmunity (Nagata et al, 2010).
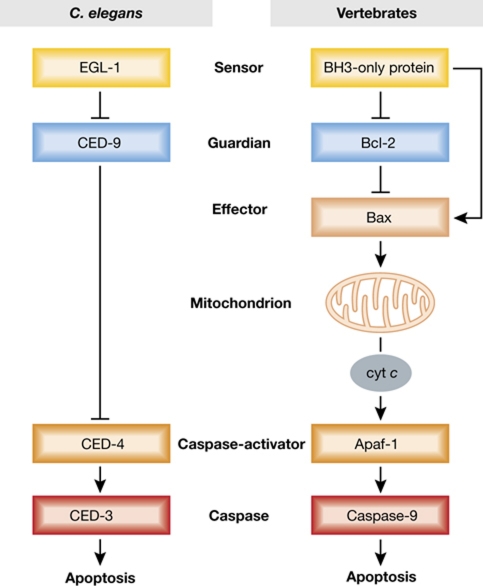
The foundation stones for current understanding of apoptosis were laid by a remarkable confluence of pioneering genetic studies in C. elegans with discoveries emerging from mammalian cancer genetics and biochemistry. The first molecularly defined cell death regulator emerged after the breakpoint of a recurrent chromosome translocation in human follicular lymphoma revealed the previously unknown gene BCL-2 (Tsujimoto et al, 1984). In a seminal discovery, enforced Bcl-2 expression was found to render cultured haemopoietic cells refractory to cell death upon cytokine deprivation (Vaux et al, 1988) and to promote B-cell accumulation in vivo (McDonnell et al, 1989; Strasser et al, 1991b), often culminating in autoimmune disease or cancer (see below). Strikingly, CED-9, which prevents developmentally programmed cell death in the worm (Hengartner et al, 1992), proved to be the functional homologue of BCL-2 (Vaux et al, 1992; Hengartner and Horvitz, 1994), heralding a partially conserved genetic programme (Figure 1). The exciting finding that the essential worm killer protein CED-3 resembled a new type of mammalian protease (Yuan et al, 1993) revealed that cell demolition relied on dedicated aspartate-specific cysteine proteases, soon termed caspases. Subsequently, elegant biochemical studies disclosed that the proteolytic cascade often initiates on the scaffold protein APAF-1 (Zou et al, 1997), which proved to be the homologue of worm CED-4 (Yuan and Horvitz, 1992). Collectively, these and other discoveries discussed below delineated the genetic programme for controlling apoptotic cell death, with immense ramifications for biology and human health.
Figure 1.
Comparison of the pathway of programmed cell death in C. elegans with the major one in vertebrates. The worm has a homologue of Bcl-2 (CED-9), of its BH3-only apoptosis inducers (EGL-1), of the caspase-activator Apaf-1 (CED-4) and of the proteolytic caspases (CED-3). However, a striking difference is that CED-9 directly binds and inhibits CED-4, until CED-4 is displaced by EGL-1, whereas vertebrate Bcl-2 does not bind to Apaf-1 but instead prevents the activation of its pro-apoptotic siblings Bax and Bak, thereby preventing their permeabilisation of the MOM and release of cytochrome c (cyt c), an essential cofactor for Apaf-1.
This review will concentrate upon apoptosis in mammalian cells, because defects in its control contribute to many human diseases (Hotchkiss et al, 2009), particularly cancer (Cory and Adams, 2002). We first briefly describe the effectors of apoptosis, the caspases, and then focus on its major regulators, particularly the Bcl-2 protein family. We discuss the physiological function of Bcl-2 family members and the remarkable ways, as yet incompletely understood, in which their interactions flip the apoptotic switch. We briefly describe the proposed link from Bcl-2 via Beclin-1 to autophagy, another ancient cell death/survival mechanism. Finally, we discuss how apoptosis is impaired in cancer, restricting current treatment modalities, and how directly targeting the apoptotic machinery is offering new hope for improved therapy for cancer and perhaps also for certain autoimmune and infectious diseases.
Caspases: the cellular demolition crew
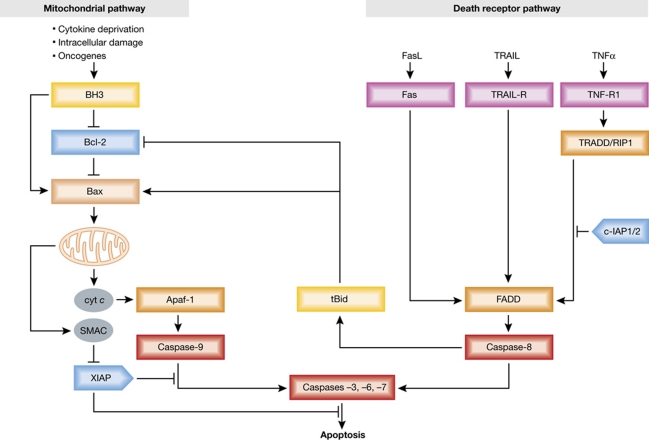
Regardless of the initiating death stimulus or cell type, apoptosis culminates in the fragmentation of several hundred proteins and the DNA. Aspartate-specific cysteinyl proteases termed caspases mediate the proteolysis (Timmer and Salvesen, 2007) and also activate the responsible DNAse, CAD (caspase-activated DNAse), by cleaving its chaperone/inhibitor ICAD (inhibitor of CAD) (Liu et al, 1997; Enari et al, 1998), allowing CAD to chop the chromatin into a characteristic ‘ladder’ of nucleosomes. By structure and function, caspases fall into two groups: initiator (or apical) caspases and effector (or executioner) caspases (Riedl and Salvesen, 2007; Timmer and Salvesen, 2007). The executioners, caspases-3, -6 and -7, which perform nearly all the cellular proteolysis and activate CAD, are synthesised as single-chain zymogens (catalytically inactive pro-forms) with short pro-domains. The proteolytic cascade is launched when an initiator caspase cleaves them into fragments of ∼20 (p20) and ∼10 (p10) kDa that assemble into the active tetrameric (p202p102) proteases. The initiator caspases, such as caspase-8 or -9, have long pro-domains that, following an apoptotic signal, target them to specific scaffold proteins (FADD/Mort1 for caspase-8 and Apaf-1 for caspase-9; Figure 2), where conformational changes provoke their activation (Riedl and Salvesen, 2007).
Figure 2.
The two major pathways to caspase activation in vertebrates: the death receptor or extrinsic pathway, engaged by the indicated members of the TNF receptor family on the cell surface, and the Bcl-2-regulated mitochondrial or intrinsic pathway. The death receptors lead, via the adaptor FADD (with help by TRADD in certain death receptors), to activation of caspase-8, which then activates the effector caspases-3, -6 and -7. Caspase-8 also processes the BH3-only protein Bid, and the truncated Bid (tBid) can then activate the Bcl-2-regulated pathway. Upon MOMP, that pathway leads to effector caspase activation via Apaf-1 and caspase-9. The cytosolic E3 ubiquitin ligase XIAP can inhibit caspases-3 and -7 (and perhaps caspase-9), but that inhibition is blocked by SMAC/DIABLO when it is released from mitochondria. E3 ubiquitin ligases cIAP1 and cIAP2 work instead in part by preventing formation of the pro-apoptotic signalling complex from TNF-R1 and by regulating pro-survival NF-κB survival pathways.
Some caspases have non-apoptotic functions. Caspase-1 and its adaptors, which process pro-IL-1β and IL-18, are critical for inflammatory responses (Martinon et al, 2002). Perplexingly, caspase-8 and its adaptor FADD are essential not only for the apoptosis induced by ‘death receptors’ (see below) but also for blood vessel development, macrophage differentiation and the proliferation of certain cell types (Newton et al, 1998; Varfolomeev et al, 1998; Zhang et al, 1998). To elicit apoptosis, caspase-8 must be processed to the p202p102 tetramer, but the non-apoptotic functions require only its non-processed activated form (Kang et al, 2008). That form can prevent the RIP1 and RIP3 kinases from provoking necroptosis (programmed necrosis) (Kaiser et al, 2011; Oberst et al, 2011; Zhang et al, 2011).
Two distinct but convergent pathways to caspase activation
Vertebrates have two distinct apoptosis signalling pathways (Strasser et al, 1995) that ultimately converge upon effector caspase activation (Figure 2). The death receptor (or extrinsic) pathway is engaged on the plasma membrane by ligation of members of the tumour necrosis factor (TNF) receptor super-family containing intracellular ‘death domains’, such as Fas and TNF-R1. As reviewed elsewhere (Strasser et al, 2009), these receptors trigger apoptosis by forming a ‘death-inducing signalling complex’ (Kischkel et al, 1995), within which the FADD adaptor protein, assisted in certain death receptors (e.g. TNF-R1) by the adaptor TRADD, recruits and activates caspase-8 (and caspase-10 in humans and certain other species but not mouse). In the Bcl-2-regulated mitochondrial (or intrinsic) pathway, developmental cues and diverse cytotoxic insults, including growth factor deprivation and exposure to DNA damage or cancer therapeutics, initiate apoptosis by activating pro-apoptotic members of the Bcl-2 protein family (see below). Their action leads to the release from the mitochondrial intermembrane space not only of cytochrome c, which triggers APAF-1-mediated activation of caspase-9, but also of other apoptogenic proteins, such as SMAC/DIABLO, which prevents the Inhibitor of Apoptosis protein XIAP from inhibiting its caspase targets (Green and Kroemer, 2004; Jost et al, 2009).
Experiments with genetically modified mice revealed that FADD and caspase-8 are essential for death receptor-induced apoptosis but dispensable for apoptosis initiated by the mitochondrial pathway (Newton et al, 1998; Varfolomeev et al, 1998; Zhang et al, 1998). Conversely, cells lacking caspase-9 or its activator APAF-1 have defects in Bcl-2-regulated apoptosis but not death receptor-induced killing (Hakem et al, 1998; Kuida et al, 1998). Interestingly, however, although loss or inhibition of caspase-8 allows long-term (clonogenic) survival of cells stimulated through a death receptor (Smith et al, 1996; Longthorne and Williams, 1997; Varfolomeev et al, 1998; Salmena et al, 2003), loss of caspase-9 or APAF-1 does not allow long-term cell survival if the mitochondrial pathway is engaged (Marsden et al, 2002, 2004, 2006; Ekert et al, 2004; van Delft et al, 2009). This is because mitochondrial outer membrane permeabilisation (MOMP) by activated Bax and Bak (see below) commits the cell to die (Green and Kroemer, 2004). Even in the absence of caspase activity, the reduced respiration following cytochrome c release soon triggers a backup cell death and clearance programme (Goldstein et al, 2000).
The Bcl-2 family: the cellular life/death switch
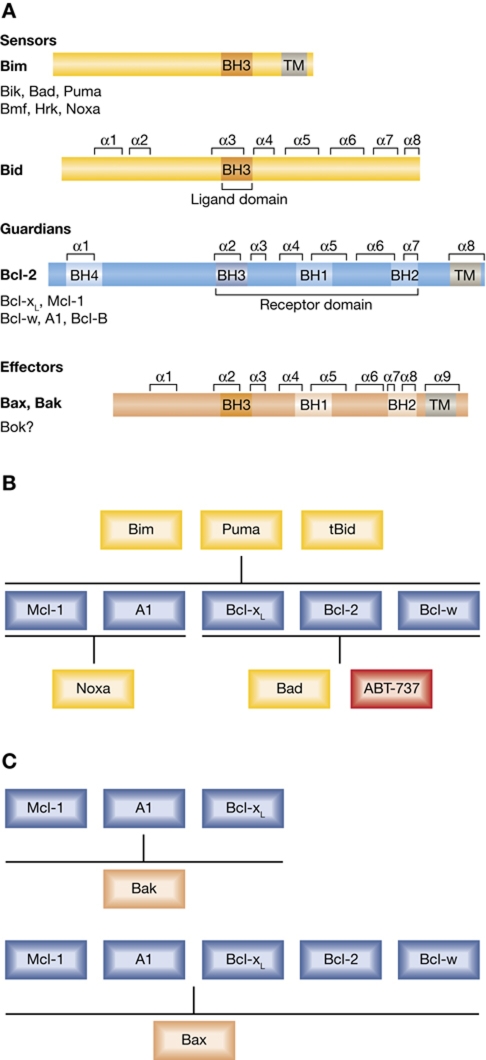
The vertebrate Bcl-2 family contains three functional subgroups (Figure 3A). Bcl-2 and its closest homologues (Bcl-xL, Bcl-w, Mcl-1, A1/Bfl1 and, in humans, Bcl-B), which contain four conserved sequence motifs (called Bcl-2 homology (BH) domains), all promote cell survival. The pro-apoptotic effectors Bax, Bak and the much less studied Bok share extensive similarity with their pro-survival relatives, including structural features of all four BH regions (Kvansakul et al, 2008). Despite this similarity, once activated, Bax and Bak damage rather than protect mitochondria, and either protein suffices for MOMP, indicative of functional redundancy (Lindsten et al, 2000). Lastly, the apoptosis initiators, the BH3-only proteins (which include Bad, Bik, Hrk, Bid, Bim, Bmf, Noxa and Puma) share with each other and the Bcl-2 family at large only the ∼26-residue BH3 domain. This amphipathic α-helix allows them to engage and inactivate their pro-survival relatives (Sattler et al, 1997) and perhaps also to transiently bind and activate Bax and Bak (Letai et al, 2002; Gavathiotis et al, 2008) (see below). The BH3-only proteins are activated by distinct cytotoxic stimuli in various ways, including enhanced transcription and post-translational modifications (Puthalakath and Strasser, 2002).
Figure 3.
(A) The three functional subgroups of the Bcl-2 family. Sequences most homologous to Bcl-2 (BH domains) and α-helical regions are indicated. The BH3-only proteins share sequence homology only within the BH3 domain, which mediates association between family members. Bid has a defined 3D structure but the others are relatively unstructured. The pro-survival group, which shares four regions of sequence homology, includes Bcl-B in humans but its mouse homologue (Boo) appears to be inactive due to a mutation of essential residues in BH1. The pro-apoptotic Bax/Bak group, which includes the little studied Bok, is remarkably similar in sequence and structure to the pro-survival group, including an α-helix resembling BH4 near the N-terminus (Kvansakul et al, 2008). Most family members contain a C-terminal hydrophobic trans-membrane (TM) region, which mediates their targeting and anchoring to the MOM and/or the ER, either constitutively (e.g. Bcl-2 or Bak) or after an apoptotic stimulus (e.g. Bcl-xL or Bax). (B,C) Predominant interactions within the Bcl-2 family, including those of BH3-only proteins with their pro-survival relatives (B) and the major interactions of the latter with Bax and Bak (C).
While most BH3-only proteins are unstructured prior to engaging pro-survival proteins (Hinds et al, 2007), Bid forms an α-helical bundle resembling Bax or Bcl-2 (Chou et al, 1999; McDonnell et al, 1999), despite the lack of sequence homology except for the BH3 domain (Youle and Strasser, 2008). Bid can link the death receptor and Bcl-2-regulated pathways because its cleavage by caspase-8 generates an active C-terminal segment termed tBid, which promotes Bax/Bak-mediated MOMP (Figure 2). This tBid-activated amplification mechanism is essential for death receptor-induced killing in so-called type 2 cells, such as hepatocytes, but dispensable in type 1 cells, such as thymocytes (Scaffidi et al, 1998; Yin et al, 1999; Kaufmann et al, 2007).
The Bcl-2 family can be regarded as a tripartite switch that sets the threshold for commitment to cell death, primarily by interactions within the family. The pro-survival members can bind with high affinity to members of both the Bax/Bak-like and the BH3-only subgroups, via association of the BH3 domain of the pro-apoptotic proteins with a hydrophobic groove on the surface of the pro-survival proteins (Sattler et al, 1997). These interactions, however, exhibit specificity (Figure 3B, C). The affinities of BH3-only proteins for the pro-survival proteins differ markedly (Chen et al, 2005; Kuwana et al, 2005): Bim, Puma and perhaps tBid bind all with high affinity, whereas other BH3-only proteins show more selectivity (Figure 3B). Most strikingly, Bad binds Bcl-2, Bcl-xL and Bcl-w but not Mcl-1 or A1, whereas Noxa interacts strongly only with Mcl-1 and A1. Accordingly, enforced expression of either Bim or Puma potently kills cells, whereas Bad and Noxa can efficiently induce cell death only if coexpressed (Chen et al, 2005). Bax and Bak also differ in their interaction with the pro-survival proteins (Figure 3C). Bak can be bound tightly by Bcl-xL and Mcl-1 but only poorly by Bcl-2 (Willis et al, 2005), whereas all the pro-survival proteins probably can constrain Bax activity (Willis et al, 2007).
How the Bcl-2 apoptotic switch is flipped
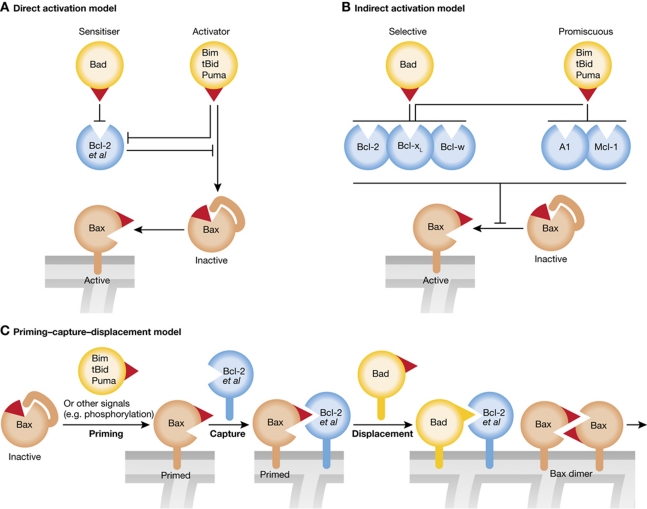
Two distinct, albeit not mutually exclusive, models have been proposed to describe how the interplay between the three Bcl-2 factions activates Bax and Bak and hence produces MOMP (Adams and Cory, 2007; Chipuk and Green, 2008). The direct activation model (Figure 4A) posits that certain BH3-only proteins (termed ‘activators’), namely Bim, tBid and probably Puma (Letai et al, 2002; Kuwana et al, 2005; Kim et al, 2009), must transiently bind and activate Bax and Bak, whereas the other BH3-only proteins, termed ‘sensitisers’ (e.g. Bad, Noxa), can only bind their pro-survival relatives (‘Bcl-2 et al’ in Figure 4A). In this model, the pro-survival Bcl-2 proteins function by sequestering the ‘activators’, and apoptosis proceeds when ‘sensitisers’ displace the activators from the pro-survival proteins, allowing them to bind and activate Bax and Bak. The activator BH3-only proteins were widely assumed to target a hydrophobic surface groove on Bax and Bak resembling that on the pro-survival proteins, but they may instead (or in addition) bind to a proposed distal site involving Bax α-helices 1 and 6 (Gavathiotis et al, 2008, 2010; Kim et al, 2009). The binding of certain BH3 domains to Bax or Bak has been described as ‘hit and run’ (transient and low affinity) and this so-called ‘rear site’ is not yet well defined. A very recent study provides strong support for direct activation of Bak by Bim, tBid and, surprisingly, Noxa, but they were clearly shown to bind to the ‘front site’ of Bak (Dai et al, 2011). Perhaps both sites can be used, one for promoting initial activation of Bax/Bak and the other for recruiting additional Bax (Bak) molecules (see below).
Figure 4.
Models for how the BH3-only proteins activate Bax and Bak. (A) In the direct activation model, the activator BH3-only proteins (Bim, tBid and probably Puma), via their BH3 domain (red triangle), can directly engage and activate Bax (or Bak), but in healthy cells the pro-survival family members (‘Bcl-2 et al’) prevent this by sequestering the BH3-only activators. After an apoptotic signal, the sensitiser BH3-only proteins (e.g. Bad, Noxa, Bik) free the activators to target Bax or Bak. Inactive cytosolic Bax has its BH3 domain buried and its C-terminal hydrophobic helix (α9) lies in its surface groove, but during activation that helix is freed and can target Bax to the membrane. (B) In the indirect activation model, the BH3-only proteins need only target their pro-survival relatives, which primarily prevent activation of Bax and Bak by sequestering any Bax or Bak that becomes active (‘primed’) by exposure of its BH3 domain (red triangle). (C) The priming–capture–displacement model proposed here incorporates features of both direct and indirect activation. In it, any Bax or Bak that becomes primed, either spontaneously or by BH3-only proteins or other potential signals (e.g. phosphorylation), is immediately captured by a pro-survival relative, until the primed Bax or Bak is displaced by further activation of BH3-only proteins (e.g. Bad or Bim). The displaced Bax or Bak can then form dimers and higher oligomers (see Figure 5).
The indirect activation model (Willis et al, 2007) (Figure 4B) posits that, probably even in healthy cells, some Bax and Bak molecules (perhaps spontaneously) assume a ‘primed conformation’, that is, one in which their BH3 domain is exposed, and that pro-survival family members prevent apoptosis by binding to this domain, thereby preventing Bax and Bak from oligomerising (see further below). In this model, the primary role of all BH3-only proteins is to bind to the pro-survival proteins, and apoptosis can only proceed when all the relevant pro-survival proteins have been neutralised, thereby liberating the ‘primed’ Bax or Bak to oligomerise and cause MOMP. Consistent with this model, Bax molecules with BH3 mutations that prevent their sequestration by pro-survival relatives provoke unrestrained apoptosis (Fletcher et al, 2008; Czabotar et al, 2011).
The direct and indirect models were recently interrogated in vivo using gene-targeted mice in which the Bim BH3 domain (binding all pro-survival Bcl-2 proteins and possibly Bax) was replaced in situ with that of Bad, Noxa or Puma, creating alleles encoding, respectively, BimBad (binding only Bcl-2, Bcl-xL, Bcl-w), BimNoxa (binding only Mcl-1, A1) and BimPuma (binding all pro-survival Bcl-2-like proteins but not Bax). The results showed that, for optimal cell death, Bim must be able to bind all anti-apoptotic Bcl-2 family members, as in the indirect model, but that its interaction with Bax may contribute to cell killing, suggesting that physiological cell death may follow both models (Merino et al, 2009). The two models could represent alternative paths to cell death or different stages of a single pathway. For example, we propose in the priming–capture–displacement model (Figure 4C) that direct activation by BH3-only proteins is at least one of the ways that ‘primed’ Bax and Bak are generated, but that pro-survival relatives immediately capture this ‘primed form’ of Bax and Bak, until BH3-only proteins displace it, as in the indirect activation model.
Interestingly, the pro-survival family members may regulate Bax and Bak in multiple ways. According to a recent report, Bax in healthy cells spontaneously translocates from the cytosol to associate peripherally with mitochondria, but Bcl-xL then binds and ‘retrotranslocates’ Bax back into the cytosol, whereupon this heterodimer disassociates (Edlich et al, 2011). Also, the ‘embedded together model’ posits that Bax and Bcl-2 interact only after both have rearranged to bury their α5 and α6 helices as well as their terminal α9 helix within the MOM (Leber et al, 2007) (see below). Furthermore, in elegant simplified systems using recombinant proteins with liposomes or isolated mitochondria, tBid rapidly bound to the membrane and recruited Bax, but its recruitment was opposed by Bcl-xL, which could sequester both tBid and Bax, until addition of Bad displaced Bax from Bcl-xL (Lovell et al, 2008).
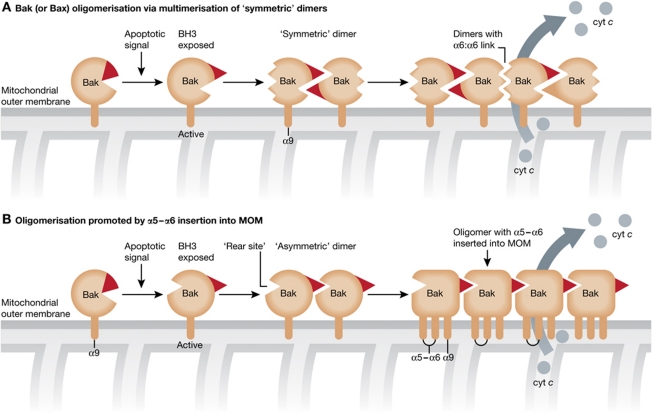
One of the great mysteries of cell death is how Bax and Bak homo-oligomerise and disrupt the MOM. Although no 3D structure of any active form of Bax or Bak is yet available, some veils have been lifted. Antibody and biochemical probes indicate that the globular structures of inactive Bax (Suzuki et al, 2000) and Bak (Moldoveanu et al, 2006) are substantially altered during their activation by several rearrangements (Kim et al, 2009; Gavathiotis et al, 2010). Notably, in an early step, Bak exposes its BH3 domain, which can then intercalate into the partial surface groove of another equivalently activated Bak molecule, forming a dimer that appears to be symmetric (Dewson et al, 2008) (Figure 5A). These novel dimers can then multimerise into larger oligomers through a separate interface involving the Bak α6 helix (Dewson et al, 2009). Bax oligomerisation is thought to proceed similarly (Westphal et al, 2011). Nevertheless, the proposed ‘rear’ site on Bax raises the possibility that Bax could instead insert an exposed BH3 domain into that site on another Bax molecule, forming ‘asymmetric’ dimers that would produce a ‘daisy chain’ of monomers (Figure 5B). This is consistent with some evidence (Shamas-Din et al, 2011) but not another study (Dewson et al, 2009). In any case, a key step in Bax and Bak oligomerisation and membrane permeabilisation may well be insertion of their α5 and α6 helices as a hairpin across the bi-layer of the MOM (Leber et al, 2007) (Figure 5B).
Figure 5.
Models for the oligomerisation of Bax and Bak. (A) In this dimer multimerisation model, upon activation by an apoptotic signal, Bak (or Bax) first extrudes its BH3 domain, which allows it to engage another ‘primed’ Bak (or Bax) molecule to form a ‘symmetric’ (face-to-face) dimer (Dewson et al, 2008). These dimers then multimerise by a different interface, such as one between α6 helices, to form the large oligomers that provoke MOMP, allowing cytochrome c (cyt c) to escape to the cytosol (Dewson et al, 2009). (B) In an alternative model (Shamas-Din et al, 2011), prompted by the proposal that certain BH3 domains can engage a novel ‘rear’ site on Bax, ‘primed’ Bak (or Bax) can engage the proposed rear site of another activated molecule to form an ‘asymmetric (face-to-back) dimer’, which could be extended by monomer recruitment. In this model, insertion of the α5–α6 hairpin as well as α9 is depicted. That insertion might also feature in the model shown in (A).
Whether Bax and Bak produce homo-oligomers of a defined size is unclear, but proteinaceous ‘pores’ large enough to allow egress of all the intermembrane proteins probably would require 8–12 mers (Green and Kroemer, 2004). However, the oligomers may instead simply create lipidic pores of undefined size by disrupting the lipid bi-layer (Green and Kroemer, 2004). To clarify these pivotal steps, there is a pressing need for the determination of 3D structures of activated forms of Bax and Bak and of their full-length heterodimers with pro-survival relatives, preferably anchored within the membrane.
An evolutionary conundrum
Given the central role of Bax/Bak-driven MOMP in vertebrate apoptosis, it is paradoxical that mitochondrial disruption plays little if any role in worms and flies. C. elegans lacks any pro-apoptotic Bax/Bak equivalent, and the worm APAF-1 homologue CED-4 does not require cytochrome c to activate the caspase CED-3 (Hengartner, 2000) (Figure 1). Instead, CED-9 directly sequesters CED-4, until EGL-1 binds to CED-9, liberating CED-4 to activate CED-3 (Hengartner, 2000). Furthermore, whereas Bcl-2 homologues have the crucial cell survival role in the worm and vertebrates, that role is dominated in Drosophila by the direct control of caspases by IAPs rather than by the two fly Bcl-2-related proteins, and mitochondrial disruption is not implicated (Dorstyn et al, 2002). Why apoptosis is tightly coupled to Bcl-2-regulated MOMP in vertebrates but not in the best-studied invertebrates remains unclear.
Physiological functions of Bcl-2 family members
Pro-survival proteins
Studies using transgenic and gene-knockout mice have greatly illuminated the biological functions of Bcl-2 family members. Demonstrating the role of the guardians, overexpression in lymphoid and other haemopoietic cells of Bcl-2 (McDonnell et al, 1989; Strasser et al, 1990b, 1991a, 1991b; Sentman et al, 1991), Bcl-xL (Grillot et al, 1995), Mcl-1 (Zhou et al, 1998; Campbell et al, 2010) or A1 (Chuang et al, 2002) protects against diverse cytotoxic signals, both physiological (e.g. cytokine deprivation) or imposed (e.g. γ-irradiation, chemotherapeutic drugs). Notably, overexpression of pro-survival proteins in lymphocytes leads to lymphadenopathy, which, in the case of Bcl-2 and Mcl-1, can progress at low (but significant) incidence to malignant lymphoma (McDonnell and Korsmeyer, 1991; Strasser et al, 1993, #1475; Zhou et al, 2001, #9379; Egle et al, 2004b; Campbell et al, 2010). Bcl-2 (but not Mcl-1) overexpression can also provoke a fatal kidney disease akin to human systemic lupus erythematosus (SLE) (Strasser et al, 1991b; Egle et al, 2004a; Campbell et al, 2010), establishing that apoptotic defects can promote autoimmune disease.
Different cell types vary in their dependence on individual pro-survival proteins, presumably due to variable expression patterns and selectivity of interactions (Figure 3B and C). For example, bcl-2−/− mice developed a fatal polycystic kidney disease (PKD), due to the death of renal epithelial stem/progenitor cells in the embryonic kidney; premature greying, due to death of melanocyte progenitors; and immunodeficiency, due to B- and T-cell attrition (Veis et al, 1993). Remarkably, all these degenerative disorders were eliminated by concomitant loss of Bim (in the case of PKD, even loss of a single bim allele) (Bouillet et al, 2001), demonstrating that Bim and Bcl-2 are the major mutually antagonistic regulators of the life/death switch in these cell types.
The cell types most affected by bcl-x loss include fetal erythroid progenitors, certain neuronal populations, male germ cells, immature (CD4+CD8+) thymocytes, hepatocytes and platelets (Motoyama et al, 1995; Kasai et al, 2003; Mason et al, 2007). The bcl-x−/− mice die around E14–15 due to severe anaemia and neuronal degeneration (Motoyama et al, 1995) and bcl-x+/−males have profoundly reduced fertility (Kasai et al, 2003). Interestingly, Bim loss restored fertility in bcl-x+/− males and prevented the erythroid attrition caused by complete bcl-x loss but did not rescue the neurons, so bim−/−bcl-x−/− embryos still die around E15 (Akhtar et al, 2008). Thus, the Bim–Bcl-xL interaction determines the fate of erythroid progenitors and male germ cells, but Bim is not the sole BH3-only protein regulating neuronal survival.
Loss of Mcl-1 has profound effects. Its complete loss caused pre-implantation embryonic lethality (Rinkenberger et al, 2000), and conditional deletion studies have shown that Mcl-1 is important for the survival of haemopoietic stem cells (Opferman et al, 2005), immature B and T lymphoid progenitors and their mature resting progeny (Opferman et al, 2003), activated germinal centre B cells (Vikstrom et al, 2010), granulocytes and activated macrophages (Steimer et al, 2009), certain neuronal populations in the embryonic brain (Arbour et al, 2008) and hepatocytes (Vick et al, 2009).
Bcl-w and A1 may have more restricted roles. Although Bcl-w is broadly expressed, its loss provoked noticeable defects only in adult spermatogenesis (Print et al, 1998; Ross et al, 1998) and in epithelial cells in the small intestine, which became more sensitive to DNA damage (Pritchard et al, 2000). The mouse has at least three expressed a1 genes and loss of one of them (a1a) accelerated the apoptosis of granulocytes (Hamasaki et al, 1998) and mast cells (Xiang et al, 2001). Targeting all the A1 genes might well reveal additional defects, since diverse stimuli induce A1 expression in many cell types.
Collectively, these results support our belief that the survival of most if not all cells in vertebrates relies upon one or more of the Bcl-2-like guardians. This is consistent with the view that death is the default for most cells unless they receive positive signals from other cells (Raff, 1992). Most likely, paracrine signals conveyed by growth factors and cell–cell or cell–matrix contact promote the survival of most cell types by up-regulating various anti-apoptotic Bcl-2 family members, as well as by curtailing expression and/or activity of particular BH3-only proteins (Youle and Strasser, 2008).
Bax/Bak
Studies with mice lacking either Bax or Bak or both have shown that their functions largely overlap. No abnormalities are discernible in bak−/− mice (Lindsten et al, 2000), except a modest rise in platelets (Mason et al, 2007), and bax−/− mice exhibit only mild lymphoid hyperplasia and male sterility, the latter because proper spermatogenesis requires that excess testicular germ cells die to achieve the proper ratio of germ to support cells (Lindsten et al, 2000). In striking contrast, most mice lacking both Bax and Bak die perinatally. Their developmental abnormalities include persisting interdigital webs and increased numbers of certain neuronal populations in the brain. A very small number of bax−/−bak−/− mice remain viable for several weeks and develop massive lymphadenopathy (Lindsten et al, 2000; Rathmell et al, 2002). Perhaps surprisingly, tissues widely thought to be shaped by apoptosis appear histologically normal in these animals, suggesting that their superfluous cells may be eliminated by Bok (Figure 3) or by non-apoptotic death processes. Cells from bax−/−bak−/− mice are highly resistant to the cytotoxic stimuli that activate the mitochondrial pathway, but, as expected, their lymphocytes (type 1 cells) remain sensitive to death receptor-induced killing (Lindsten et al, 2000; Rathmell et al, 2002).
BH3-only proteins
Death stimuli can induce multiple BH3-only proteins, which can also vary with cell type (Huang and Strasser, 2000; Puthalakath and Strasser, 2002; Youle and Strasser, 2008). Nonetheless, analysis of gene-targeted mice has revealed that individual BH3-only proteins have distinctive physiological roles. For example, Bim (O’Connor et al, 1998), one of the most prominent players, is essential in many cell types for apoptosis induced by growth factor deprivation (Bouillet et al, 1999) and for the deletion of autoreactive thymocytes (Bouillet et al, 2002) and immature B cells (Enders et al, 2003). Consequently, bim−/− mice develop progressive lymphadenopathy and, on a mixed C57BL/6x129SV background, a fatal SLE-like autoimmune disease (Bouillet et al, 1999). Bim also drives most of the cell death induced by endoplasmic reticulum (ER) stress (Puthalakath et al, 2007) but has less importance in apoptosis induced by DNA damage (Bouillet et al, 1999), which is largely p53 driven.
The puma and noxa genes are both direct transcriptional targets of the tumour suppressor p53 (Vousden and Lane, 2007). Significantly, lymphocytes and certain other cell types from puma−/− mice are highly resistant to γ-irradiation and DNA damage-inducing chemotherapeutic drugs (e.g. etoposide) (Jeffers et al, 2003; Villunger et al, 2003), demonstrating that Puma is the major mediator of the p53 apoptotic response in those cells. Puma also drives the apoptosis induced by several p53-independent insults, such as growth factor deprivation or treatment with glucocorticoids or phorbol ester (Jeffers et al, 2003; Villunger et al, 2003; Erlacher et al, 2005). Curiously, while Noxa loss only modestly impaired γ-radiation- or etoposide-induced apoptosis of lymphocytes and fibroblasts (Shibue et al, 2003; Villunger et al, 2003), its loss had more impact than Puma loss on UV-radiation-induced apoptosis of fibroblasts and keratinocytes (Naik et al, 2007).
Importantly, mice lacking both Puma and Noxa showed that they account for all of the pro-apoptotic activity induced by p53 in lymphocytes with damaged DNA (Michalak et al, 2008), challenging the physiological significance in that response of ‘mitochondrial p53’ (reviewed in Vousden and Lane, 2007). Indeed, mutations that ablate p53's transcriptional activation function in vivo eliminated all its biological functions, including tumour suppression (Brady et al, 2011). Puma and Bim appear to be the most potent BH3-only proteins in many contexts, and their combined loss renders many cell types more refractory to cell death stimuli than loss of either alone (Erlacher et al, 2006).
Activation of Bid requires its processing by caspase-8 or certain other proteases (e.g. caspase-3, granzyme B) (Li et al, 1998; Luo et al, 1998) (Figure 2). Accordingly, Bid is essential for the death induced by FasL or TNF in certain cell types (type 2 cells), including hepatocytes and pancreatic β cells (Yin et al, 1999; Kaufmann et al, 2007, 2009; Jost et al, 2009), but claims that Bid is needed for the responses to DNA damage, replicative stress or cell-cycle arrest have been challenged (Kaufmann et al, 2007).
Although studies in cell lines implicated Bmf in anoikis (apoptosis on detachment of adherent cells) (Puthalakath et al, 2001), endothelial cells and fibroblasts from bmf−/− mice proved normally sensitive to this stress (Labi et al, 2008), possibly because other BH3-only proteins, notably Bim (Mailleux et al, 2007), cooperate with Bmf in anoikis. Bmf-deficient lymphocytes are, however, refractory to glucocorticoids, and older bmf−/− mice accumulate excess B cells (Labi et al, 2008), demonstrating a role for Bmf in B-cell homeostasis.
The other BH3-only proteins seem to have more limited roles. No abnormalities appeared in bik−/− mice or cells (Coultas et al, 2004), but males lacking both Bim and Bik were infertile due to impaired apoptosis of immature testicular progenitor cells (Coultas et al, 2005), as in bax−/− males (see above). Although Bad reportedly is regulated by growth factor signalling, Bad-deficient cells remained sensitive to cytokine deprivation and diverse other cytotoxic insults (Kelly et al, 2010). The minor rise in platelets in bad−/− mice (Kelly et al, 2010) probably reflects reduced blockade of its target Bcl-xL, the major regulator of platelet survival (Mason et al, 2007). Hrk is expressed largely (perhaps exclusively) in the brain and only delayed death of certain hrk−/− neuronal cell types in culture has been reported (Imaizumi et al, 2004; Coultas et al, 2007).
Beclin-1: an apparent link from Bcl-2 to autophagy
The Bcl-2 family may control not only commitment to apoptosis but also the initiation of autophagy, an evolutionarily conserved process for maintaining cell survival (e.g. when nutrients are limiting) by self-cannibalism of cellular components, including organelles (Kang et al, 2011). Beclin-1, which is essential for initiation of autophagy, was reported to bind to anti-apoptotic Bcl-2 family members (Pattingre et al, 2005) via a BH3-like domain (Maiuri et al, 2007; Oberstein et al, 2007). The association of Beclin-1 with Bcl-2, Bcl-xL or Mcl-1 reportedly blocks its ability to activate the PI3 kinase Vps34 and trigger autophagy (Pattingre et al, 2005; Maiuri et al, 2007). Accordingly, a BH3-only protein of higher affinity (as most are), or a BH3 mimetic compound such as ABT-737 (see below), can displace Beclin-1 and allow autophagy. Beclin-1 can also be freed by several other mechanisms (Kang et al, 2011), including mono-ubiquitination or phosphorylation of its BH3 domain. Curiously, only Bcl-2 on the ER and not that on mitochondria seemed to block autophagy induction (Pattingre et al, 2005). These findings may have implications for tumourigenesis and therapy (see below).
The roles of the Bcl-2 family in tumourigenesis
The cancer connection was first made when overexpression of bcl-2, then newly discovered as the gene involved in the t[14;18] chromosomal translocation found in most human follicular lymphomas (Tsujimoto et al, 1984), was shown to prevent the death of haemopoietic cells deprived of cytokine in vitro and to cooperate with myc in immortalisation of lymphoid cells (Vaux et al, 1988) and in lymphomagenesis (Strasser et al, 1990a). This was the first identification of a gene that regulates cell survival (in any species) and the first evidence that evasion of apoptosis contributes to neoplastic transformation.
Bcl-2 transgenes linked to an Igh gene enhancer, mimicking the human t[14;18] translocation, produced plasma cell tumours and lymphocytic leukaemias but not, disappointingly, follicular lymphoma (McDonnell and Korsmeyer, 1991; Strasser et al, 1993). More recently, however, a bcl-2 transgene expressed widely in the haemopoietic compartment did provoke follicular lymphoma, preceded by a florid germinal centre hyperplasia dependent on CD4+ T cells, which also accumulated due to the high Bcl-2 levels (Egle et al, 2004a). These observations indicate that the bcl-2 translocation by itself probably is insufficient to cause human follicular lymphoma and that chronic T-cell stimulation may contribute. Further, the long latency of tumour development (McDonnell and Korsmeyer, 1991; Strasser et al, 1993) pointed to a need for additional oncogenic mutations.
As indicated above, myc and bcl-2 exhibit striking synergy in malignant transformation (Vaux et al, 1988; Strasser et al, 1990a). Pertinently, the tumours that develop spontaneously in bcl-2 transgenic mice often exhibit myc gene rearrangements (McDonnell and Korsmeyer, 1991; Strasser et al, 1993), and some highly aggressive human lymphomas harbour both a myc (t8;14) and a bcl-2 (t14;18) translocation (Lee et al, 1989). The basis for this synergy is that Myc overexpression causes not only increased cell proliferation, but under conditions of stress (e.g. growth factor limitation) can also promote apoptosis (Evan et al, 1992). By blocking this apoptosis, Bcl-2 allows the increased expansion of cycling cells at risk of acquiring further mutations.
Mcl-1 and Bcl-xL are also important players in tumourigenesis. Mcl-1 overexpression predisposes mice to diverse B-cell lymphomas (Zhou et al, 2001) and, notably, haemopoietic stem/progenitor cell tumours (Campbell et al, 2010). Also, Bcl-xL (Cheung et al, 2004; Swanson et al, 2004; Boylan et al, 2007) and Mcl-1 (Campbell et al, 2010) synergise with Myc in lymphomagenesis and plasmacytomagenesis, and all the pro-survival family members accelerate Myc-induced myeloid leukaemia (Beverly and Varmus, 2009). In humans, Mcl-1 expression is strikingly high in many cases of acute myeloid leukaemia (AML) and multiple myeloma, and diverse cancers exhibit amplified Mcl-1 or Bcl-x genes (Beroukhim et al, 2010).
Just as anti-apoptotic Bcl-2 proteins promote tumourigenesis, pro-apoptotic family members can function as tumour suppressors. Thus, Myc-induced lymphomagenesis was accelerated by loss of Bax (Eischen et al, 2001), Bim (Egle et al, 2004b), Puma (Garrison et al, 2008; Michalak et al, 2009) and Bmf or Bad (Frenzel et al, 2010), because nearly all contribute to Myc-induced apoptosis. Myc activates Puma through Arf-dependent up-regulation of p53 (reviewed in Vousden and Lane, 2007), but how Myc up-regulates the other BH3-only proteins is not yet known. Bax loss also accelerated brain tumour development in a transgenic model (Yin et al, 1997), and γ-radiation-induced thymic lymphomagenesis was enhanced by loss of Noxa (Michalak et al, 2010), Bmf (Labi et al, 2008) or both Bim and Bad (Kelly et al, 2010). Mice lacking both Bim and Puma have enhanced lymphocyte accumulation and some spontaneously develop lymphoma (Erlacher et al, 2006). However, mice lacking even both of p53's major apoptotic targets, puma and noxa, are not tumour prone (Michalak et al, 2009), probably because p53 transcriptional targets mediating additional functions (e.g. growth arrest and senescence) also contribute to its tumour suppressor activity (Brady et al, 2011).
Importantly, loss or suppression of pro-apoptotic family members is also found in human cancer. Bax frameshift mutations appear in ∼50% of colon carcinomas in patients with a DNA mismatch repair defect, due to slippage during replication of an eight G run in the human Bax gene (Rampino et al, 1997). Moreover, 17% of mantle cell lymphomas have homozygous bim deletions (Tagawa et al, 2005), and the bok and puma genes have suffered allelic deletions in diverse cancers (Beroukhim et al, 2010). In many Burkitt lymphomas, bim or puma is epigenetically silenced, often by hypermethylation (Garrison et al, 2008; Richter-Larrea et al, 2010). Notably, the bim hypermethylation correlated with lower remission and shorter survival, and histone-deacetylase inhibitors overcame the chemoresistance (Richter-Larrea et al, 2010).
Defects in Fas death receptor signalling are also tumourigenic. Mutations in Fas or Fas ligand in mice or in patients with autoimmune lymphoproliferative syndrome not only cause progressive lymphadenopathy and systemic autoimmune disease but the longer-term survivors are also predisposed to lymphoma, plasma cell tumours and histiocytic sarcoma (Watanabe-Fukunaga et al, 1992; Davidson et al, 1998; Straus et al, 2001; O’Reilly et al, 2009). Some human non-lymphoid malignancies also display Fas mutations, including 28% of bladder cancers (Lee et al, 1999).
Such findings have led to the now widespread view that evasion of apoptosis is a hallmark of cancer (Hanahan and Weinberg, 2000). However, since a relatively small proportion of human malignancies carry abnormalities that directly affect genes of the Bcl-2 or death receptor families, the survival of cells undergoing neoplastic transformation most likely often relies upon upstream oncogenic pathways that alter expression of Bcl-2 family members. For example, gene expression databases show high Bcl-2 levels in most human lymphoid malignancies, including not only follicular lymphoma (due to the t[14;18] translocation), but also B-chronic lymphocytic leukaemia (CLL) and multiple myeloma. Notably, over half of B-CLL cases exhibit deletions or mutations in micro-RNAs (miR-15a, miR-16-1) that down-regulate Bcl-2 mRNA (Iorio and Croce, 2009). Also, the deregulated NF-κB activity in many lymphomas (Lenz and Staudt, 2010) can up-regulate A1 (Grumont et al, 1999) and Bcl-xL (Chen et al, 2000). Since loss of even a single bim allele substantially accelerates Myc-induced lymphomagenesis (Egle et al, 2004b), it will be important to examine human malignancies for changes to regulators that reduce its expression even two-fold.
Upstream mutations can also affect the stability and therefore the activity of Bcl-2 family members. In particular, Mcl-1 is very labile, but its level is elevated in diverse tumours by loss of the FBW7 tumour suppressor, the substrate-binding component of an E3 ubiquitin ligase that can promote Mcl-1 degradation (Wertz et al, 2011). Also, in many B lymphoid tumours, Mcl-1 is elevated by increased expression of the de-ubiquitinase USP9X, which spares Mcl-1 from degradation (Schwickart et al, 2010).
Perhaps surprisingly, although Bcl-2 overexpression enhances Myc-induced lymphomagenesis (Strasser et al, 1990a) and is required for sustained growth of such lymphomas (Letai et al, 2004), loss of endogenous Bcl-2 does not impair Myc-induced lymphomagenesis (Kelly et al, 2007). Presumably, other pro-survival relatives sustain the pre-leukaemic cells while they acquire the mutations allowing transformation and progression. Pertinently, loss of even a single mcl-1 allele protected mice from developing Myc-induced AML (Xiang et al, 2010). Identifying the pro-survival family members critical for development and sustained growth of distinct tumour types should provide valuable clues for early intervention and improved treatment of malignant disease (see below).
Although evasion of cell death contributes importantly to cancer development, in certain circumstances excessive cell death actually promotes tumourigenesis. Repeated low-dose γ-irradiation in certain mouse strains causes thymic lymphoma, which arises from bone marrow-derived haemopoietic stem cells that have sustained oncogenic mutations. Remarkably, protection of the differentiated leukocytes from the γ-radiation-induced apoptosis, either by loss of Puma or by Bcl-2 overexpression, ablated lymphomagenesis by obviating the compensatory proliferation of mutated haemopoietic stem/progenitor cells (Labi et al, 2010; Michalak et al, 2010). Potential parallels in human malignancies include hepatocellular carcinoma in patients with viral- or alcoholism-induced cirrhosis, which develops from stem or progenitor cells repeatedly driven into action through cycles of cell death and regeneration (Farazi and DePinho, 2006). Pertinently, liver-specific loss of Mcl-1 in mice provoked sustained hepatic apoptosis, regeneration and ultimately carcinoma (Vick et al, 2009). Furthermore, many cancer survivors eventually develop new primary tumours provoked by their courses of radiotherapy or chemotherapy, which elicit cycles of depletion of differentiated cells replenished by recruitment of stem/progenitor cells, some of which will have sustained oncogenic mutations (Allan and Travis, 2005).
The crucial roles of the Bcl-2 family in cancer therapy
It has long been recognised that most types of tumour cells exposed to cytotoxic cancer therapies exhibit hallmarks of apoptosis and that this process could be critical for the therapeutic response (Kerr et al, 1972). Indeed, overexpression of Bcl-2, Bcl-xL or Mcl-1 in mice protects lymphoid and myeloid cells against many therapeutic agents (e.g. γ-irradiation, glucocorticoids, etoposide) (Strasser et al, 1991a; Grillot et al, 1995; Zhou et al, 1997; Campbell et al, 2010). Verifying the essential role of the Bcl-2-regulated pathway, combined loss of Bax and Bak renders diverse cell types highly resistant to such cytotoxic insults (Lindsten et al, 2000; Rathmell et al, 2002), whereas crippling the death receptor pathway had no impact (Varfolomeev et al, 1998; Newton and Strasser, 2000; Salmena et al, 2003). Pertinently, in diverse human cancer types, elevated Bcl-2, Bcl-xL, Mcl-1 or A1/Bfl1 correlates strongly with chemoresistance (reviewed in Cory and Adams, 2002; Youle and Strasser, 2008).
Gene-targeted mice have revealed that distinct BH3-only proteins are required to initiate apoptosis in response to different anti-cancer therapeutics (reviewed in Adams and Cory, 2007; Youle and Strasser, 2008). For DNA-damaging cancer therapeutics, the crucial role of p53 (Vousden and Lane, 2007) implicated its direct transcriptional targets puma and noxa, and indeed Puma and to a lesser extent Noxa proved critical in many cell types (Jeffers et al, 2003; Shibue et al, 2003; Villunger et al, 2003; Erlacher et al, 2005; Michalak et al, 2008). Apoptosis elicited by glucocorticoids, which are used to treat certain lymphoid malignancies, requires Bim, Puma and Bmf (Bouillet et al, 1999; Jeffers et al, 2003; Villunger et al, 2003; Erlacher et al, 2005; Labi et al, 2008), and HDAC inhibitors kill in a Bim- and Bmf-dependent manner (Labi et al, 2008). Taxol-induced cell death also relies heavily on Bim (Bouillet et al, 1999).
These findings for non-transformed cells have generally held for tumour-derived cell lines (Michalak et al, 2009; Happo et al, 2010), although, surprisingly, efficient killing of Myc-driven B lymphoid tumours was found to depend not only on Puma and Noxa but also on Bim (Happo et al, 2010). Since DNA damage of transformed cells can engage the Bcl-2-regulated apoptotic pathway through both p53-dependent and p53-independent routes (Strasser et al, 1994), Bim might be involved in the latter, as p53 does not appear to regulate it directly (Egle et al, 2004b; Kelly et al, 2010).
The discovery that certain oncogenic proteins are critical for sustained growth of a tumour (‘oncogene addiction’) has prompted development of designer drugs that target these key factors, which include several oncogenic kinases (Sharma et al, 2006). Like traditional chemotherapeutics, many designer drugs act at least in part by inducing apoptosis. Thus, the death of chronic myelogenous leukaemia (CML)-derived cell lines treated with imatinib (Gleevec), which inhibits Bcr–Abl, the kinase that causes this disease, proved to require Bim and, to a lesser extent, Bad (Kuroda et al, 2006). Bcr–Abl shutdown may activate Bim both by impairing PI3K-Akt signalling, thereby allowing FOXO3A to induce bim, and by preventing ERK-mediated phosphorylation of Bim, which can target it for ubiquitination and proteasomal degradation (reviewed in Ley et al, 2005). Additional pre-clinical studies have shown that Bim is also critical for the killing of tumour-derived cell lines by drugs that target other oncogenic kinases: EGF receptors in non-small cell lung cancer (Costa et al, 2007; Cragg et al, 2007; Gong et al, 2007), mutant B-Raf in melanoma and colon cancer (Cragg et al, 2008) and JAK2 in myeloproliferative neoplasms (Kobayashi et al, 2010). Thus, although non-apoptotic processes (e.g. senescence) may well contribute to the efficacy of these cancer therapies, activation of apoptosis via BH3-only proteins plays a crucial role (Cragg et al, 2009).
Novel anti-cancer therapeutics that directly trigger the apoptotic machinery
BH3 mimetics
The efficacy of most chemotherapeutic drugs and γ-radiation is blunted by anti-apoptotic abnormalities in the tumour cells, for example, mutant p53 or elevated Bcl-2, Bcl-xL or Mcl-1. Hence, chemical mimetics of BH3-only proteins that can bypass these defects and directly flip the Bcl-2-regulated apoptotic switch are now avidly sought (reviewed in Lessene et al, 2008). Although a number of compounds have been proposed to function as BH3 mimetics, only ABT-737 (Oltersdorf et al, 2005) and its orally available derivative ABT-263 (navitoclax) (Tse et al, 2008), which is now undergoing clinical trials, have clearly been shown to kill cells through a Bax/Bak-dependent mechanism. The others kill bax−/−bak−/− cells as well as wt cells, demonstrating off-target toxicity (van Delft et al, 2006; Lessene et al, 2008).
ABT-737 and ABT-263 both bind with high (low nanomolar) affinity to Bcl-2, Bcl-xL and Bcl-w but not to Mcl-1 or A1 (Oltersdorf et al, 2005; Tse et al, 2008). Accordingly, these drugs show significant single-agent efficacy in culture and in xeno-transplant mouse models against human tumour cells that express high levels of Bcl-2 (or Bcl-xL) and low levels of Mcl-1 (Oltersdorf et al, 2005; van Delft et al, 2006), particularly lymphoid malignancies and small cell lung carcinoma (Oltersdorf et al, 2005). ABT-737 also cooperates potently with many standard chemotherapeutic drugs, such as etoposide or cyclophosphamide, and their combined action can even kill refractory tumour cells with high levels of Mcl-1 (Mason et al, 2008), often because those agents promote Mcl-1 degradation (van Delft et al, 2006) or activate BH3-only proteins that can neutralise it (Cragg et al, 2008). For example, agents that provoke mitotic arrest, such as docetaxel, lead to Mcl-1 degradation (Wertz et al, 2011). Pertinently, xenografts of human breast tumours that exhibited elevated Bcl-2 were sensitised by ABT-737 to docetaxel treatment (Oakes et al, 2011), as were lung carcinoma xenografts with elevated Bcl-xL by navitoclax (Tan et al, 2011). Remarkably, hypoxic regions of tumours, often the most refractory to standard therapy, seem particularly vulnerable to ABT-737, probably because their Mcl-1 levels are lower (Harrison et al, 2011). Importantly, ABT-737 spares most normal cells but preferentially kills older platelets, which depend upon Bcl-xL (Mason et al, 2007). Hence, transient thrombocytopenia has been the dose-limiting but manageable toxicity in clinical trials of ABT-263. Some normal lymphoid and myeloid populations, such as mast cells, are also relatively sensitive (Carrington et al, 2010).
Of note, ABT-737 synergises potently with drugs designed to target specific oncoproteins, including inhibitors of oncogenic kinases. Examples include synergy with the bcr-abl inhibitor imatinib in killing CML-derived cell lines (Kuroda et al, 2006) and inhibitors of mutant B-Raf signalling in cell lines derived from melanoma or colon carcinoma (Cragg et al, 2008). Thus, combining a BH3 mimetic with an inhibitor of an oncoprotein that targets only the cancer cells should be highly effective at eliminating the tumour cells while limiting collateral damage to healthy tissues (Cragg et al, 2009). Recent work indicates that anti-VEGF therapy of solid tumours destroys the tumour vasculature and abates tumour growth by up-regulating Bim in the endothelial cells (Naik et al, 2011). Thus, some BH3 mimetics may prove to be efficacious in combination therapy by killing not only the tumour cells but also their support cells.
Since BH3 mimetics may promote autophagy by freeing Beclin-1 (see above), this cell survival mechanism might limit the efficacy of BH3 mimetic therapy in some circumstances. Treatment might be improved in such cases by including well-tolerated inhibitors of autophagy, such as chloroquine, which can enhance therapy of pancreatic cancer (Yang et al, 2011).
In principle, a BH3 mimetic drug that attacks a larger subset of pro-survival proteins than ABT-737 (e.g. including Mcl-1) would be more efficacious against cancer cells. However, such agents might well damage too many normal tissues, and BH3 mimetics specific for single pro-survival targets could well have greater clinical utility. Pertinently, GDC-0199, a novel BH3 mimetic from Abbott and Genentech specific for Bcl-2, which is now entering clinical trials for lymphoid malignancies, should avoid the dose-limiting thrombocytopenia that navitoclax provokes by its inhibition of Bcl-xL.
Once the molecular mechanisms for activation of Bax and Bak are better understood (see above), it may also be possible to design compounds that directly trigger this critical step in apoptosis signalling to enhance the killing of cancer cells or unwanted cells in other diseases, such as autoreactive lymphocytes in autoimmunity. However, whether a therapeutic window exists for such agents remains to be determined.
Death receptor agonists
Engaging the death receptor pathway also has considerable therapeutic potential. Although prohibitive liver damage probably precludes activation of Fas (Ogasawara et al, 1993; Huang et al, 1999), in vitro and xeno-transplant studies suggest that stimulation of TRAIL receptor(s) is safe and efficacious with diverse tumour types, including glioblastoma and certain haemopoietic malignancies (Gonzalvez and Ashkenazi, 2010). Hence, TRAIL and agonistic antibodies against its receptors are in early clinical trials. Since killing through this pathway in most cell types relies upon amplification via the Bcl-2-regulated pathway (see above), combination therapy with BH3 mimetics should improve efficacy.
IAP antagonists
Finally, the elevated IAP levels found in many cancers have stimulated the development of small molecule inhibitors that mimic the action of the natural IAP antagonist SMAC/DIABLO (Du et al, 2000; Verhagen et al, 2000), and these ‘SMAC mimetics’ are now in clinical trial (Straub, 2011). Their key target was expected to be XIAP, because it potently inhibits the effector caspases and perhaps caspase-9. However, cIAP1 and cIAP2, components of the TNF-R1 signalling complex, have also proved to be important targets, because their engagement reduces pro-survival signalling from this receptor and, via NF-κB activation, provokes autocrine production of TNFα, which then elicits TNF-R1-mediated apoptosis (Gaither et al, 2007; Varfolomeev et al, 2007; Vince et al, 2007). Critical questions now are which tumour types are most susceptible to IAP inhibitors and how can they best be combined with other agents? There is evidence for their synergy with a death receptor agonist and cooperation with BH3 mimetics also seems highly likely (Straub, 2011).
Autoimmune, infectious and degenerative diseases
Since defects in apoptosis contribute to the development of autoimmune diseases (Strasser et al, 1991b; Watanabe-Fukunaga et al, 1992; Bouillet et al, 1999), directly targeting the apoptotic machinery could well aid their treatment. Indeed, ABT-737 has shown considerable promise in mouse models of SLE, rheumatoid arthritis and transplant rejection (Bardwell et al, 2009; Carrington et al, 2010). Furthermore, the distinctive Bcl-2 homologues found in many viruses (Cuconati and White, 2002) suggest that virus-specific BH3 mimetics could limit viral infections, including that of viruses such as Epstein–Barr virus that contribute to human cancer. Similarly, parasites, such as schistosomes, have Bcl-2 homologues that could be targeted as a new approach to parasite elimination (Lee et al, 2011). Finally, since excess cell death is the hallmark of degenerative disorders, such as Alzheimer's disease, a deeper understanding of the underlying cell death mechanisms should eventually suggest strategies to block their progression.
Concluding remarks
Over the past 25 years, work from hundreds of laboratories has laid a solid framework for understanding the pathways to cell death, their mechanisms and physiological impact, as well as insights into the types of diseases that can be caused or exacerbated by defects in these processes. Armed with this knowledge, the field is now poised to develop novel therapeutic strategies for cancer, autoimmune diseases and other disorders.
Acknowledgments
We thank all present and past colleagues, particularly Drs D Vaux, D Huang, P Bouillet, P Colman, A Harris, R Kluck, J Silke, H Puthalakath, A Villunger, L O’Reilly and C Scott for their outstanding contributions to apoptosis research. We dedicate this review to the memory of late Alan W Harris, who had a major influence on our research. Our research is supported by fellowships and grants from the Australian NHMRC (461221, 461299, 516703, 637326), NIH (CA43540), Leukemia and Lymphoma Society (SCOR 7413-07) and the JDRF/NHMRC.
Footnotes
The authors declare that their research at the Walter and Eliza Hall Institute includes a joint programme with Genentech Inc. and Abbott Labs to develop novel anti-cancer therapeutics.
References
- Adams JM, Cory S (2007) The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 26: 1324–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar RS, Klocke BJ, Strasser A, Roth KA (2008) Loss of BH3-only protein Bim inhibits apoptosis of hemopoietic cells in the fetal liver and male germ cells but not neuronal cells in bcl-x-deficient mice. J Histochem Cytochem 56: 921–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan JM, Travis LB (2005) Mechanisms of therapy-related carcinogenesis. Nat Rev Cancer 5: 943–955 [DOI] [PubMed] [Google Scholar]
- Arbour N, Vanderluit JL, Le Grand JN, Jahani-Asl A, Ruzhynsky VA, Cheung EC, Kelly MA, MacKenzie AE, Park DS, Opferman JT, Slack RS (2008) Mcl-1 is a key regulator of apoptosis during CNS development and after DNA damage. J Neurosci 28: 6068–6078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell PD, Gu J, McCarthy D, Wallace C, Bryant S, Goess C, Mathieu S, Grinnell C, Erickson J, Rosenberg SH, Schwartz AJ, Hugunin M, Tarcsa E, Elmore SW, McRae B, Murtaza A, Wang LC, Ghayur T (2009) The Bcl-2 family antagonist ABT-737 significantly inhibits multiple animal models of autoimmunity. J Immunol 182: 7482–7489 [DOI] [PubMed] [Google Scholar]
- Beroukhim R, Mermel C, Porter D, Wei G, Raychaudhuri S, Donovan J, Barretina J, Boehm J, Dobson J, Urashima M, Mc Henry K, Pinchback R, Ligon A, Cho Y-J, Haery L, Greulich H, Reich M, Winckler W, Lawrence M, Weir B et al. (2010) The landscape of somatic copy-number alteration across human cancers. Nature 463: 899–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverly LJ, Varmus HE (2009) MYC-induced myeloid leukemogenesis is accelerated by all six members of the antiapoptotic BCL family. Oncogene 28: 1274–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillet P, Cory S, Zhang L-C, Strasser A, Adams JM (2001) Degenerative disorders caused by Bcl-2 deficiency are prevented by loss of its BH3-only antagonist Bim. Dev Cell 1: 645–653 [DOI] [PubMed] [Google Scholar]
- Bouillet P, Metcalf D, Huang DCS, Tarlinton DM, Kay TWH, Köntgen F, Adams JM, Strasser A (1999) Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 286: 1735–1738 [DOI] [PubMed] [Google Scholar]
- Bouillet P, Purton JF, Godfrey DI, Zhang L-C, Coultas L, Puthalakath H, Pellegrini M, Cory S, Adams JM, Strasser A (2002) BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature 415: 922–926 [DOI] [PubMed] [Google Scholar]
- Boylan KL, Gosse MA, Staggs SE, Janz S, Grindle S, Kansas GS, Van Ness BG (2007) A transgenic mouse model of plasma cell malignancy shows phenotypic, cytogenetic, and gene expression heterogeneity similar to human multiple myeloma. Cancer Res 67: 4069–4078 [DOI] [PubMed] [Google Scholar]
- Brady CA, Jiang D, Mello SS, Johnson TM, Jarvis LA, Kozak MM, Broz DK, Basak S, Park EJ, McLaughlin ME, Karnezis AN, Attardi LD (2011) Distinct p53 transcriptional programs dictate acute DNA-damage responses and tumor suppression. Cell 145: 571–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KJ, Bath ML, Turner ML, Vandenberg CJ, Bouillet P, Metcalf D, Scott CL, Cory S (2010) Elevated Mcl-1 perturbs lymphopoiesis, promotes transformation of hematopoietic stem/progenitor cells, and enhances drug resistance. Blood 116: 3197–3207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington EM, Vikstrom IB, Light A, Sutherland RM, Londrigan SL, Mason KD, Huang DC, Lew AM, Tarlinton DM (2010) BH3 mimetics antagonizing restricted prosurvival Bcl-2 proteins represent another class of selective immune modulatory drugs. Proc Natl Acad Sci USA 107: 10967–10971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Edelstein LC, Gelinas C (2000) The Rel/NF-κB family directly activates expression of the apoptosis inhibitor Bcl-xL. Mol Cell Biol 20: 2687–2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DCS (2005) Differential targeting of pro-survival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell 17: 393–403 [DOI] [PubMed] [Google Scholar]
- Cheung WC, Kim JS, Linden M, Peng L, Van Ness B, Polakiewicz RD, Janz S (2004) Novel targeted deregulation of c-Myc cooperates with Bcl-X(L) to cause plasma cell neoplasms in mice. J Clin Invest 113: 1763–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk JE, Green DR (2008) How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol 18: 157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou JJ, Li H, Salvesen GS, Yuan J, Wagner G (1999) Solution structure of BID, an intracellular amplifier of apoptotic signaling. Cell 96: 615–624 [DOI] [PubMed] [Google Scholar]
- Chuang PI, Morefield S, Liu CY, Chen S, Harlan JM, Willerford DM (2002) Perturbation of B-cell development in mice overexpressing the Bcl-2 homolog A1. Blood 99: 3350–3359 [DOI] [PubMed] [Google Scholar]
- Cory S, Adams JM (2002) The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2: 647–656 [DOI] [PubMed] [Google Scholar]
- Costa DB, Halmos B, Kumar A, Schumer ST, Huberman MS, Boggon TJ, Tenen DG, Kobayashi S (2007) BIM mediates EGFR tyrosine kinase inhibitor-induced apoptosis in lung cancers with oncogenic EGFR mutations. PLoS Med 4: e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultas L, Bouillet P, Loveland KL, Meachem S, Perlman H, Adams JM, Strasser A (2005) Concomitant loss of proapoptotic BH3-only Bcl-2 antagonists Bik and Bim arrests spermatogenesis. EMBO J 24: 3963–3973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultas L, Bouillet P, Stanley EG, Brodnicki TC, Adams JM, Strasser A (2004) Proapoptotic BH3-only Bcl-2 family member Bik/Blk/Nbk is expressed in hemopoietic and endothelial cells but is redundant for their programmed death. Mol Cell Biol 24: 1570–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultas L, Terzano S, Thomas T, Voss A, Reid K, Stanley EG, Scott CL, Bouillet P, Bartlett P, Ham J, Adams JM, Strasser A (2007) Hrk/DP5 contributes to the apoptosis of select neuronal populations but is dispensable for haematopoietic cell apoptosis. J Cell Sci 120(Part 12): 2044–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg MS, Harris C, Strasser A, Scott CL (2009) Unleashing the power of inhibitors of oncogenic kinases through BH3 mimetics. Nat Rev Cancer 9: 321–326 [DOI] [PubMed] [Google Scholar]
- Cragg MS, Jansen ES, Cook M, Harris C, Strasser A, Scott CL (2008) Treatment of B-RAF mutant human tumor cells with a MEK inhibitor requires Bim and is enhanced by a BH3 mimetic. J Clin Invest 118: 3651–3659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg MS, Kuroda J, Puthalakath H, Huang DCS, Strasser A (2007) Gefitinib-induced killing of NSCLC cell lines expressing mutant EGFR requires Bim and can be enhanced by BH3 Mimetics. PLoS Med 4: 1681–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuconati A, White E (2002) Viral homologs of BCL-2: role of apoptosis in the regulation of virus infection. Genes Dev 16: 2465–2478 [DOI] [PubMed] [Google Scholar]
- Czabotar PE, Lee EF, Thompson GV, Wardak AZ, Fairlie WD, Colman PM (2011) Mutations to Bax beyond the BH3 domain disrupts interactions with pro-survival proteins and promotes apoptosis. J Biol Chem 286: 7123–7131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H, Smith A, Meng XW, Schneider PA, Pang YP, Kaufmann SH (2011) Transient binding of an activator BH3 domain to the Bak BH3-binding groove initiates Bak oligomerization. J Cell Biol 194: 39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson WF, Giese T, Fredrickson TN (1998) Spontaneous development of plasmacytoid tumors in mice with defective Fas-Fas ligand interactions. J Exp Med 187: 1825–1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewson G, Kratina T, Czabotar P, Day CL, Adams JM, Kluck RM (2009) Bak activation for apoptosis involves oligomerization of dimers via their alpha6 helices. Mol Cell 36: 696–703 [DOI] [PubMed] [Google Scholar]
- Dewson G, Kratina T, Sim HW, Puthalakath H, Adams JM, Colman PM, Kluck RM (2008) To trigger apoptosis, Bak exposes its BH3 domain and homodimerizes via BH3:groove interactions. Mol Cell 30: 369–380 [DOI] [PubMed] [Google Scholar]
- Dorstyn L, Read S, Cakouros D, Huh JR, Hay BA, Kumar S (2002) The role of cytochrome c in caspase activation in Drosophila melanogaster cells. J Cell Biol 156: 1089–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C, Fang M, Li Y, Li L, Wang X (2000) Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102: 33–42 [DOI] [PubMed] [Google Scholar]
- Edlich F, Banerjee S, Suzuki M, Cleland MM, Arnoult D, Wang C, Neutzner A, Tjandra N, Youle RJ (2011) Bcl-x(L) retrotranslocates Bax from the mitochondria into the cytosol. Cell 145: 104–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton M, Scollay R, Shortman K (1990) Kinetics of mature T cell development in the thymus. Proc Natl Acad Sci USA 87: 2579–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egle A, Harris AW, Bath ML, O’Reilly L, Cory S (2004a) VavP-Bcl2 transgenic mice develop follicular lymphoma preceded by germinal center hyperplasia. Blood 103: 2276–2283 [DOI] [PubMed] [Google Scholar]
- Egle A, Harris AW, Bouillet P, Cory S (2004b) Bim is a suppressor of Myc-induced mouse B cell leukemia. Proc Natl Acad Sci USA 101: 6164–6169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eischen CM, Roussel MF, Korsmeyer SJ, Cleveland JL (2001) Bax loss impairs Myc-induced apoptosis and circumvents the selection of p53 mutations during Myc-mediated lymphomagenesis. Mol Cell Biol 21: 7653–7662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekert PG, Read SH, Silke J, Marsden VS, Kaufmann H, Hawkins CJ, Gerl R, Kumar S, Vaux DL (2004) Apaf-1 and caspase-9 accelerate apoptosis, but do not determine whether factor-deprived or drug-treated cells die. J Cell Biol 165: 835–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S (1998) A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391: 43–50 [DOI] [PubMed] [Google Scholar]
- Enders A, Bouillet P, Puthalakath H, Xu Y, Tarlinton DM, Strasser A (2003) Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim inhibits BCR stimulation-induced apoptosis and deletion of autoreative B cells. J Exp Med 198: 1119–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlacher M, Laabi V, Manzl C, Bock G, Tzankov A, Haecker G, Strasser A, Villunger A (2006) Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. J Exp Med 203: 2939–2951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlacher M, Michalak EM, Kelly PN, Labi V, Niederegger H, Coultas L, Adams JM, Strasser A, Villunger A (2005) BH3-only proteins Puma and Bim are rate-limiting for {gamma}—radiation and glucocorticoid-induced apoptosis of lymphoid cells in vivo. Blood 106: 4131–4138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan GI, Wyllie AH, Gilbert CS, Littlewood TD, Land H, Brooks M, Waters CM, Penn LZ, Hancock DC (1992) Induction of apoptosis in fibroblasts by c-myc protein. Cell 69: 119–128 [DOI] [PubMed] [Google Scholar]
- Farazi PA, DePinho RA (2006) Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer 6: 674–687 [DOI] [PubMed] [Google Scholar]
- Fletcher JI, Meusburger S, Hawkins CJ, Riglar DT, Lee EF, Fairlie WD, Huang DC, Adams JM (2008) Apoptosis is triggered when prosurvival Bcl-2 proteins cannot restrain Bax. Proc Natl Acad Sci USA 105: 18081–18087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenzel A, Labi V, Chmelewskij W, Ploner C, Geley S, Fiegl H, Tzankov A, Villunger A (2010) Suppression of B-cell lymphomagenesis by the BH3-only proteins Bmf and Bad. Blood 115: 995–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaither A, Porter D, Yao Y, Borawski J, Yang G, Donovan J, Sage D, Slisz J, Tran M, Straub C, Ramsey T, Iourgenko V, Huang A, Chen Y, Schlegel R, Labow M, Fawell S, Sellers WR, Zawel L (2007) A Smac mimetic rescue screen reveals roles for inhibitor of apoptosis proteins in tumor necrosis factor-alpha signaling. Cancer Res 67: 11493–11498 [DOI] [PubMed] [Google Scholar]
- Garrison SP, Jeffers JR, Yang C, Nilsson JA, Hall MA, Rehg JE, Yue W, Yu J, Zhang L, Onciu M, Sample JT, Cleveland JL, Zambetti GP (2008) Selection against PUMA gene expression in Myc-driven B-cell lymphomagenesis. Mol Cell Biol 28: 5391–5402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavathiotis E, Reyna DE, Davis ML, Bird GH, Walensky LD (2010) BH3-triggered structural reorganization drives the activation of proapoptotic BAX. Mol Cell 40: 481–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, Tu HC, Kim H, Cheng EH, Tjandra N, Walensky LD (2008) BAX activation is initiated at a novel interaction site. Nature 455: 1076–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JC, Waterhouse NJ, Juin P, Evan GI, Green DR (2000) The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat Cell Biol 2: 156–162 [DOI] [PubMed] [Google Scholar]
- Golstein P (1998) Cell death in us and others. Science 281: 1283. [DOI] [PubMed] [Google Scholar]
- Gong Y, Somwar R, Politi K, Balak M, Chmielecki J, Jiang X, Pao W (2007) Induction of BIM is essential for apoptosis triggered by EGFR kinase inhibitors in mutant EGFR-dependent lung adenocarcinomas. PLoS Med 4: e294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalvez F, Ashkenazi A (2010) New insights into apoptosis signaling by Apo2L/TRAIL. Oncogene 29: 4752–4765 [DOI] [PubMed] [Google Scholar]
- Green DR, Kroemer G (2004) The pathophysiology of mitochondrial cell death. Science 305: 626–629 [DOI] [PubMed] [Google Scholar]
- Grillot DAM, Merino R, Nuñez G (1995) Bcl-xL displays restricted distribution during T cell development and inhibits multiple forms of apoptosis but not clonal deletion in transgenic mice. J Exp Med 182: 1973–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumont RJ, Rourke IJ, Gerondakis S (1999) Rel-dependent induction of A1 transcription is required to protect B cells from antigen receptor ligation-induced apoptosis. Genes Dev 13: 400–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakem R, Hakem A, Duncan GS, Henderson JT, Woo M, Soengas MS, Elia A, de la Pompa JL, Kagi D, Khoo W, Potter J, Yoshida R, Kaufman SA, Lowe SW, Penninger JM, Mak TW (1998) Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell 94: 339–352 [DOI] [PubMed] [Google Scholar]
- Hamasaki A, Sendo F, Nakayama K, Ishida N, Negishi I, Nakayama K-I, Hatakeyama S (1998) Accelerated neutrophil apoptosis in mice lacking A1-a, a subtype of the bcl-2-related A1 gene. J Exp Med 188: 1985–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100: 57–70 [DOI] [PubMed] [Google Scholar]
- Happo L, Cragg MS, Phipson B, Haga JM, Jansen ES, Herold MJ, Dewson G, Michalak EM, Vandenberg CJ, Smyth GK, Strasser A, Cory S, Scott CL (2010) Maximal killing of lymphoma cells by DNA-damage inducing therapy requires not only the p53 targets Puma and Noxa but also Bim. Blood 116: 5256–5267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison LR, Micha D, Brandenburg M, Simpson KL, Morrow CJ, Denneny O, Hodgkinson C, Yunus Z, Dempsey C, Roberts D, Blackhall F, Makin G, Dive C (2011) Hypoxic human cancer cells are sensitized to BH-3 mimetic-induced apoptosis via downregulation of the Bcl-2 protein Mcl-1. J Clin Invest 121: 1075–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengartner MO (2000) The biochemistry of apoptosis. Nature 407: 770–776 [DOI] [PubMed] [Google Scholar]
- Hengartner MO, Ellis RE, Horvitz HR (1992) Caenorhabditis elegans gene ced-9 protects cells from programmed cell death. Nature 356: 494–499 [DOI] [PubMed] [Google Scholar]
- Hengartner MO, Horvitz HR (1994) C. elegans cell survival gene ced-9 encodes a functional homolog of the mammalian proto-oncogene bcl-2. Cell 76: 665–676 [DOI] [PubMed] [Google Scholar]
- Hinds MG, Smits C, Fredericks-Short R, Risk JM, Bailey M, Huang DC, Day CL (2007) Bim, Bad and Bmf: intrinsically unstructured BH3-only proteins that undergo a localized conformational change upon binding to prosurvival Bcl-2 targets. Cell Death Differ 14: 128–136 [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS, Strasser A, McDunn JE, Swanson PE (2009) Cell death. N Engl J Med 361: 1570–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DC, Hahne M, Schroeter M, Frei K, Fontana A, Villunger A, Newton K, Tschopp J, Strasser A (1999) Activation of Fas by FasL induces apoptosis by a mechanism that cannot be blocked by Bcl-2 or Bcl-xL. Proc Natl Acad Sci USA 96: 14871–14876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DCS, Strasser A (2000) BH3-only proteins—essential initiators of apoptotic cell death. Cell 103: 839–842 [DOI] [PubMed] [Google Scholar]
- Imaizumi K, Benito A, Kiryu-Seo S, Gonzalez V, Inohara N, Lieberman AP, Kiyama H, Nunez G, Leiberman AP (2004) Critical role for DP5/Harakiri, a Bcl-2 homology domain 3-only Bcl-2 family member, in axotomy-induced neuronal cell death. J Neurosci 24: 3721–3725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorio MV, Croce CM (2009) MicroRNAs in cancer: small molecules with a huge impact. J Clin Oncol 27: 5848–5856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J, MacLean KH, Han J, Chittenden T, Ihle JN, McKinnon PJ, Cleveland JL, Zambetti GP (2003) Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell 4: 321–328 [DOI] [PubMed] [Google Scholar]
- Jost PJ, Grabow S, Gray D, McKenzie MD, Nachbur U, Huang DC, Bouillet P, Thomas HE, Borner C, Silke J, Strasser A, Kaufmann T (2009) XIAP discriminates between type I and type II FAS-induced apoptosis. Nature 460: 1035–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES (2011) RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471: 368–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang R, Zeh HJ, Lotze MT, Tang D (2011) The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ 18: 571–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang TB, Oh GS, Scandella E, Bolinger B, Ludewig B, Kovalenko A, Wallach D (2008) Mutation of a self-processing site in caspase-8 compromises its apoptotic but not its nonapoptotic functions in bacterial artificial chromosome-transgenic mice. J Immunol 181: 2522–2532 [DOI] [PubMed] [Google Scholar]
- Kasai S, Chuma S, Motoyama N, Nakatsuji N (2003) Haploinsufficiency of Bcl-x leads to male-specific defects in fetal germ cells: differential regulation of germ cell apoptosis between the sexes. Dev Biol 264: 202–216 [DOI] [PubMed] [Google Scholar]
- Kaufmann T, Jost PJ, Pellegrini M, Puthalakath H, Gugasyan R, Gerondakis S, Cretney E, Smyth MJ, Silke J, Hakem R, Bouillet P, Mak TW, Dixit VM, Strasser A (2009) Fatal hepatitis mediated by tumor necrosis factor TNFalpha requires caspase-8 and involves the BH3-only proteins Bid and Bim. Immunity 30: 56–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann T, Tai L, Ekert PG, Huang DC, Norris F, Lindemann RK, Johnstone RW, Dixit VM, Strasser A (2007) The BH3-only protein bid is dispensable for DNA damage- and replicative stress-induced apoptosis or cell-cycle arrest. Cell 129: 423–433 [DOI] [PubMed] [Google Scholar]
- Kelly PN, Puthalakath H, Adams JM, Strasser A (2007) Endogenous bcl-2 is not required for the development of Eμ-myc-induced B-cell lymphoma. Blood 109: 4907–4913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PN, White MJ, Goschnick MW, Fairfax KA, Tarlinton DM, Kinkel SA, Bouillet P, Adams JM, Kile BT, Strasser A (2010) Individual and overlapping roles of BH3-only proteins Bim and Bad in apoptosis of lymphocytes and platelets and in suppression of thymic lymphoma development. Cell Death Differ 17: 1655–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JFR, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26: 239–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Tu HC, Ren D, Takeuchi O, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH (2009) Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol Cell 36: 487–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, Peter ME (1995) Cytotoxicity-dependent APO-1 (Fas/CD95)—associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J 14: 5579–5588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi I, Ono H, Moritomo T, Kano K, Nakanishi T, Suda T (2010) Comparative gene expression analysis of zebrafish and mammals identifies common regulators in hematopoietic stem cells. Blood 115: e1–e9 [DOI] [PubMed] [Google Scholar]
- Kuida K, Haydar TF, Kuan CY, Gu Y, Taya C, Karasuyama H, Su MS, Rakic P, Flavell RA (1998) Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell 94: 325–337 [DOI] [PubMed] [Google Scholar]
- Kuroda J, Puthalakath H, Cragg MS, Kelly PN, Bouillet P, Huang DC, Kimura S, Ottmann OG, Druker BJ, Villunger A, Roberts AW, Strasser A (2006) Bim and Bad mediate imatinib-induced killing of Bcr/Abl+ leukemic cells, and resistance due to their loss is overcome by a BH3 mimetic. Proc Natl Acad Sci USA 103: 14907–14912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD (2005) BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell 17: 525–535 [DOI] [PubMed] [Google Scholar]
- Kvansakul M, Yang H, Fairlie WD, Czabotar PE, Fischer SF, Perugini MA, Huang DC, Colman PM (2008) Vaccinia virus anti-apoptotic F1L is a novel Bcl-2-like domain-swapped dimer that binds a highly selective subset of BH3-containing death ligands. Cell Death Differ 15: 1564–1571 [DOI] [PubMed] [Google Scholar]
- Labi V, Erlacher M, Kiessling S, Manzl C, Frenzel A, O’Reilly L, Strasser A, Villunger A (2008) Loss of the BH3-only protein Bmf impairs B cell homeostasis and accelerates gamma irradiation-induced thymic lymphoma development. J Exp Med 205: 641–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labi V, Erlacher M, Krumschnabel G, Manzl C, Tzankov A, Pinon J, Egle A, Villunger A (2010) Apoptosis of leukocytes triggered by acute DNA damage promotes lymphoma formation. Genes Dev 24: 1602–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leber B, Lin J, Andrews DW (2007) Embedded together: the life and death consequences of interaction of the Bcl-2 family with membranes. Apoptosis 12: 897–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EF, Clarke OB, Evangelista M, Feng Z, Speed TP, Tchoubrieva EB, Strasser A, Kalinna BH, Colman PM, Fairlie WD (2011) Discovery and molecular characterization of a Bcl-2-regulated cell death pathway in schistosomes. Proc Natl Acad Sci USA 108: 6999–7003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JT, Innes DJJ, Williams ME (1989) Sequential bcl-2 and c-myc oncogene rearrangements associated with the clinical transformation of non-Hodgkin's lymphoma. J Clin Invest 84: 1454–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Shin MS, Park WS, Kim SY, Dong SM, Pi JH, Lee HK, Kim HS, Jang JJ, Kim CS, Kim SH, Lee JY, Yoo NJ (1999) Alterations of Fas (APO-1/CD95) gene in transitional cell carcinomas of urinary bladder. Cancer Res 59: 3068–3072 [PubMed] [Google Scholar]
- Lenz G, Staudt LM (2010) Aggressive lymphomas. N Engl J Med 362: 1417–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessene G, Czabotar PE, Colman PM (2008) BCL-2 family antagonists for cancer therapy. Nat Rev Drug Discov 7: 989–1000 [DOI] [PubMed] [Google Scholar]
- Letai A, Bassik M, Walensky L, Sorcinelli M, Weiler S, Korsmeyer S (2002) Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2: 183–192 [DOI] [PubMed] [Google Scholar]
- Letai A, Sorcinelli MD, Beard C, Korsmeyer SJ (2004) Antiapoptotic BCL-2 is required for maintenance of a model leukemia. Cancer Cell 6: 241–249 [DOI] [PubMed] [Google Scholar]
- Ley R, Ewings KE, Hadfield K, Cook SJ (2005) Regulatory phosphorylation of Bim: sorting out the ERK from the JNK. Cell Death Differ 12: 1008–1014 [DOI] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu C-J, Yuan J (1998) Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94: 491–501 [DOI] [PubMed] [Google Scholar]
- Lindsten T, Ross AJ, King A, Zong W, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, Chen Y, Wei M, Eng VM, Adelman DM, Simon MC, Ma A, Golden JA, Evan G, Korsmeyer SJ, MacGregor GR et al. (2000) The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol Cell 6: 1389–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zou H, Slaughter C, Wang X (1997) DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell 89: 175–184 [DOI] [PubMed] [Google Scholar]
- Longthorne VL, Williams GT (1997) Caspase activity is required for commitment to Fas-mediated apoptosis. EMBO J 16: 3805–3812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell JF, Billen LP, Bindner S, Shamas-Din A, Fradin C, Leber B, Andrews DW (2008) Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell 135: 1074–1084 [DOI] [PubMed] [Google Scholar]
- Luo X, Budlhardjo I, Zou H, Slaughter C, Wang X (1998) Bid, a Bcl-2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94: 481–490 [DOI] [PubMed] [Google Scholar]
- Mailleux AA, Overholtzer M, Schmelzle T, Bouillet P, Strasser A, Brugge JS (2007) BIM regulates apoptosis during mammary Ductal morphogenesis, and its absence reveals alternative cell death mechanisms. Dev Cell 12: 221–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F, Juin P, Tasdemir E, Pierron G, Troulinaki K, Tavernarakis N, Hickman JA, Geneste O, Kroemer G (2007) Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J 26: 2527–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden V, O’Connor L, O’Reilly LA, Silke J, Metcalf D, Ekert P, Huang DCS, Cecconi F, Kuida K, Tomaselli KJ, Roy S, Nicholson DW, Vaux DL, Bouillet P, Adams JM, Strasser A (2002) Apoptosis initiated by Bcl–2-regulated caspase activation independently of the cytochrome c/Apaf–1/caspase–9 apoptosome. Nature 419: 634–637 [DOI] [PubMed] [Google Scholar]
- Marsden VS, Ekert PG, Van Delft M, Vaux DL, Adams JM, Strasser A (2004) Bcl-2-regulated apoptosis and cytochrome c release can occur independently of both caspase-2 and caspase-9. J Cell Biol 165: 775–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden VS, Kaufmann T, O’Reilly LA, Adams JM, Strasser A (2006) Apaf-1 and Caspase-9 are required for cytokine withdrawal-induced apoptosis of mast cells but dispensable for their functional and clonogenic death. Blood 107: 1872–1877 [DOI] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol Cell 10: 417–426 [DOI] [PubMed] [Google Scholar]
- Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S, Kelly PN, Ekert PG, Metcalf D, Roberts AW, Huang DC, Kile BT (2007) Programmed anuclear cell death delimits platelet life span. Cell 128: 1173–1186 [DOI] [PubMed] [Google Scholar]
- Mason KD, Vandenberg CJ, Scott CL, Wei AH, Cory S, Huang DC, Roberts AW (2008) In vivo efficacy of the Bcl-2 antagonist ABT-737 against aggressive Myc-driven lymphomas. Proc Natl Acad Sci USA 105: 17961–17966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell JM, Fushman D, Milliman CL, Korsmeyer SJ, Cowburn D (1999) Solution structure of the proapoptotic molecule BID: a structural basis for apoptotic agonists and antagonists. Cell 96: 625–634 [DOI] [PubMed] [Google Scholar]
- McDonnell TJ, Deane N, Platt FM, Nuñez G, Jaeger U, McKearn JP, Korsmeyer SJ (1989) bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell 57: 79–88 [DOI] [PubMed] [Google Scholar]
- McDonnell TJ, Korsmeyer SJ (1991) Progression from lymphoid hyperplasia to high-grade malignant lymphoma in mice transgenic for the t(14;18). Nature 349: 254–256 [DOI] [PubMed] [Google Scholar]
- Merino D, Giam M, Hughes PD, Siggs OM, Heger K, O’Reilly LA, Adams JM, Strasser A, Lee EF, Fairlie WD, Bouillet P (2009) The role of BH3-only protein Bim extends beyond inhibiting Bcl-2-like prosurvival proteins. J Cell Biol 186: 355–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak EM, Jansen ES, Happo L, Cragg MS, Tai L, Smyth GK, Strasser A, Adams JM, Scott CL (2009) Puma and to a lesser extent Noxa are suppressors of Myc-induced lymphomagenesis. Cell Death Differ 16: 684–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak EM, Vandenberg CJ, Delbridge ARD, Wu L, Scott CL, Adams JM, Strasser A (2010) Apoptosis-promoted tumorigenesis: gamma-irradiation-induced thymic lymphomagenesis requires Puma-driven leukocyte death. Genes Dev 24: 1608–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak EM, Villunger A, Adams JM, Strasser A (2008) In several cell types the tumour suppressor p53 induces apoptosis largely via Puma but Noxa can contribute. Cell Death Differ 15: 1019–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldoveanu T, Liu Q, Tocilj A, Watson MH, Shore G, Gehring K (2006) The X-ray structure of a BAK homodimer reveals an inhibitory zinc binding site. Mol Cell 24: 677–688 [DOI] [PubMed] [Google Scholar]
- Motoyama N, Wang FP, Roth KA, Sawa H, Nakayama K, Nakayama K, Negishi I, Senju S, Zhang Q, Fujii S, Loh DY (1995) Massive cell death of immature hematopoietic cells and neurons in Bcl-x deficient mice. Science 267: 1506–1510 [DOI] [PubMed] [Google Scholar]
- Nagata S, Hanayama R, Kawane K (2010) Autoimmunity and the clearance of dead cells. Cell 140: 619–630 [DOI] [PubMed] [Google Scholar]
- Naik E, Michalak EM, Villunger A, Adams JM, Strasser A (2007) UV-radiation triggers apoptosis of fibroblasts and skin keratinocytes mainly via the BH3-only protein Noxa. J Cell Biol 176: 415–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik E, O’Reilly LA, Asselin-Labat ML, Merino D, Lin A, Cook M, Coultas L, Bouillet P, Adams JM, Strasser A (2011) Destruction of tumor vasculature and abated tumor growth upon VEGF blockade is driven by proapoptotic protein Bim in endothelial cells. J Exp Med 208: 1351–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K, Harris AW, Bath ML, Smith KGC, Strasser A (1998) A dominant interfering mutant of FADD/Mort1 enhances deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes. EMBO J 17: 706–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K, Strasser A (2000) Ionizing radiation and chemotherapeutic drugs induce apoptosis in lymphocytes in the absence of fas or FADD/MORT1 signaling: implications for cancer therapy. J Exp Med 191: 195–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor L, Strasser A, O’Reilly LA, Hausmann G, Adams JM, Cory S, Huang DCS (1998) Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J 17: 384–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly LA, Tai L, Lee L, Kruse EA, Grabow S, Fairlie WD, Haynes NM, Tarlinton DM, Zhang JG, Belz GT, Smyth MJ, Bouillet P, Robb L, Strasser A (2009) Membrane-bound Fas ligand only is essential for Fas-induced apoptosis. Nature 461: 659–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes SR, Vaillant F, Lim E, Lee L, Breslin K, Feleppa F, Deb S, Ritchie ME, Takano E, Ward T, Fox SB, Generali D, Smyth GK, Strasser A, Huang DC, Visvader JE, Lindeman GJ (2011) Breast cancer special feature: sensitization of BCL-2-expressing breast tumors to chemotherapy by the BH3 mimetic ABT-737. Proc Natl Acad Sci USA (advance online publication 18 July 2011; doi:10.1073/pnas.1104778108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR (2011) Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 471: 363–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberstein A, Jeffrey P, Shi Y (2007) Crystal structure of the Bcl-XL-Beclin 1 peptide complex: Beclin 1 is a novel BH3-only protein. J Biol Chem 282: 13123–13132 [DOI] [PubMed] [Google Scholar]
- Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S (1993) Lethal effect of the anti-Fas antibody in mice. Nature 364: 806–809 [DOI] [PubMed] [Google Scholar]
- Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, Joseph MK, Kitada S, Korsmeyer SJ, Kunzer AR, Letai A, Li C, Mitten MJ, Nettesheim DG, Ng S, Nimmer PM et al. (2005) An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435: 677–681 [DOI] [PubMed] [Google Scholar]
- Opferman J, Iwasaki H, Ong CC, Suh H, Mizuno S, Akashi K, Korsmeyer SJ (2005) Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science 307: 1101–1104 [DOI] [PubMed] [Google Scholar]
- Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ (2003) Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature 426: 671–676 [DOI] [PubMed] [Google Scholar]
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B (2005) Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 122: 927–939 [DOI] [PubMed] [Google Scholar]
- Print CG, Loveland KL, Gibson L, Meehan T, Stylianou A, Wreford N, de Kretser D, Metcalf D, Köntgen F, Adams JM, Cory S (1998) Apoptosis regulator Bcl-w is essential for spermatogenesis but appears otherwise redundant. Proc Natl Acad Sci USA 95: 12424–12431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard DM, Print C, O’Reilly L, Adams JM, Potten CS, Hickman JA (2000) Bcl-w is an important determinant of damage-induced apoptosis in epithelia of small and large intestine. Oncogene 19: 3955–3959 [DOI] [PubMed] [Google Scholar]
- Puthalakath H, O’Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A (2007) ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 129: 1337–1349 [DOI] [PubMed] [Google Scholar]
- Puthalakath H, Strasser A (2002) Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ 9: 505–512 [DOI] [PubMed] [Google Scholar]
- Puthalakath H, Villunger A, O’Reilly LA, Beaumont JG, Coultas L, Cheney RE, Huang DCS, Strasser A (2001) Bmf: a pro-apoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science 293: 1829–1832 [DOI] [PubMed] [Google Scholar]
- Raff MC (1992) Social controls on cell survival and cell death. Nature 356: 397–400 [DOI] [PubMed] [Google Scholar]
- Rampino N, Yamamoto H, Ionov Y, Li Y, Sawai H, Reed JC, Perucho M (1997) Somatic frameshift mutations in the bax gene in colon cancers of the microsatellite mutator phenotype. Science 275: 967–969 [DOI] [PubMed] [Google Scholar]
- Rathmell JC, Lindsten T, Zong W-X, Cinalli RM, Thompson CB (2002) Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis. Nat Immunol 3: 932–939 [DOI] [PubMed] [Google Scholar]
- Richter-Larrea JA, Robles EF, Fresquet V, Beltran E, Rullan AJ, Agirre X, Calasanz MJ, Panizo C, Richter JA, Hernandez JM, Roman-Gomez J, Prosper F, Martinez-Climent JA (2010) Reversion of epigenetically mediated BIM silencing overcomes chemoresistance in Burkitt lymphoma. Blood 116: 2531–2542 [DOI] [PubMed] [Google Scholar]
- Riedl SJ, Salvesen GS (2007) The apoptosome: signalling platform of cell death. Nat Rev Mol Cell Biol 8: 405–413 [DOI] [PubMed] [Google Scholar]
- Rinkenberger JL, Horning S, Klocke B, Roth K, Korsmeyer SJ (2000) Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes Dev 14: 23–27 [PMC free article] [PubMed] [Google Scholar]
- Ross AJ, Waymire KG, Moss JE, Parlow AF, Skinner MK, Russell LD, MacGregor GR (1998) Testicular degeneration in Bclw-deficient mice. Nat Genet 18: 251–256 [DOI] [PubMed] [Google Scholar]
- Salmena L, Lemmers B, Hakem A, Matysiak-Zablocki E, Murakami K, Au B, Berry DM, Tamblyn L, Shehabeldin EM, Migon E, Wakeham A, Bouchard D, Chen WY, McGlade JC, Ohashi PS, Hakem R (2003) Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev 17: 883–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, Minn AJ, Thompson CB, Fesik SW (1997) Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science 275: 983–986 [DOI] [PubMed] [Google Scholar]
- Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin K-M, Krammer PH, Peter ME (1998) Two CD95 (APO-1/Fas) signaling pathways. EMBO J 17: 1675–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwickart M, Huang X, Lill JR, Liu J, Ferrando R, French DM, Maecker H, O’Rourke K, Bazan F, Eastham-Anderson J, Yue P, Dornan D, Huang DC, Dixit VM (2010) Deubiquitinase USP9X stabilizes MCL1 and promotes tumour cell survival. Nature 463: 103–107 [DOI] [PubMed] [Google Scholar]
- Sentman CL, Shutter JR, Hockenbery D, Kanagawa O, Korsmeyer SJ (1991) bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell 67: 879–888 [DOI] [PubMed] [Google Scholar]
- Shamas-Din A, Brahmbhatt H, Leber B, Andrews DW (2011) BH3-only proteins: orchestrators of apoptosis. Biochim Biophys Acta 1813: 508–520 [DOI] [PubMed] [Google Scholar]
- Sharma SV, Gajowniczek P, Way IP, Lee DY, Jiang J, Yuza Y, Classon M, Haber DA, Settleman J (2006) A common signaling cascade may underlie ‘addiction’ to the Src, BCR-ABL, and EGF receptor oncogenes. Cancer Cell 10: 425–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibue T, Takeda K, Oda E, Tanaka H, Murasawa H, Takaoka A, Morishita Y, Akira S, Taniguchi T, Tanaka N (2003) Integral role of Noxa in p53-mediated apoptotic response. Genes Dev 17: 2233–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KGC, Strasser A, Vaux DL (1996) CrmA expression in T lymphocytes of transgenic mice inhibits CD95 (Fas/APO-1)-transduced apoptosis, but does not cause lymphadenopathy or autoimmune disease. EMBO J 15: 5167–5176 [PMC free article] [PubMed] [Google Scholar]
- Steimer DA, Boyd K, Takeuchi O, Fisher JK, Zambetti GP, Opferman JT (2009) Selective roles for anti-apoptotic MCL-1 during granulocyte development and macrophage effector function. Blood 113: 2805–2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A, Harris AW, Bath ML, Cory S (1990a) Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature 348: 331–333 [DOI] [PubMed] [Google Scholar]
- Strasser A, Harris AW, Cory S (1991a) Bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell 67: 889–899 [DOI] [PubMed] [Google Scholar]
- Strasser A, Harris AW, Cory S (1993) Eμ-bcl-2 transgene facilitates spontaneous transformation of early pre-B and immunoglobulin-secreting cells but not T cells. Oncogene 8: 1–9 [PubMed] [Google Scholar]
- Strasser A, Harris AW, Huang DCS, Krammer PH, Cory S (1995) Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J 14: 6136–6147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A, Harris AW, Jacks T, Cory S (1994) DNA damage can induce apoptosis in proliferating lymphoid cells via p53-independent mechanisms inhibitable by Bcl-2. Cell 79: 329–339 [DOI] [PubMed] [Google Scholar]
- Strasser A, Harris AW, Vaux DL, Webb E, Bath ML, Adams JM, Cory S (1990b) Abnormalities of the immune system induced by dysregulated bcl-2 expression in transgenic mice. Curr Top Microbiol Immunol 166: 175–181 [DOI] [PubMed] [Google Scholar]
- Strasser A, Jost PJ, Nagata S (2009) The many roles of FAS receptor signaling in the immune system. Immunity 30: 180–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A, O’Connor L, Dixit VM (2000) Apoptosis signaling. Ann Rev Biochem 69: 217–245 [DOI] [PubMed] [Google Scholar]
- Strasser A, Whittingham S, Vaux DL, Bath ML, Adams JM, Cory S, Harris AW (1991b) Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc Natl Acad Sci USA 88: 8661–8665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub CS (2011) Targeting IAPs as an approach to anti-cancer therapy. Curr Top Med Chem 11: 291–316 [DOI] [PubMed] [Google Scholar]
- Straus SE, Jaffe ES, Puck JM, Dale JK, Elkon KB, Rosen-Wolff A, Peters AM, Sneller MC, Hallahan CW, Wang J, Fischer RE, Jackson CM, Lin AY, Baumler C, Siegert E, Marx A, Vaishnaw AK, Grodzicky T, Fleisher TA, Lenardo MJ (2001) The development of lymphomas in families with autoimmune lymphoproliferative syndrome with germline Fas mutations and defective lymphocyte apoptosis. Blood 98: 194–200 [DOI] [PubMed] [Google Scholar]
- Suzuki M, Youle RJ, Tjandra N (2000) Structure of Bax: coregulation of dimer formation and intracellular localization. Cell 103: 645–654 [DOI] [PubMed] [Google Scholar]
- Swanson PJ, Kuslak SL, Fang W, Tze L, Gaffney P, Selby S, Hippen KL, Nunez G, Sidman CL, Behrens TW (2004) Fatal acute lymphoblastic leukemia in mice transgenic for B cell-restricted bcl-xL and c-myc. J Immunol 172: 6684–6691 [DOI] [PubMed] [Google Scholar]
- Tagawa H, Karnan S, Suzuki R, Matsuo K, Zhang X, Ota A, Morishima Y, Nakamura S, Seto M (2005) Genome-wide array-based CGH for mantle cell lymphoma: identification of homozygous deletions of the proapoptotic gene BIM. Oncogene 24: 1348–1358 [DOI] [PubMed] [Google Scholar]
- Tan N, Malek M, Zha J, Kassees R, Yue P, Berry L, Fairbrother W, Sampath D, Belmont LD (2011) Navitoclax enhances the efficacy of Taxanes in non-small cell lung cancer models. Clin Cancer Res 17: 1394–1404 [DOI] [PubMed] [Google Scholar]
- Timmer JC, Salvesen GS (2007) Caspase substrates. Cell Death Differ 14: 66–72 [DOI] [PubMed] [Google Scholar]
- Trambas CM, Griffiths GM (2003) Delivering the kiss of death. Nat Immunol 4: 399–403 [DOI] [PubMed] [Google Scholar]
- Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, Johnson EF, Marsh KC, Mitten MJ, Nimmer P, Roberts L, Tahir SK, Xiao Y, Yang X, Zhang H, Fesik S, Rosenberg SH, Elmore SW (2008) ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 68: 3421–3428 [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y, Yunis J, Onorato-Showe L, Erikson J, Nowell PC, Croce CM (1984) Molecular cloning of the chromosomal breakpoint of B-cell lymphomas and leukemias with the t(11;14) chromosome translocation. Science 224: 1403–1406 [DOI] [PubMed] [Google Scholar]
- van Delft MF, Smith DP, Lahoud MH, Huang DC, Adams JM (2009) Apoptosis and non-inflammatory phagocytosis can be induced by mitochondrial damage without caspases. Cell Death Differ 17: 821–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, Willis SN, Scott CL, Day CL, Cory S, Adams JM, Roberts AW, Huang DCS (2006) The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell 10: 389–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, Zobel K, Dynek JN, Elliott LO, Wallweber HJ, Flygare JA, Fairbrother WJ, Deshayes K, Dixit VM, Vucic D (2007) IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell 131: 669–681 [DOI] [PubMed] [Google Scholar]
- Varfolomeev EE, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann JS, Mett IL, Rebrikov D, Brodianski VM, Kemper OC, Kollet O, Lapidot T, Soffer D, Sobe T, Avraham KB, Goncharov T, Holtmann H, Lonai P, Wallach D (1998) Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity 9: 267–276 [DOI] [PubMed] [Google Scholar]
- Vaux DL, Cory S, Adams JM (1988) Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 335: 440–442 [DOI] [PubMed] [Google Scholar]
- Vaux DL, Haecker G, Strasser A (1994) An evolutionary perspective on apoptosis. Cell 76: 777–779 [DOI] [PubMed] [Google Scholar]
- Vaux DL, Weissman IL, Kim SK (1992) Prevention of programmed cell death in Caenorhabditis elegans by human bcl-2. Science 258: 1955–1957 [DOI] [PubMed] [Google Scholar]
- Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ (1993) Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell 75: 229–240 [DOI] [PubMed] [Google Scholar]
- Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, Moritz RL, Simpson RJ, Vaux DL (2000) Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing inhibitor of apoptosis (IAP) proteins. Cell 102: 43–53 [DOI] [PubMed] [Google Scholar]
- Vick B, Weber A, Urbanik T, Maass T, Teufel A, Krammer PH, Opferman JT, Schuchmann M, Galle PR, Schulze-Bergkamen H (2009) Knockout of myeloid cell leukemia-1 induces liver damage and increases apoptosis susceptibility of murine hepatocytes. Hepatology 49: 627–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikstrom I, Carotta S, Luethje K, Peperzak V, Jost PJ, Glaser S, Busslinger M, Bouillet P, Strasser A, Nutt SL, Tarlinton DM (2010) Mcl-1 is essential for germinal center formation and B cell memory. Science 330: 1095–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villunger A, Michalak EM, Coultas L, Müllauer F, Böck G, Ausserlechner MJ, Adams JM, Strasser A (2003) p53- and drug-induced apoptotic responses mediated by BH3-only proteins Puma and Noxa. Science 302: 1036–1038 [DOI] [PubMed] [Google Scholar]
- Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, Benetatos CA, Chunduru SK, Condon SM, McKinlay M, Brink R, Leverkus M, Tergaonkar V, Schneider P, Callus BA, Koentgen F, Vaux DL, Silke J (2007) IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell 131: 682–693 [DOI] [PubMed] [Google Scholar]
- Vousden KH, Lane DP (2007) p53 in health and disease. Nat Rev Mol Cell Biol 8: 275–283 [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S (1992) Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 356: 314–317 [DOI] [PubMed] [Google Scholar]
- Wertz IE, Kusam S, Lam C, Okamoto T, Sandoval W, Anderson DJ, Helgason E, Ernst JA, Eby M, Liu J, Belmont LD, Kaminker JS, O’Rourke KM, Pujara K, Kohli PB, Johnson AR, Chiu ML, Lill JR, Jackson PK, Fairbrother WJ et al. (2011) Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature 471: 110–114 [DOI] [PubMed] [Google Scholar]
- Westphal D, Dewson G, Czabotar PE, Kluck RM (2011) Molecular biology of Bax and Bak activation and action. Biochim Biophys Acta 1813: 521–531 [DOI] [PubMed] [Google Scholar]
- Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, Adams JM, Huang DC (2005) Pro-apoptotic Bak is sequestered by Mc1-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev 19: 1294–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, Ierino H, Lee EF, Fairlie WD, Bouillet P, Strasser A, Kluck RM, Adams JM, Huang DC (2007) Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science 315: 856–859 [DOI] [PubMed] [Google Scholar]
- Xiang Z, Ahmed AA, Moller C, Nakayama K, Hatakeyama S, Nilsson G (2001) Essential role of the prosurvival bcl-2 homologue A1 in mast cell survival after allergic activation. J Exp Med 194: 1561–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z, Luo H, Payton JE, Cain J, Ley TJ, Opferman JT, Tomasson MH (2010) Mcl1 haploinsufficiency protects mice from Myc-induced acute myeloid leukemia. J Clin Invest 120: 2109–2118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Wang X, Contino G, Liesa M, Sahin E, Ying H, Bause A, Li Y, Stommel JM, Dell’antonio G, Mautner J, Tonon G, Haigis M, Shirihai OS, Doglioni C, Bardeesy N, Kimmelman AC (2011) Pancreatic cancers require autophagy for tumor growth. Genes Dev 25: 717–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin CY, Knudson CM, Korsmeyer SJ, Van Dyke T (1997) Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature 385: 637–640 [DOI] [PubMed] [Google Scholar]
- Yin X-M, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, Roth KA, Korsmeyer SJ (1999) Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature 400: 886–891 [DOI] [PubMed] [Google Scholar]
- Youle RJ, Strasser A (2008) The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9: 47–59 [DOI] [PubMed] [Google Scholar]
- Yuan J, Horvitz HR (1992) The Caenorhabditis elegans cell death gene ced-4 encodes a novel protein and is expressed during the period of extensive programmed cell death. Development 116: 309–320 [DOI] [PubMed] [Google Scholar]
- Yuan J, Kroemer G (2010) Alternative cell death mechanisms in development and beyond. Genes Dev 24: 2592–2602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Shaham S, Ledoux S, Ellis HM, Horvitz HR (1993) The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1β-converting enzyme. Cell 75: 641–652 [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhou X, McQuade T, Li J, Chan FK, Zhang J (2011) Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature 471: 373–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Cado D, Chen A, Kabra NH, Winoto A (1998) Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature 392: 296–300 [DOI] [PubMed] [Google Scholar]
- Zhou P, Levy NB, Xie H, Qian L, Lee CY, Gascoyne RD, Craig RW (2001) MCL1 transgenic mice exhibit a high incidence of B-cell lymphoma manifested as a spectrum of histologic subtypes. Blood 97: 3902–3909 [DOI] [PubMed] [Google Scholar]
- Zhou P, Qian L, Bieszczad CK, Noelle R, Binder M, Levy NB, Craig RW (1998) Mcl-1 in transgenic mice promotes survival in a spectrum of hematopoietic cell types and immortalization in the myeloid lineage. Blood 92: 3226–3239 [PubMed] [Google Scholar]
- Zhou P, Qian L, Kozopas KM, Craig RW (1997) Mcl-1, a Bcl-2 family member, delays the death of hematopoietic cells under a variety of apoptosis-inducing conditions. Blood 89: 630–643 [PubMed] [Google Scholar]
- Zou H, Henzel WJ, Liu X, Lutschg A, Wang X (1997) Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of Caspase-3. Cell 90: 405–413 [DOI] [PubMed] [Google Scholar]