Abstract
Objective
To examine a large, single-surgeon series of patients with prostate cancer who underwent retropubic radical prostatectomy (RRP) for men with postoperative bladder neck contractures (BNCs).
Patients and methods
From 1983 to 2007, 4132 men underwent RRP for prostate cancer by one surgeon. All patients had BN reconstruction with mucosal eversion. The bladder to membranous urethral anastomosis was made using six 2/0 chromic catgut sutures over an 18 F Foley catheter. The catheter was left in place for 10 days. Data from these men is stored in a prospective database, which was reviewed in this study for men with BNCs after RRP. Men with BNCs were compared with all other men in the series to determine risk factors for BNC development.
Results
Overall, BNCs developed in 110 patients (2.5%). Examining our last 500 patients there was a contemporary BNC rate of < 1%. The median (range) follow-up was 44 (12–233) months. Tumour characteristics were similar in the men with BNCs and those with no BNCs, and the rates of organ-confined disease were also similar (65% vs 70%, P = 0.27). Men with BNCs had higher median preoperative prostate-specific antigen (PSA) levels (6.7 vs 5.7 mg/dL; P = 0.009) and were more likely to have PSA failure after RRP (30% vs 16%, P < 0.001). On multivariate analysis, non-nerve sparing (P = 0.003) and a surgical date before 1992 (P < 0.001) were significant predictors of BNC. Patients with BNCs had lower potency rates (49% vs 63%, P < 0.003) and continence rates (88% vs 94%, P = 0.07) at the 18-month follow-up.
Conclusions
BNCs are rare, occurring in < 1% in our modern series. The important surgical factors in preventing BNCs are to avoid closing the BN too tightly and attaining good apposition of the BN with the urethral stump with a watertight closure. BNCs are more common with non-nerve-sparing surgery and early in a surgeon's experience.
Keywords: prostate cancer, bladder neck contracture, retropubic radical prostatectomy
Introduction
Nearly 220 000 men were diagnosed with prostate cancer in 2008 and nearly a third of them elected definitive surgical therapy [1]. Because more of these men are being diagnosed at an earlier age with the potential for ≥ 20 years of cancer-free life remaining, minimizing their complications is of utmost importance.
One of the more problematic complications to result from the retropubic radical prostatectomy (RRP) is a bladder neck contracture (BNC). BNCs have been associated with higher rates of urinary incontinence [2], the need for subsequent and often life-long urological interventions [3] and can decrease the postoperative quality of life [4].
The reported rates of BNCs vary from 0.5% to 31%, with higher rates generally being found in both earlier and patient reported data series [2,5]. Fortunately, the published rates of BNCs after RP continues to decrease, a finding that has widely been attributed to improved open surgical technique [6]. We have previously reported a BNC rate of 2.7% [7] in 3477 consecutive men undergoing RRP and now update this consecutive RRP series with a specific focus on the men that developed a BNC.
Patients and methods
All patients undergoing RRP by one surgeon (W.J.C.) between May 1983 and June 2007 with ≥ 1 year of follow-up were included in the study.
Surgical technique
The anatomical RRP was performed in all men as previously described [8] and a bilateral nerve-sparing approach was performed when surgically appropriate. The surgical technique of the vesico-urethral anastomosis (VUA) was modified over the course of the series. Specifically, the number of sutures was increased from four to five, with additional sutures being placed as necessary to help prevent anastomotic leaks. We also take great care in ensuring that all knots are tied are under direct visualization with the help of a sturdy, straight-bladed, hand-held retractor that serves to both depress the bladder and to approximate the bladder to the urethra.
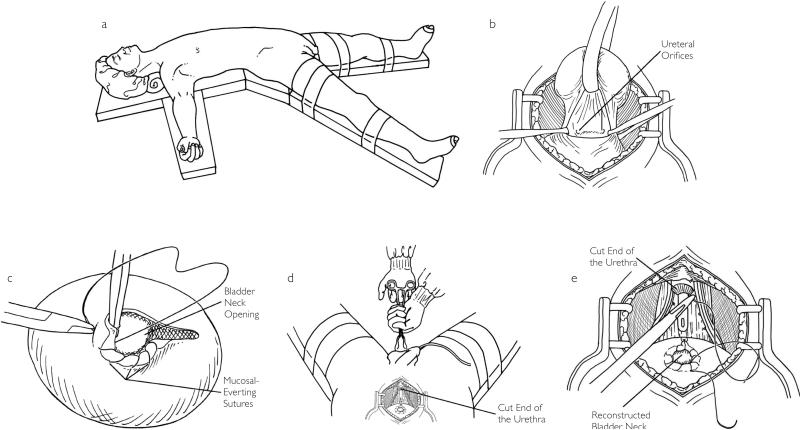
Important aspects of our current VUA technique are shown in Fig. 1. Specifically, the prostate is removed using a non-BN-sparing approach. The BN is then reconstructed using a 2/0 chromic catgut suture in a ‘tennis racquet’ fashion to a size of 22–24 F. A running, 3/0 chromic, mucosal-eversion suture is placed from the 9 o'clock to 3 o'clock positions, which serve to both display the mucosa and improve haemostasis at the BN. The anastomosis is then made using five 2/0 chromic catgut sutures placed at the 2, 4, 6, 8 and 10 o'clock positions.
Fig 1.
(a) The patient is placed supine and the table is flexed to help with pelvic visualization. The legs are placed in spreader bars to allow for perineal access to aid with distal urethral stump visualization. (b) The anterior BN has been opened with a non-BN-sparing approach. (c) A 3/0 chromic catgut suture is run along the anterior BN to evert the BN mucosa. This suture is also useful for BN haemostasis. (d) Pressure is applied in the perineum with a sponge-stick, which helps to elevate the distal urethral stump into the surgical field. This step can improve stump visualization before the anastomotic sutures are placed. (e) The anastomosis is performed using five 2/0 chromic catgut sutures at the 2, 4, 6, 8 and 10 o'clock positions.
Urethral stump visualization is improved by meticulous haemostasis in the prostatic bed using 4/0 chromic catgut sutures and perineal pressure applied with a sponge-forceps. An 18 F-silicone Foley catheter is inserted under direct visualization before the sutures are tied. Sutures are tied under direct visualization as described above. After the sutures are tied, the catheter is then irrigated to ensure a watertight closure; additional sutures are placed if leakage is seen. We place the catheter on slight traction on the evening of surgery. The catheter is removed after 10 days if there is no evidence of a urine leak (persistent suction drain output). If a urine leak is suspected, a cystogram is taken to confirm the absence of a leak before the catheter is removed. Our database does not contain information correlating the presence of a postoperative urine leak with BNC formation.
Follow-up
The follow-up schedule includes a bi-annual PSA measurement and a yearly DRE. All postoperative patients are mailed a yearly questionnaire that includes questions on sexual functioning, urinary continence, urinary symptoms and the need for further surgical intervention or therapy after their RRP. All data is stored in a prospectively maintained, Institutional Review Board-approved database.
BNC diagnosis
Patients seen at follow-up with urinary complaints such as slow or sprayed urinary stream or with complaints of UTI or incomplete voiding are evaluated with cystoscopy. The diagnosis of a BNC is given when it is not possible to easily navigate a standard 17 F cystoscope into the bladder. If a BNC is found, initial management is urethral dilatation to 22 F with urethral sounds or over a wire with cystoscopic guidance. The catheter is then left in place for 72 h. If a recurrent BNC is found, a formal operative direct vision internal urethrotomy usually with injection of triamcinolone through an endoscopic needle is generally performed.
The clinical details of the management and outcomes data for men with BNCs was available for only the most recent 654 patients who had a RRP (2003–2006).
Chi-square, Fisher's exact, or Wilcoxon rank-sum tests were used to determine if clinical and surgical factors differed for men who developed BNC compared with those who did not.
We also used a multivariate logistic regression model predicting BNC based on significant variables from the univariate analysis (P < 0.05). Continuously scaled variables were entered as simple linear effects and categorical variables were entered as dummy-coded predictors. We report the adjusted odds ratios (ORs) and CIs for the model.
Results
In all, 4132 men were included in the study, of which 110 (2.5%) had a BNC after RRP. The median (range) follow-up was 44 (0– 233) months. Demographics of the two groups of men are included in Table 1. Patients with BNCs (BNC +) were older (65 vs 61 years, P < 0.001) had a higher clinical stage (P = 0.04) and a higher median PSA level (6.7 vs 5.7 mg/dL, P = 0.009) but had similar rates of positive surgical margins (22% vs 20%, P = 0.72) and organ-confined disease as patients with no BNCs (65% vs 70%, P = 0.27). Patients with BNCs were more likely to have biochemical evidence of cancer progression (30% vs 16%, P < 0.001) and require adjuvant or salvage radiation (26% vs 15%, P = 0.003). Potency rates in the patients with BNCs, not selecting for preoperative potency rates, follow-up times or the need for radiation and/or hormonal therapy after RRP were lower (49% vs 63%, P = 0.003) as were urinary continence rates (88% vs 94%, P = 0.07) than patients with no BNCs. Patients with BNCs were far more likely to be operated on early in the series (median year of surgery 1992 vs 1998, P < 0.001). On multivariate analysis, only the age at time of surgery (OR 1.04, P = 0.03), median PSA level (OR 1.02, P = 0.05), year of surgery < 1992 (OR 4.0, P < 0.001) and non-nerve-sparing surgical technique (OR 2.1, P = 0.003) were significant predictors of BNC development (Table 2).
Table 1.
Univariate analysis of risk factors for BNC formation after RRP
| BNC |
|||
|---|---|---|---|
| Variable | Yes | No | P |
| Number of patients | 110 | 4022 | |
| Median (range): | |||
| age, years | 65 (48–77) | 61 (36–80) | < 0.001 |
| PSA level, mg/dL | 6.7 (0.6–37.2) | 5.7 (0.0–98) | 0.009 |
| year of surgery | 1992 (1983–2005) | 1998 (1983–2006) | < 0.001 |
| Race (White), n | 107 (97) | 3790 (94) | 0.21 |
| N (%): | |||
| Clinical stage | |||
| T1 | 52 (47) | 2308 (57) | 0.04 |
| T2 | 57 (52) | 1669 (42) | |
| T3 | 1 (1) | 37 (1) | |
| Prior TURP* | 0 | 5 (0.8) | 1.0 |
| CAD* | 2 (18.2) | 96 (15.1) | 0.676 |
| Smoking history* | 1 (9.1) | 89 (13.9) | 1.0 |
| Surgical factors | |||
| N (%): | |||
| organ-confined disease | 72 (65) | 2812 (70) | 0.27 |
| bilateral nerve sparing | 84 (76) | 3668 (92) | < 0.001 |
| adjuvant/salvage radiation | 28 (26) | 573 (15) | 0.03 |
| PSA progression | 33 (30) | 634 (16) | < 0.001 |
| potency | 51 (49) | 1922 (63) | 0.003 |
| Continence | 92 (88) | 2876 (94) | 0.07 |
Data available on last 654 patients
CAD, coronary artery disease.
Table 2.
Multivariate model with all significant variables on univariate analysis
| Variable | Odds ratio (95% CI) | P |
|---|---|---|
| Age | 1.0 (1.0–1.1) | 0.03 |
| Clinical stage | 0.7 (0.4–1.1) | 0.11 |
| Median PSA level | 1.0 (1.0–1.0) | 0.05 |
| Surgery date < 1992 | 4.0 (2.3–6.8) | < 0.001 |
| Bilateral nerve sparing | 2.1 (1.3–3.5) | 0.003 |
| Adjuvant/salvage radiation therapy | 1.5 (0.7–2.8) | 0.27 |
| PSA recurrence | 0.7 (0.4–1.4) | 0.36 |
| Potency | 1.2 (0.7–1.9) | 0.48 |
The rate of BNCs decreased significantly over time, from an initial rate of > 17% to a contemporary rate of < 1%. (Fig. 2). Considering our last 500 patients who underwent RRP, there was BNC in only five (1%) patients. The mean (range) time to BNC development in these five patients was 100 (58–129) days. The recent BNCs were of minimal severity. All were managed with a single outpatient dilatation in an office setting and none has had BNC recurrence at a median (range) follow-up of 24 (12– 48) months.
Fig. 2.
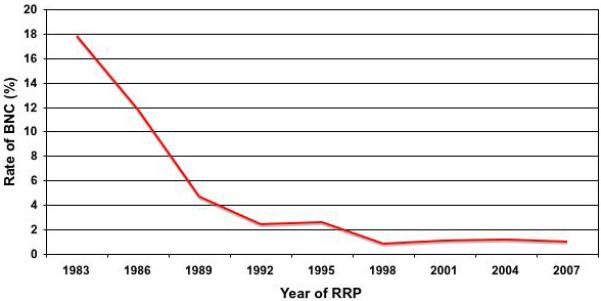
BNC rates over time
Discussion
The exact mechanism of BNC formation is unknown, though their formation has been associated with many operative and postoperative variables such as large volume blood loss, pelvic haematoma formation, urine leakage at the VUA and a history of hypertrophic scar formation [2,9].
The unifying theme for each of these risk factors seems to be their potential to alter normal healing of the VUA. Each can generate a peri-VUA inflammatory response and put undue stress on the fresh anastomosis with resulting scar formation. An adequate microvascular environment of the healing anastomosis also appears to be important, as patients with comorbidities, such as current cigarette smoking, coronary artery disease and diabetes mellitus have been reported to be more likely to have BNCs [10]. Poor microcirculation may also help explain why patients with a history of prior TURP have been reported to have higher BNC rates, as these surgeries may disrupt the peri-bladder neck vascular supply and impair healing after RRP [9].
In the present series, the most important independent risk factor for BNC formation was the timing of the patient's RRP. Men undergoing RRP early in our series had a significantly higher risk of developing a postoperative BNC. There are multiple explanations for this finding. First, surgeon experience has clearly been shown to play a role in the rate and severity of complications after RRP, and BNCs are no exception. Hu et al. [11] reported that surgeons who performed ≥ 40 RRPs per year had nearly half the risk of peri-operative complications as compared with those who performed ≤ 40 surgeries (OR 0.53) and surgeons in high volume hospitals (≥ 60 RRP's per year) had significantly fewer anastomotic strictures (26.8% vs 19.8%). Begg et al. [12] reported similar findings when evaluating the Surveillance, Epidemiology, and End Results database, reporting significantly fewer late urinary complications (BNCs, urethral strictures, urinary fistulas) for high volume surgeons (> 16 RRPs/year) vs low volume surgeons (< 4 RRPs/year) at 20% vs 28% respectively (P = 0.001). A similar ‘learning curve’ has been seen in the early laparoscopic RP series with BNC rates as high as 15% and significant improvement with increasing experience [13]. We think that the apical dissection is the most difficult part of the RRP to master and because this component of the operation is so crucial to a good VUA, lack of experience undoubtedly leads to higher rates of BNC formation.
The decrease in the present BNC rate over time was also affected by modifications of our surgical technique. The present series first reports on men undergoing RRP in 1983, only 1 year after Walsh [14] first described the anatomical, nerve-sparing RRP, and many modifications of the original technique have been described since then, most of which have aimed to decrease the perioperative morbidity associated with RRP. Eastham et al. [6] noted that after changes were made to their anastomotic technique in 1990, which included less surgical manipulation of the urethra, BN reconstruction to 24 F and full thickness mucosal-eversion sutures, urinary continence rates (found in the study to be inversely related to BNC rates) improved dramatically. Other studies that have evaluated outcomes in relation to changes in surgical technique show a similar improvement in the BNC rate [15].
In 1999, we published on the outcomes from 1870 consecutive patients undergoing RRP and found that after we changed the size of the reconstructed BN from 18 F to 24 F, our BNC rates decreased from nearly 10% to ≈1% [16]. While this modification was not studied in a controlled manner and the improvements seen were undoubtedly multifactorial, it further highlights the importance that surgical technique plays in outcomes after RRP.
Other components of our present surgical technique that we feel help to minimize BNCs deserve further mention. We have used a non-BN-sparing approach to our RRP throughout the present series. While this approach adds the additional step of BN reconstruction to the operation, we feel the main advantage to not sparing the BN is the subsequent ability to tailor the BN to a specific dimension (22–24 F), giving us a uniform size BN for the VUA in every case. This makes the VUA technique more predictable and possibly more successful. Although BN sparing has been reported to achieve a slightly quicker return of continence, overall continence rates are no different and concerns have been raised about higher positive margin rates with BN sparing [17].
We also use mucosal-eversion sutures along the BN to help ensure a good mucosa-to-mucosa and watertight anastomosis. A prospective, randomized study performed by Srougi et al. reported that continence rates (92% vs 92%, P = 0.856) and BNC rates (0% vs 2%, P = 0.495) were not different between those that had eversion sutures and those that did not, respectively [18]. However, while we acknowledge that eversion sutures themselves may not decrease BNC rates, we feel this step helps to ensure full thickness suture bites are taken at the BN, which probably improves perfusion to the anastomosis.
Finally, when tying the anastomotic sutures, it is imperative that good BN to urethral stump approximation is achieved. We find that with the patient positioned on spreader bars, perineal pressure using a sponge-forceps can improve the exposure and the length of the distal urethral stump available for suturing. The use of perineal pressure can also aid in haemostasis deep in the pelvis, improving visualization. Once the sutures are placed, we always attempt to tie the knots under direct visualization. This can often be hastened by downward and inferior pressure on the bladder using a straight blade retractor. The increase in the number of sutures at the VUA has also probably played a role in the present decreasing BNC rate by decreasing the amount of urine leakage at the VUA, a reported risk factor for BNCs. Unfortunately, urine leaks were not prospectively recorded in this series and could not be evaluated as a risk factor.
An interesting finding from the present study that deserves further analysis is the association between non-neurovascular bundle (NVB)-sparing surgery and BNC formation. Only 76% of the men with BNCs after RRP had a bilateral nerve-sparing RRP vs 92% without BNC (P < 0.001). The goal of the nerve-sparing RRP was initially to preserve the neural and vascular supply to the erectile bodies with the hopes of preserving erections. However, numerous studies evaluating the nerve-sparing RRP have shown that cavernosal NVB preservation not only improves erection recovery rates but also continence rates [6,14]. These findings suggest that preservation of the NVBs may protect neural and vascular input to other parts of the pelvis, including the urinary sphincter mechanism and indeed, anatomical studies have shown that branches from the NVB to the external sphincter do exist [19]. However, whether or not preservation of these branches directly results in improved continence rates is conjecture. Similarly, it is unclear how NVB preservation would directly decrease BNC rates, though it is likely that the meticulous apical dissection required for a good nerve-sparing RRP better preserves the peri-anastomotic tissue and therefore, the blood supply to the VUA.
Ideally, changes in our surgical technique would have been evaluated in a controlled fashion to determine which steps were most responsible for our improvement in BNC rates. Also, because the medical records were readily available for only the most recent 654 patients, a full evaluation of risk factors known to predispose to BNCs, such as blood loss and urine leaks was not possible. Similarly, BNC management and outcomes could not be evaluated for most of the men, though it was interesting to note that all of our recent BNCs were amenable to a single dilatation. This had not been our experience with BNCs early in the present series and again, it is likely that the decrease in the severity of our BNCs is a result of our refined surgical technique.
Finally, there were a significant number of men with BNCs that required radiation therapy after RRP, a known risk factor for BNC formation. Although multivariate analysis did not show radiation to be an independent risk factor for BNC formation, had we been able to evaluate the timing of BNC formation in relation to the radiation exposure, we may have uncovered a stronger correlation. Additionally, we acknowledge that the ideal study would need to include postoperative cystoscopic evaluation of the VUA of all patients. To our knowledge, though, the present study represents the largest series to date that specifically evaluates BNCs after RRP and provides insights into ways to prevent BNC formation in both open and laparoscopic RRP.
In conclusion, in the present series BNCs rates decreased dramatically over time and now occur in < 1% of patients after RRP. Improved surgical technique and surgeon experience appear to be the factors most responsible for this decrease, and surgeons finding higher rates in their own series should carefully examine how they perform their VUA. The nerve-sparing RRP appears to protect against BNC formation, which is probably secondary to the meticulous apical dissection required to adequately spare the NVBs.
Acknowledgements
This clinical research was supported in part by the Urological Research Foundation, the prostate SPORE grant (P50 CA90386– 05S2) and the Robert H. Lurie Comprehensive Cancer Center (P30 CA60553).
Abbreviations
- (R)RP
(retropubic) radical prostatectomy
- BN(C)
bladder neck (contracture)
- VUA
vesico-urethral anastomosis
- NVB
neurovascular bundle
Footnotes
Conflict of Interest
None declared.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Park R, Martin S, Goldberg JD, Lepor H. Anastomotic strictures following radical prostatectomy. insights into incidence, effectiveness of intervention, effect on continence, and factors predisposing to occurrence. Urology. 2001;57:742–6. doi: 10.1016/s0090-4295(00)01048-7. [DOI] [PubMed] [Google Scholar]
- 3.Anger JT, Raj GV, Delvecchio FC, Webster GD. Anastomotic contracture and incontinence after radical prostatectomy: a graded approach to management. J Urol. 2005;173:1143–6. doi: 10.1097/01.ju.0000155624.48337.a5. [DOI] [PubMed] [Google Scholar]
- 4.Herr HW. Quality of life of incontinent men after radical prostatectomy. J Urol. 1994;151:652–4. doi: 10.1016/s0022-5347(17)35038-3. [DOI] [PubMed] [Google Scholar]
- 5.Kao TC, Cruess DF, Garner D, et al. Multicenter patient self-reporting questionnaire on impotence, incontinence and stricture after radical prostatectomy. J Urol. 2000;163:858–64. [PubMed] [Google Scholar]
- 6.Eastham JA, Kattan MW, Rogers E, et al. Risk factors for urinary incontinence after radical prostatectomy. J Urol. 1996;156:1707–13. [PubMed] [Google Scholar]
- 7.Kundu SD, Roehl KA, Eggener SE, Antenor JA, Han M, Catalona WJ. Potency, continence and complications in 3,477 consecutive radical retropubic prostatectomies. J Urol. 2004;172:2227–31. doi: 10.1097/01.ju.0000145222.94455.73. [DOI] [PubMed] [Google Scholar]
- 8.McCarthy JF, Catalona WJ. Nerve-sparing radical retropubic prostatectomy. In: Marshall FF, editor. Textbook of Operative Urology, chapt. 65. W.B. Saunders; Philadelphia: 1996. pp. 537–44. [Google Scholar]
- 9.Surya BV, Provet J, Johanson KE, Brown J. Anastomotic strictures following radical prostatectomy: risk factors and management. J Urol. 1990;143:755–8. doi: 10.1016/s0022-5347(17)40082-6. [DOI] [PubMed] [Google Scholar]
- 10.Borboroglu PG, Sands JP, Roberts JL, Amling CL. Risk factors for vesicourethral anastomotic stricture after radical prostatectomy. Urology. 2000;56:96–100. doi: 10.1016/s0090-4295(00)00556-2. [DOI] [PubMed] [Google Scholar]
- 11.Hu JC, Gold KF, Pashos CL, Mehta SS, Litwin MS. Role of surgeon Volume in radical prostatectomy outcomes. J Clin Oncol. 2003;21:401–5. doi: 10.1200/JCO.2003.05.169. [DOI] [PubMed] [Google Scholar]
- 12.Begg CB, Riedel ER, Bach PB, et al. Variations in morbidity after radical prostatectomy. N Engl J Med. 2002;346:1138–44. doi: 10.1056/NEJMsa011788. [DOI] [PubMed] [Google Scholar]
- 13.Hu JC, Wang Q, Pashos CL, Lipsitz SR, Keating NL. Utilization and outcomes of minimally invasive radical prostatectomy. J Clin Oncol. 2008;26:2278–84. doi: 10.1200/JCO.2007.13.4528. [DOI] [PubMed] [Google Scholar]
- 14.Walsh PC. Anatomic radical prostatectomy: evolution of the surgical technique. J Urol. 1998;160:2418–24. doi: 10.1097/00005392-199812020-00010. [DOI] [PubMed] [Google Scholar]
- 15.Orvieto MA, Zorn KC, Gofrit ON, Anderson J, Zagaja GP, Brendler CB. Surgical modifications in bladder neck reconstruction and vesicourethral anastomosis during radical retropubic prostatectomy to reduce bladder neck contractures. Can J Urol. 2006;13:3353–7. [PubMed] [Google Scholar]
- 16.Catalona WJ, Carvalhal GF, Mager DE, Smith DS. Potency, continence and complication rates in 1,870 consecutive radical retropubic prostatectomies. J Urol. 1999;162:433–8. [PubMed] [Google Scholar]
- 17.Selli C, De Antoni P, Moro U, et al. Role of bladder neck preservation in urinary continence following radical retropubic prostatectomy. Scand J Urol Nephrol. 2004;38:32–7. doi: 10.1080/00365590310017280. [DOI] [PubMed] [Google Scholar]
- 18.Srougi M, Paranhos M, Leite KM, Dall'Oglio M, Nesrallah L. The influence of bladder neck mucosal eversion and early urinary extravasation on patient outcome after radical retropubic prostatectomy: a prospective controlled trial. BJU Int. 2005;95:757–60. doi: 10.1111/j.1464-410X.2005.05395.x. [DOI] [PubMed] [Google Scholar]
- 19.Narayan P, Konety B, Aslam K, Aboseif S, Blumenfeld W, Tanagho E. Neuroanatomy of the external urethral sphincter: implications for urinary continence preservation during radical prostate surgery. J Urol. 1995;153:337–41. doi: 10.1097/00005392-199502000-00012. [DOI] [PubMed] [Google Scholar]