Abstract
Background
Although recent guidelines call for expanded routine screening for HIV, resources for antiretroviral treatment (ART) are limited and all eligible people are not currently being treated.
Objective
To evaluate the effects on the U.S. HIV epidemic of expanded ART, HIV screening, or interventions to reduce risk behavior.
Design
Dynamic mathematical model of HIV transmission and disease progression, and cost-effectiveness analysis.
Data Sources
Published literature.
Target Population
High-risk (injection drug users, men who have sex with men) and low-risk individuals aged 15 to 64 in the U.S.
Time Horizon
20 years and lifetime (costs and QALYs).
Perspective
Societal.
Interventions
Expanded HIV screening and counseling, treatment with ART, or both.
Outcome Measures
New HIV infections, discounted costs and quality-adjusted life years (QALYs), and incremental cost-effectiveness ratios.
Results Base-Case Analysis
One-time HIV screening of low-risk individuals coupled with annual screening of high-risk individuals could prevent 6.7% of a projected 1.23 million new infections and cost $22,382/QALY gained, assuming a 20% reduction in sexual activity post-screening. Expanding ART utilization to 75% of eligible individuals prevents 10.3% of infections and costs $20,300/QALY gained. A combination strategy prevents 17.3% of infections and costs $21,580/QALY gained.
Results Sensitivity Analysis
With no reduction in sexual activity, expanded screening prevents 3.7% of infections. Earlier ART initiation when CD4>350 cells/mL prevents 20–28% of infections. Additional efforts to halve high-risk behavior could reduce infections by 65%.
Limitations
Simplified model of disease progression and treatment; exclusion of acute HIV screening.
Conclusions
Expanding HIV screening and treatment simultaneously offers the greatest health benefit and is cost-effective. However, even substantial expansion of HIV screening and treatment programs is not sufficient to markedly reduce the U.S. HIV epidemic without significant reductions in risk behavior.
Primary Funding Source
National Institute on Drug Abuse, United States National Institutes of Health (R-01-DA-15612) and the United States Department of Veterans Affairs.
Introduction
Approximately 56,000 people in the United States acquire human immunodeficiency virus (HIV) annually, which has not decreased in recent years and highlights the need for expanded HIV screening and treatment (1, 2). Routine HIV screening facilitates early identification of HIV infection, linking infected individuals with access to life-saving treatments. HIV screening, if accompanied by an effective counseling program, may reduce sexual activity and other risky behavior among participants (3–7). Once identified, HIV-infected individuals eligible to receive antiretroviral therapy can benefit from substantially reduced mortality and improved quality of life. Moreover, suppressive antiretroviral therapy (ART) may reduce overall HIV transmission in the population by reducing a recipient’s blood plasma viral load and subsequent infectivity (8–14).
The United States Centers for Disease Control and Prevention (CDC) estimates that 21% of the approximately 1.1 million people living with HIV in the U.S. are unaware of their disease status, implying that expanded screening could directly benefit almost 250,000 people and their partners (15). In 2006, the CDC published revised guidelines calling for routine HIV screening in all healthcare settings of patients aged 13 to 64 years, regardless of potential risk behaviors, unless HIV prevalence is less than 0.1% among undiagnosed patients (3). Many other professional organizations have endorsed this policy (16), and the American College of Physicians recently advised routine screening of patients (17).
Prior studies have demonstrated that HIV screening is cost-effective. Older analyses focused on specific high-risk groups (18, 19) or settings with a relatively high prevalence (20). Because ART therapy is now much more effective than it was early in the epidemic, more recent studies show that HIV screening is cost-effective even in low-prevalence settings where HIV prevalence exceeds 0.1% to 0.2% (21–25), in patients older than 55 years of age (26), and with either conventional or rapid testing (24, 27). These studies demonstrate that the cost-effectiveness of screening is critically dependent on the availability of ART. Despite the 2009 Department of Health and Human Services guidelines recommending ART initiation in patients with CD4 counts between 350 and 500 cells/mL (28), universal ART utilization has not been fully realized. Between 2007 and 2009, the CDC’s Expanded Testing Initiative newly diagnosed 10,000 people with HIV, linking 75% to care (29). However, among individuals newly diagnosed with HIV, 36% develop AIDS within one year and 45% develop AIDS within three years (30), suggesting that many individuals who are linked to care have advanced HIV disease.
Because neither universal HIV screening nor universal treatment for identified individuals has been achieved yet, we evaluated how expanding either screening, treatment, or both would affect the HIV epidemic in the U.S. Unlike most prior analyses (21, 22, 26, 27), we used a modeling framework that can assess the impact of these programs on the entire U.S. population by tracking HIV transmission among multiple risk groups and the general population. Our goal was to understand whether expansion of screening, treatment, or both could significantly diminish the HIV epidemic in the U.S., and whether allocating resources to screening or to treatment was more effective and efficient. We also evaluated the impact of reductions in risk behavior on the epidemic.
Methods
Study Design
We extended a dynamic HIV epidemic model that we previously developed to estimate the health benefits and costs of expanded HIV screening and ART in the U.S. (31). We integrated epidemiologic, clinical, and economic data and calculated population-level outcomes that accrue from varying combinations of the two interventions. We calibrated our model to match empiric estimates of incidence and prevalence (Appendix). We estimated HIV prevalence, incidence, QALYs, and healthcare costs over a 20-year time horizon, using a societal perspective. Our base-case analysis estimates HIV infections averted and incremental cost-effectiveness ratios associated with implementing each program individually or jointly. Because the effects of HIV screening and treatment with ART on risk behavior and disease transmission are uncertain, we varied each intervention’s effectiveness. All costs are in 2009 U.S. dollars, and both costs and QALYs were discounted at an annual rate of 3% (32). We programmed the model using Matlab R2010a.
HIV Epidemic Model
Our HIV epidemic model captures HIV transmission and progression in the population (Appendix). We partitioned the adult population aged 15 to 64 years into compartments based on: gender; risk behavior (men who have sex with men (MSM), injection drug users (IDUs), low-risk individuals); HIV-infection status; CD4 cell count if infected; treatment status if infected (receiving ART, not receiving ART); screening status (unidentified, identified); and male circumcision status.
We included HIV transmission via heterosexual and homosexual contact and via needle-sharing associated with injection drug use. Heterosexual contact occurs within risk groups (e.g., both partners low-risk, both IDUs) and across groups (e.g., female IDU with low-risk male partner, low-risk female with MSM partner). We assumed proportional mixing, where individuals with many sexual partners are more likely to select a partner who similarly has many partners. We estimated the average annual number of sexual partnerships (33–42), average condom use (33, 38–44), and the transmission probability per partnership (8, 45–55), for each sexual behavior mode, based on published data. The model captures HIV transmission via needle-sharing in a similar manner, as a function of the annual number of injections (56–58), average needle-sharing rates (41, 44, 57, 59), and the probability of transmission per shared needle (57, 60, 61). The probability of HIV transmission between two individuals depends on the infected individual’s gender, disease status, and treatment status, and the uninfected individual’s gender and circumcision status. Finally, the model accounts for changes in risky behavior due to effective HIV screening and counseling (4, 5, 62, 63).
Upon acquiring HIV, individuals progress through a set of health states at a rate inversely proportional to the average time spent in each health state, based on a Markov model of the natural history of HIV infection (21). The health states are defined approximately according to an individual’s CD4 T-cell count: asymptomatic HIV (CD4 >350 cells/mL), symptomatic HIV (CD4 200–350 cells/mL), and AIDS (CD4 <200 cells/mL).
HIV Screening and Treatment
We considered alternative combinations of HIV screening and treatment by varying screening frequency, targeted risk groups, and ART utilization. Based on CDC guidelines (3), we considered one strategy offering one-time screening of low-risk individuals, which mostly detects prevalent cases, accompanied by annual screening of high-risk individuals, which detects prevalent cases initially and incident cases thereafter. For each strategy, we adjusted the appropriate model parameters to account for changes in sexual behavior and infection transmissibility. We assumed that HIV-infected individuals are eligible to initiate ART at a CD4 count of 350 cells/mL, although we also estimated the effect of initiating ART earlier (28). Individuals receiving ART benefit from reduced disease progression and mortality and improved quality of life (21, 64–67). The partners of infected individuals may also benefit because ART suppresses an individual’s viral load, thus reducing the chance of transmitting the virus. We assumed that ART reduces sexual infectivity by 90% (4, 5, 8, 45, 68–71) and injection drug use-related infectivity by 50% (21, 56). We varied these parameters widely in sensitivity analysis.
Before initiating an ART regimen, HIV-infected individuals must first be identified through an HIV screening program or via symptom-based case-finding. We assumed a standard rapid HIV testing protocol of an initial enzyme-linked immunosorbent assay (ELISA), followed by a Western blot test to confirm HIV infection. We included the effects of post-test counseling to accompany screening, and we assumed that only HIV-infected individuals reduce their sexual partnerships by 20% (4, 5, 62, 63), but varied this assumption in sensitivity analysis.
Target Populations
We assessed relevant demographic data for each risk group, including population sizes, HIV prevalence, entry and maturation rates, and mortality rates (Table 1). We populated the model using 2007 data and we numerically solved the dynamic system to estimate population compartment sizes over a 20-year time horizon. Initial HIV prevalence was 12.6% (MSM), 18.8% (MSM/IDUs), 12.9% (male IDUs), 17.3% (female IDUs), 0.10% (low-risk men), and 0.22% (low-risk women). Although HIV prevalence varies across geographic regions, we applied a U.S. average to serve as a basis for estimating the benefits of a national HIV screening and treatment campaign.
Table 1.
Summary of Key Model Parameters
| Parameter | Value | Range | Source |
|---|---|---|---|
| Demographics | |||
| Total population (15 to 64 years) | |||
| Male IDU | 1,000,000 | 0.5–1.5 million | Calculated (1, 72–75) |
| Male MSM | 4,057,194 | 3–6 million | Calculated (1, 72, 73, 76) |
| Male IDU/MSM | 300,000 | 200,000–500,000 | Calculated (1, 43, 72–77) |
| Male Other | 96,022,652 | 95–100 million | Calculated (1, 72, 73) |
| Female IDU | 450,000 | 300,00–600,000 | Calculated (1, 72–75) |
| Female Other | 101,632,781 | 100–105 million | Calculated (1, 72, 73) |
| HIV prevalence | |||
| Male IDU | 12.9% | 10–20% | Calculated (1, 72–75) |
| Male MSM | 12.6% | 5–20% | Calculated (1, 72, 73, 76) |
| Male IDU/MSM | 18.8% | 15–30% | Calculated (1, 43, 72–76) |
| Male Other | 0.10% | 0.05–0.25% | Calculated (1, 72, 73) |
| Female IDU | 17.3% | 15–30% | Calculated (1, 72–75) |
| Female Other | 0.22% | 0.10–0.40% | Calculated (1, 72, 73) |
| Mortality rate | |||
| Male | 0.0041 | 0.002–0.005 | (78) |
| Female | 0.0024 | 0.002–0.005 | (78) |
| IDU (excess) | 0.025 | 0–0.05 | (57) |
| Maturation rate | |||
| Male | 0.0111 | 0.01–0.02 | (73) |
| Female | 0.0122 | 0.01–0.02 | (73) |
| Entry rate | |||
| Male | 0.0227 | 0.01–0.05 | (73) |
| Female | 0.0213 | 0.01–0.05 | (73) |
| Sexual Transmission | |||
| Transmission probability per partnership | |||
| Heterosexual (FHIV+→MHIV−) | |||
| Asymptomatic HIV | 0.02 | 0.01–0.04 | (8, 45–52) |
| Symptomatic HIV | 0.03 | 0.01–0.04 | (8, 45–52) |
| AIDS | 0.05 | 0.03–0.06 | (8, 45–52) |
| Heterosexual (MHIV+→FHIV−) | |||
| Asymptomatic HIV | 0.03 | 0.02–0.05 | (8, 45–52) |
| Symptomatic HIV | 0.04 | 0.02–0.05 | (8, 45–52) |
| AIDS | 0.08 | 0.05–0.10 | (8, 45–52) |
| Homosexual (MHIV+→MHIV−) | |||
| Asymptomatic HIV | 0.04 | 0.03–0.06 | (49, 53–55) |
| Symptomatic HIV | 0.05 | 0.03–0.06 | (49, 53–55) |
| AIDS | 0.10 | 0.08–0.15 | (49, 53–55) |
| Annual number of same-sex partners | |||
| Male MSM | 3.0 | 2.0–5.0 | (37, 40–42) |
| Male IDU/MSM | 3.0 | 2.0–5.0 | (40–42) |
| Condom usage with same-sex partners | |||
| Male MSM | 40% | 30–60% | (37, 40–43) |
| Male IDU/MSM | 40% | 30–50% | (41) |
| Annual number of opposite-sex partners | |||
| Male IDU | 3.0 | 2.0–5.0 | (39) |
| Male MSM | 0.1 | 0–1.0 | (37) |
| Male IDU/MSM | 0.1 | 0–1.0 | (33) |
| Male Other | 1.1 | 0.5–2.0 | (34–38) |
| Female IDU | 3.5 | 2.0–5.0 | (39) |
| Female Other | 1.1 | 0.5–2.0 | (34–36, 38) |
| Condom usage with opposite-sex partners | |||
| Male IDU | 25% | 15–35% | (33, 43) |
| Male MSM | 30% | 20–50% | (41, 43) |
| Male IDU/MSM | 30% | 30–50% | (33, 41) |
| Male Other | 20% | 10–40% | (38) |
| Female IDU | 25% | 20–50% | (39, 44) |
| Female Other | 20% | 10–40% | (38) |
| Reduction in heterosexual HIV transmission due to male circumcision | 50% | 48–60% | (79–81) |
| Injection Drug Use Transmission | |||
| Transmission probability per shared injection | |||
| Asymptomatic HIV | 0.002 | 0.001–0.005 | (57, 60, 61) |
| Symptomatic HIV | 0.003 | 0.001–0.005 | (57, 60, 61) |
| AIDS | 0.003 | 0.001–0.005 | (57, 60, 61) |
| Average injections per year | 200 | 100–500 | (57, 58) |
| Fraction of injections that are shared | 20% | 10–40% | (41, 44, 57, 59) |
| HIV Screening | |||
| Fraction of population tested in past 12 months | |||
| High-risk individuals | 23% | 10–30% | (82) |
| Low-risk individuals | 10% | 5–20% | (82) |
| Annual probability of symptom-based case finding | |||
| HIV | 10% | 0–30% | (21) |
| AIDS | 20% | 10–60% | (21) |
| Reduction in sexual behavior among HIV+ identified individuals due to screening | 20% | 0–50% | (4, 5, 62, 63) |
| Antiretroviral Therapy | |||
| Fraction starting ART at CD4=350 cells/mL | 50% | 25–75% | Assumed (29, 83) |
| Annual ART entry rate if CD4<350 cells/mL | 0.05 | 0–0.10 | Assumed (83) |
| Reduction in injection infectivity due to ART | 50% | 25–75% | (21, 56) |
| Reduction in sexual infectivity due to ART | 90% | 50–99% | (8–14) |
| Quality-of-life Multipliers | |||
| Uninfected | 1.0 | --- | (84) |
| Asymptomatic HIV – Unidentified | 0.91 | 0.85–0.95 | (64, 65, 67, 85) |
| Asymptomatic HIV – Identified (Year 1) | 0.84 | 0.85–0.95 | (21, 64, 65, 67, 85) |
| Asymptomatic HIV – Identified (Years 2+) | 0.89 | 0.85–0.95 | (21, 64, 65, 67, 85) |
| Symptomatic HIV – Unidentified | 0.79 | 0.70–0.80 | (64, 65, 67, 85) |
| Symptomatic HIV – Identified | 0.72 | 0.70–0.80 | (64, 65, 67, 85) |
| Symptomatic HIV – Treated with ART | 0.83 | 0.82–0.87 | (64, 65, 67, 85) |
| AIDS – Unidentified | 0.72 | 0.60–0.75 | (64, 65, 67, 85) |
| AIDS – Identified | 0.72 | 0.60–0.75 | (64, 65, 67, 85) |
| AIDS – Treated with ART | 0.82 | 0.82–0.87 | (64, 65, 67, 85) |
| IDU (multiplier)* | 0.90 | 0.80–1.0 | (56, 57) |
| Costs (2009 USD) | |||
| Annual HIV-related healthcare costs | |||
| Asymptomatic HIV – Untreated | $4,125 | $3,000–$6,000 | (86, 87) |
| Symptomatic HIV – Untreated | $6,925 | $5,000–$9,000 | (86, 87) |
| Symptomatic HIV – Treated with ART | $6,174 | $5,000–$7,000 | (86, 87) |
| AIDS – Untreated | $21,838 | $15,000–$25,000 | (86–88) |
| AIDS – Treated with ART | $9,938 | $6,000–$17,000 | (21, 87) |
| Annual non-HIV-related healthcare costs | $7,576 | $5,000–$8,000 | (89) |
| Annual cost of ART | $15,571 | $12,000–$18,000 | (21, 22, 87, 88) |
| Cost of HIV ELISA antibody test | $12 | $10–20 | (90) |
| Cost of confirmatory Western Blot test | $19 | $10–50 | (90) |
| Cost of behavior counseling | $60 | $50–$100 | (21, 22, 24) |
| Annual cost of ancillary IDU services | $2,500 | $1,000–$4,000 | (57) |
| Annual discount rate | 3% | 0–5% | (32) |
IDU = Injection drug user, MSM = Men who have sex with men, Other = General population.
ART = Antiretroviral therapy, ELISA = enzyme linked immunosorbent assay.
Quality of life for all injection drug users is multiplied by this quantity.
We estimated that approximately 50% of infected individuals receive ART at CD4 <350 cells/mL (29, 30) and, thereafter, an additional 5% enter treatment regimens annually, resulting in approximately 85% of eligible individuals eventually on ART. We calculated an overall undiagnosed HIV prevalence of 0.11% and an annual incidence rate of 0.03% in our target populations.
Economic Model
We calculated net present health benefits (QALYs) and costs for each strategy. We estimated quality of life for each health state, and assessed the incremental gain in health benefits due to reduced morbidity and mortality. We measured lifetime health costs based on HIV disease progression and mortality rates, and the associated costs of each health state. Finally, we included the per person cost of HIV screening and counseling and the annual cost of ART. We calculated the incremental cost-effectiveness ratio (ICER) for each intervention, relative to the next-best alternative.
Role of the Funding Source
This work was supported by a grant from the National Institute on Drug Abuse, National Institutes of Health (R-01-DA-15612) and the Department of Veterans Affairs. Each organization had no role in the design, conduct, and reporting of the study.
Results
HIV Infections Prevented
Individual Interventions
Our model projects that approximately 1.23 million new HIV infections will occur over 20 years, with 74% occurring among high-risk individuals. Although MSM comprise 4% of adult men, they account for 49% of total new infections, similar to the CDC’s estimate of 53% (76).
One-time screening of low-risk individuals combined with annual screening of high-risk individuals prevents 81,991 infections (6.7% of the projected total), including 23,099 and 58,892 infections among low-risk and high-risk groups, respectively (Table 2). For comparison, targeted annual screening of only MSM could avert 58,513 infections, of which 95% are among MSM, although the feasibility of risk-based screening is questionable (3). Routine screening of all individuals every three years is less effective, preventing 2.3% of infections, because high-risk individuals are screened less frequently. A treatment-focused strategy that instead increases ART utilization (at CD4 <350 cells/mL) from 50% to 75% prevents 125,775 infections (10.3% of the total).
Table 2.
Population Benefits and Costs over 20 Years of Expanded HIV Screening and Treatment
| Strategy | HIV infections over 20 years* |
Incremental Costs (billions) |
Incremental QALYs (millions) |
ICER | |
|---|---|---|---|---|---|
| Status quo | Next best | ||||
| Status quo | 1,225,380 | --- | --- | --- | |
| Expanded screening only | |||||
| Screening (low-risk once, high-risk annually) | −81,991 (6.7%) | $26.9 | 1.2 | $22,382 | $22,382 |
| Screening (low-risk 3-years, high-risk annually) | −89,438 (7.3%) | $71.7 | 1.4 | $51,040 | Dominated |
| Screening (every 3 years) | −28,132 (2.3%) | $60.7 | 0.5 | $112,094 | Dominated |
| Screening (annually) | −94,538 (7.7%) | $225.7 | 1.6 | $143,930 | Dominated |
| Expanded ART only | |||||
| ART (75% utilization) | −125,775 (10.3%) | $63.8 | 3.1 | $20,300 | $20,300 |
| Combination screening and ART | |||||
| Screening (low-risk once, high-risk annually) and ART (75% utilization) | −212,291 (17.3%) | $92.6 | 4.5 | $20,682 | $21,580 |
| Screening (low-risk 3-years, high-risk annually) and ART (75% utilization) | −220,027 (18.0%) | $138.2 | 4.7 | $29,327 | $192,139 |
| Screening (every 3 years) and ART (75% utilization) | −155,958 (12.7%) | $126.5 | 3.8 | $33,488 | Dominated |
| Screening (annually) and ART (75% utilization) | −225,242 (18.4%) | $293.1 | 4.9 | $59,691 | $788,706 |
Under the status quo, the total number of projected infections over 20 years is given. For the remaining strategies, a negative number refers to infections prevented, compared to the status quo.
The values in parentheses are the fraction of total HIV infections prevented.
Incremental costs, quality-adjusted life years (QALYs) are relative to the status quo.
ICER = incremental cost-effectiveness ratio relative to the status quo or next-best alternative.
Dominated = the strategy is dominated by another strategy and is not an efficient use of resources.
Combination Interventions
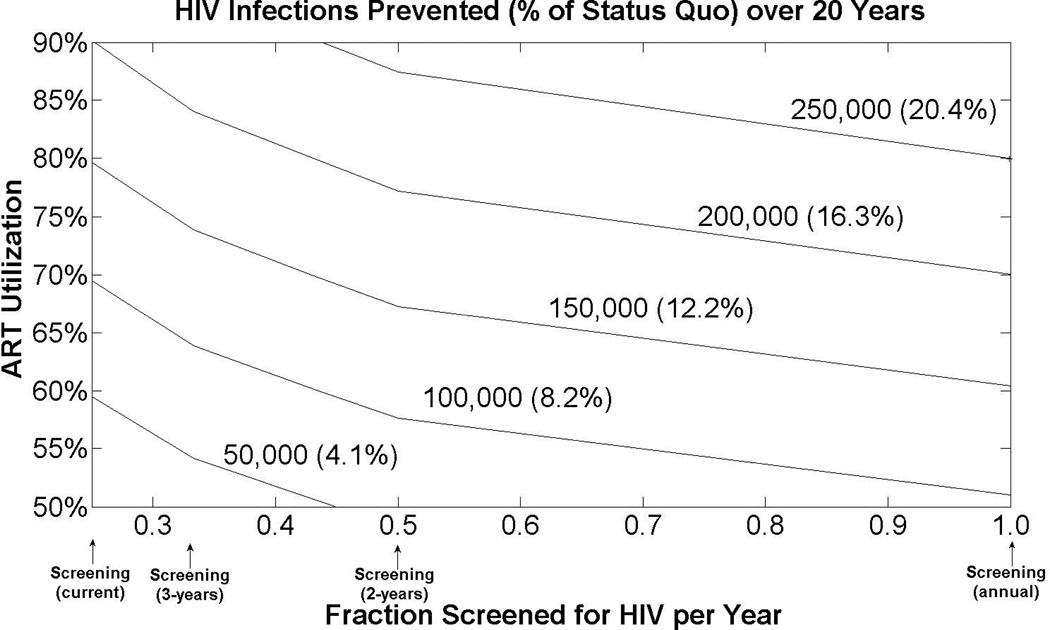
In general, we find that combination strategies that increase both screening and treatment prevent more HIV infections than the sum prevented from each individual strategy (Figure 1). A joint strategy that simultaneously offers one-time screening to low-risk individuals and annual screening of high-risk individuals, and increases ART utilization to 75% prevents 212,291 infections (17.3% of the total).
Figure 1. Complementary Effects of Expanded HIV Screening and Treatment.
The horizontal axis displays varying levels of HIV screening (current levels, every three years, every two years, or annually), and the vertical axis displays antiretroviral therapy (ART) utilization levels (50%, 60%, 70%, 80%, or 90% utilization). The curves are iso-contours showing a given number (and fraction) of HIV infections prevented over 20 years, compared to the status quo. The point at the origin corresponds to current screening and treatment levels. Assumes HIV screening reduces sexual behavior by 20% among HIV+ identified individuals, and treatment with ART reduces sexual infectivity by 90%. Under the status quo, an estimated 1.23 million HIV infections occur over 20 years.
We also examined whether a universal annual HIV screening and treatment with ART (with 90% utilization) could theoretically eliminate the HIV epidemic within the next two decades. Such a strategy could prevent 24% of new infections, but would fail to prevent more than 40,000 new infections each year. To substantially reduce HIV incidence, expanded screening and treatment programs must be augmented with programs aimed at reducing risky sexual behavior. HIV incidence drops by 65% if in addition to universal annual HIV screening and 90% ART utilization, high-risk MSM halve their number of sexual partners and IDUs halve their needle-sharing frequency.
False Diagnoses
With annual screening of high-risk individuals and one-time screening of low-risk individuals, a number of false positive and false negative diagnoses will occur. We assumed a testing sequence sensitivity of 0.995 and specificity of 0.999994 (21). In the first year of the intervention, approximately 1,162 false positives and 1,034 false negatives occur, but these decrease to 176 and 189 per year, respectively, over 20 years because low-risk individuals are screened only once with this strategy. High-risk individuals with a false negative test will likely be detected on a future annual screening.
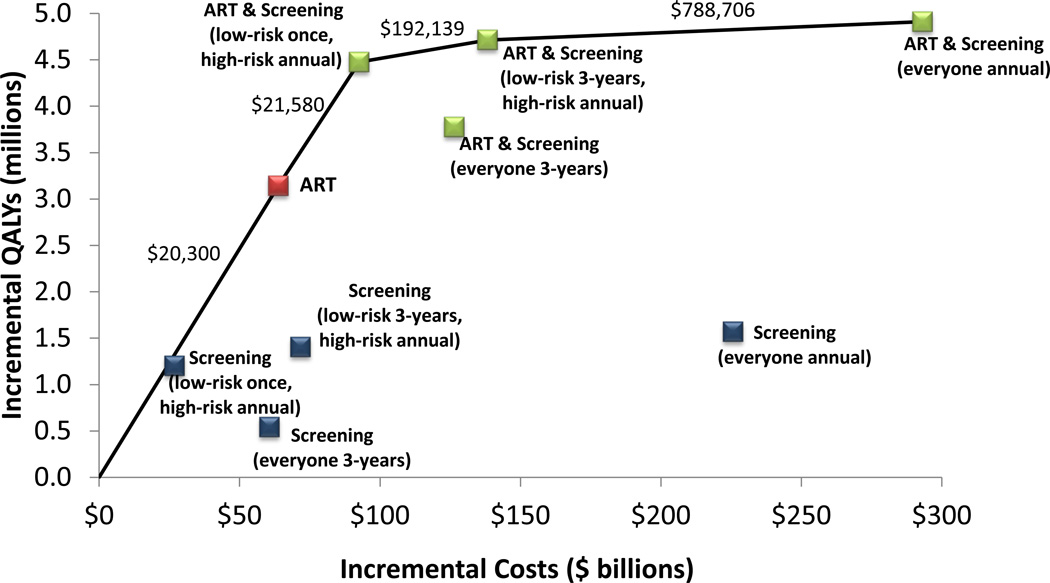
Cost-effectiveness Analysis
In general, strategies that target screening frequency based on risk status, and/or expand ART are cost-effective (Figure 2 and Table 2). One-time screening of low-risk groups and annual screening of high-risk groups adds 1.2 million QALYs over 20 years, at a cost of $26.9 billion (discounted), or $22,382/QALY gained, compared to the status quo. During its first year, this program costs $12.4 billion because of the one-time screening component. Routine screening every three years among all risk groups adds 540,000 QALYs and has an ICER of $112,094/QALY gained compared to the status quo. This strategy is less effective and more expensive than the former strategy. Annual HIV screening of all adults is less cost-effective at $143,930/QALY gained.
Figure 2. Cost-Effectiveness of Alternative Screening and Treatment Strategies.
Incremental costs and quality-adjusted life years (QALYs) of expanded HIV screening and counseling, expanded access to antiretroviral therapy (ART), or a combination of screening and ART. Expanded screening occurs once, annually, or every three years. Expanded ART includes treatment utilization of 75%. The cost-effectiveness frontier (solid line) includes strategies that may be cost-effective if the incremental cost-effectiveness ratio (ICER) is less than the accepted threshold. Strategies that are not on the frontier are dominated, meaning that these are not an efficient use of resources. Costs and QALYs are calculated over a 20-year time horizon and are discounted to the present.
Alternatively, increasing ART utilization to 75% adds 3.1 million QALYs with an ICER of $20,300/QALY gained compared to the status quo. A combination program of one-time low-risk and annual high-risk screening and 75% ART utilization adds 4.5 million QALYs, more than the sum of implementing each program separately, at a cost of $92.6 billion over 20 years ($12.9 billion in the first year), or $21,580/QALY gained compared to expanded treatment only (Table 2).
Sensitivity Analysis
Health outcomes were most sensitive to changes in screening and treatment effectiveness. The effect of viral resistance and other sensitivity analyses are discussed in the Appendix.
Screening Effectiveness
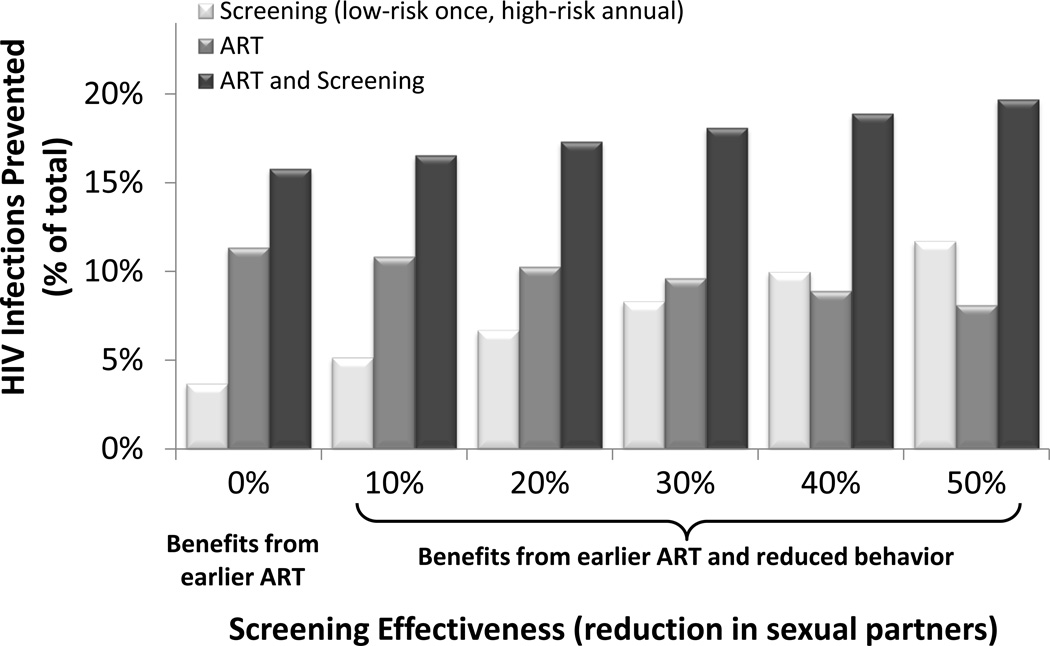
Expanded HIV screening can reduce new infections via two mechanisms: 1) infected individuals are identified at an earlier stage and can initiate treatment sooner, thereby reducing the chance of infecting their partners, and 2) screened individuals may reduce their sexual behavior due to counseling. In the base case, we assumed that both mechanisms are in effect, but that only HIV-infected individuals reduce their risky behavior after screening and counseling.
Under these assumptions, one-time low-risk and annual high-risk screening prevents 6.7% of projected HIV infections. With no reduction in sexual partners after screening, nearly 4% of new infections are still prevented due to identified individuals initiating ART at an earlier stage (Figure 3). This strategy remains cost-effective ($31,615 /QALY gained).
Figure 3. Fraction of HIV Infections Prevented with Varying HIV Screening Effectiveness.
Each bar corresponds to the fraction of HIV infections prevented over 20 years, with varying degrees of screening effectiveness at reducing sexual partners among HIV+ identified individuals (base case: 20%). The benefits from earlier ART are due to infected individuals who are identified through a screening program and can initiate treatment, thereby reducing their infectivity. The benefits from reduced behavior result from reduced sexual partnerships among HIV+ identified individuals – and hence reduced HIV transmission – following screening and counseling. Expanded screening occurs once for low-risk individuals and annually for high-risk individuals. Expanded ART includes treatment utilization of 75%. Under the status quo, an estimated 1.23 million HIV infections occur over 20 years.
Conversely, a counseling program that successfully reduces partnerships by 50% prevents a significant number of new infections (11.7%) with one-time low-risk and annual high-risk screening, and costs $16,321/QALY gained. Under this assumption, universal screening every year costs approximately $100,000/QALY gained.
ART Effectiveness
We considered variations in ART’s effectiveness at reducing infectivity via sexual transmission. If ART reduces infectivity by 50% instead of 90%, then expanded ART utilization prevents 59,281 infections over 20 years (versus 125,775 in the base case) and costs $27,585/QALY gained. With no reduction in sexual infectivity, expanding ART costs $41,367/ QALY gained because of the enormous gain in health benefits from reduced morbidity and mortality among infected individuals. The cost-effectiveness of expanded treatment improves to $16,000/QALY gained if ART reduces sexual and needle-sharing infectivity completely. Combination screening and treatment cost between $19,000/QALY and $42,000/QALY gained if ART is more or less effective, respectively, compared to the next-best alternative.
ART Starting CD4 Count
Based on recent guidelines recommending earlier treatment of HIV in some patients (28), we considered variations in CD4 count at initiation of ART. Because the effect on mortality of ART initiation at a CD4 <500 cells/mL is uncertain, we performed exploratory analysis assuming a 50% survival gain with early initiation of ART (91). If 5% of asymptomatic HIV-infected individuals enter treatment programs each year, more than twice as many (20.6%) HIV infections are prevented (Table 2). Under this scenario, 30% of individuals with CD4 >350 cells/mL would be receiving ART after ten years. If 10% of asymptomatic patients begin treatment each year (i.e., 50% receive ART after ten years), 28% of new infections are prevented.
Epidemic Elimination
We assessed whether the HIV epidemic could theoretically be eliminated under the following optimistic assumptions: annual screening of all risk groups, 50% reduction in sexual partners among HIV-infected status-aware individuals, 100% ART utilization at CD4 <350 cells/mL, and rapid scale-up of ART for CD4>350 cells/mL (60% after one year, 80% after two years, 95% after four years). This prevents 69% of new infections, but 18,000 infections still occur each year, with over 60% occurring among IDUs, largely because ART only reduces needle-sharing infectivity by 50%. If both sexual and needle-sharing infectivity are reduced by 90%, then 9,000 infections occur each year. Even with these unrealistic assumptions, it is not possible to eliminate the HIV epidemic in the United States without additional concomitant preventive measures and/or behavior modification.
Initial HIV Prevalence
In general, as HIV prevalence increases, expanded screening becomes more cost-effective and vice versa. With 50% lower HIV prevalence levels, expanded screening (one-time low-risk, annual high-risk) costs $31,789/QALY gained (versus $22,382 in the base case) but is dominated by expanded ART, which costs $19,829/QALY gained. Conversely, if HIV prevalence is doubled, both strategies cost less than $22,000/QALY gained. HIV screening is more sensitive to variations in prevalence because of the higher cost of detecting an infected person in a low-prevalence U.S. setting.
Discussion
Our study aimed to assess the population-wide effects of expanded HIV treatment and screening on the HIV epidemic in the U.S. Although prior studies have addressed the effectiveness and cost-effectiveness of either expanded HIV screening (21, 22, 24, 26) or treatment (92–94), those analyses were not designed to fully evaluate how such programs would influence HIV transmission in the overall population or the course of the epidemic. Our study is the first to evaluate the population-wide effects (new HIV infections and other health outcomes) and the cost-effectiveness of alternative combinations of HIV screening and treatment in the U.S.
Our study has several key findings. First, we find that expanding HIV screening and treatment could prevent 200,000 to 300,000 infections over 20 years, or approximately 17% to 24% of new infections, adding up to 6.8 million quality-adjusted life years to the population. To prevent 24% of new infections, routine HIV screening would need to occur annually, with antiretroviral treatment available for essentially all symptomatic patients. Our analysis assumes that people identified as HIV-infected reduce risk behaviors by 20%; even with modest reductions in risk behavior, expansion of screening and treatment would provide enormous health benefit. If HIV-infected people reduce risk behavior further as some studies suggest (7), the health benefit could be substantially higher than we have estimated. Annual HIV screening and counseling leading to 50% behavior reduction in infected individuals, along with 90% ART initiation in symptomatic patients, reduces new infections to fewer than 35,000 per year. Even under such optimistic assumptions, the U.S. HIV epidemic is unlikely to be completely eliminated without additional preventive measures.
Second, our analysis highlights the importance of emphasizing risk behavior reduction as HIV screening and treatment becomes increasingly available. For example, in addition to expanded screening and treatment, a 50% reduction in sexual risk behaviors among MSM and needle-sharing among IDUs could prevent 65% of new infections, reducing HIV incidence to approximately 20,000 cases per year. This suggests that programs to reduce risk behavior among high-risk individuals are likely to be a key component of a successful prevention program. If, however, uninfected individuals increase risk behavior post-screening, some of the benefits would be attenuated.
Our third finding is that the net benefit of implementing both interventions is greater than the sum from implementing each program individually. A substantial increase in HIV screening or treatment could prevent 95,000 or 198,000 new infections, respectively, whereas a combination program could avert 300,000 infections (a gain of 7,000 infections prevented or 2%). Programs to expand screening and treatment will be most effective if they are implemented together, because this realizes the complementary effects of each program. Essential to achieving these levels of infections averted is patient receipt of test results following diagnosis, as well as linkage to care, which has been shown to improve with nurse-initiated counseling (95) and health worker follow-up interviews with newly diagnosed patients (96).
The effectiveness of screening and counseling in reducing sexual activity will likely vary within healthcare settings due to differences in risk behavior, and the length, content, and intensity of counseling services. Even with no reduction in risk behavior, one-time screening of low-risk groups and annual screening of high-risk groups could prevent nearly 4% of new infections, by identifying infected individuals and linking them to treatment programs. Augmenting this strategy with expanded ART prevents 16% of new infections. This suggests that preventing future infections through increased ART becomes increasingly important as the effectiveness of screening and counseling diminishes. In settings where counseling is unavailable or ineffectual, increased utilization of ART can help ensure that expanded screening will lead to reductions in HIV transmission.
Finally, we find that expanded utilization of ART (to 75% or 90% initiating ART at CD4 <350 cells/mL) is very cost-effective, as is one-time screening of low-risk groups and annual screening of high-risk groups. Combination strategies prevent more HIV infections and increase QALYs more than either individual strategy. As noted, our analysis specifically accounts for the effect of combination screening and treatment on population-wide HIV transmission, which is a strength of our modeling framework. As routine HIV screening for adults increases across healthcare settings due to recently revised CDC guidelines (3), it is important to ensure that ART utilization increases at a concomitant rate. Further expanding HIV screening and counseling services, without expanding the proportion of infected individuals on ART, fails to realize the potential benefits of implementing these two complementary interventions.
Compared to other disease screening programs in the United States, one-time HIV screening of low-risk individuals and annual screening of high-risk individuals is economically attractive with a cost-effectiveness ratio less than $23,000/QALY gained. This compares favorably with other accepted interventions, including screening for type-2 diabetes (97) and breast cancer mammography (98).
Our study has several limitations. First, we assumed proportional mixing among sexual partners and needle-sharing contacts, which simplifies the complex network structure of partnership formation and dissolution. Second, although we stratified the population according to gender and risk behavior, we did not include variations by race or ethnicity. To fully account for such granularity, we would need to accurately estimate sexual and needle-sharing behavior within and between races, which would be difficult to obtain. Moreover, significant disparities in treatment rates, background mortality, and co-morbidities exist, and our model cannot account for these additional factors. A third limitation of our study is the omission of acute HIV screening, which would require a different model structure and specific assumptions about the benefits and costs associated with identification and treatment of acute HIV infection. The degree to which acute infection contributes to transmission is uncertain and estimates vary (47, 99–103). Fourth, we used a simplified HIV treatment model that does not include the intricacies of individual HIV disease management, drug toxicities, CD4 monitoring, or the presence of co-morbidities such as coronary heart disease, diabetes, and various cancers. Our results, however, are broadly consistent with those from more complicated models of HIV disease progression (21–24, 92, 104). Finally, we did not explicitly model development of resistance to ART, although we believe our assumptions about the benefits of ART are conservative given the introduction of new classes of antiretroviral therapy, such as integrase inhibitors and entry inhibitors, and we evaluated scenarios that included resistance in sensitivity analyses.
Expanded HIV screening and counseling in the United States can prevent a substantial number of new HIV infections, adding millions of QALYs to the population. Programs that simultaneously expand antiretroviral therapy utilization can prevent more HIV infections than expanding either intervention alone. Our analysis indicates that over the next two decades, HIV incidence in the U.S. could be reduced by 24% with a comprehensive expansion of screening and treatment. If these programs are accompanied by additional interventions that halve risky sexual and needle-sharing behavior, the epidemic could be reduced by 65%, suggesting the need for a comprehensive portfolio of HIV prevention, screening, and treatment.
Acknowledgment
This work was supported by a grant from the National Institute on Drug Abuse, United States National Institutes of Health (R-01-DA-15612) and the United States Department of Veterans Affairs.
Footnotes
This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to www.annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.
Research Material Availability
Protocol: not available
Statistical Code: Additional details may be available with written consent by contacting Dr. Long at elisa.long@yale.edu, although the exact code is not available in the public domain.
Data: not available
Contributor Information
Elisa F. Long, Yale School of Management, New Haven, Connecticut
Margaret L. Brandeau, Department of Management Science and Engineering, Stanford University, Stanford, California
Douglas K. Owens, Department of Veterans Affairs Palo Alto Health Care System, Palo Alto, California Center for Primary Care and Outcomes Research, Stanford University, Stanford, California
References
- 1.Centers for Disease Control and Prevention (CDC) Estimates of New HIV Infections in the United States. [Accessed August 1, 2010];2008 http://www.cdc.gov/hiv/topics/surveillance/resources/factsheets/incidence.htm.
- 2.Hall HI, Song R, Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300(5):520–529. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1–17. [PubMed] [Google Scholar]
- 4.The National Institute of Mental Health (NIMH) Multisite HIV Prevention Trial Group. The NIMH Multisite HIV Prevention Trial: reducing HIV sexual risk behavior. Science. 1998;280(5371):1889–1894. doi: 10.1126/science.280.5371.1889. [DOI] [PubMed]
- 5.Kamb ML, Fishbein M, Douglas JM, Jr, et al. Efficacy of risk-reduction counseling to prevent human immunodeficiency virus and sexually transmitted diseases: a randomized controlled trial. Project RESPECT Study Group. JAMA. 1998;280(13):1161–1167. doi: 10.1001/jama.280.13.1161. [DOI] [PubMed] [Google Scholar]
- 6.Koblin B, Chesney M, Coates T. Effects of a behavioural intervention to reduce acquisition of HIV infection among men who have sex with men: the EXPLORE randomised controlled study. Lancet. 2004;364(9428):41–50. doi: 10.1016/S0140-6736(04)16588-4. [DOI] [PubMed] [Google Scholar]
- 7.Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005;39(4):446–453. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- 8.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342(13):921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 9.Vernazza P, Hirschel B, Bernasconi E, Flepp M. Les personnes seropositives ne souff rant d'aucune autre MST et suivant un traitment antiretroviral effi cie ne transmettent pas le VIH par voie sexuelle. Bull Med Suisses. 2008;89:165–169. [Google Scholar]
- 10.Castilla J, Del Romero J, Hernando V, Marincovich B, Garcia S, Rodriguez C. Effectiveness of highly active antiretroviral therapy in reducing heterosexual transmission of HIV. J Acquir Immune Defic Syndr. 2005;40(1):96–101. doi: 10.1097/01.qai.0000157389.78374.45. [DOI] [PubMed] [Google Scholar]
- 11.Porco TC, Martin JN, Page-Shafer KA, et al. Decline in HIV infectivity following the introduction of highly active antiretroviral therapy. AIDS. 2004;18(1):81–88. doi: 10.1097/01.aids.0000096872.36052.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vernazza PL, Troiani L, Flepp MJ, et al. Potent antiretroviral treatment of HIV-infection results in suppression of the seminal shedding of HIV. The Swiss HIV Cohort Study. AIDS. 2000;14(2):117–121. doi: 10.1097/00002030-200001280-00006. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Dornadula G, Beumont M, et al. Human immunodeficiency virus type 1 in the semen of men receiving highly active antiretroviral therapy. N Engl J Med. 1998;339(25):1803–1809. doi: 10.1056/NEJM199812173392502. [DOI] [PubMed] [Google Scholar]
- 14.Gallant JE, Staszewski S, Pozniak AL, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292(2):191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention (CDC) HIV and AIDS in the United States. [Accessed August 1, 2010];2010 http://www.cdc.gov/hiv/resources/factsheets/us.htm.
- 16.Bartlett JG, Branson BM, Fenton K, Hauschild BC, Miller V, Mayer KH. Opt-out testing for human immunodeficiency virus in the United States: progress and challenges. JAMA. 2008;300(8):945–951. doi: 10.1001/jama.300.8.945. [DOI] [PubMed] [Google Scholar]
- 17.Qaseem A, Snow V, Shekelle P, Hopkins R, Jr, Owens DK. Screening for HIV in health care settings: a guidance statement from the American College of Physicians and HIV Medicine Association. Ann Intern Med. 2009;150(2):125–131. doi: 10.7326/0003-4819-150-2-200901200-00300. [DOI] [PubMed] [Google Scholar]
- 18.Brandeau ML, Owens DK, Sox CH, Wachter RM. Screening women of childbearing age for human immunodeficiency virus. A cost-benefit analysis. Arch Intern Med. 1992;152(11):2229–2237. [PubMed] [Google Scholar]
- 19.Brandeau ML, Owens DK, Sox CH, Wachter RM. Screening women of childbearing age for human immunodeficiency virus. A model-based policy analysis. Manage Sci. 1993;39:72–92. [Google Scholar]
- 20.Owens DK, Nease RF, Jr, Harris RA. Cost-effectiveness of HIV screening in acute care settings. Arch Intern Med. 1996;156(4):394–404. [PubMed] [Google Scholar]
- 21.Sanders GD, Bayoumi AM, Sundaram V, et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med. 2005;352(6):570–585. doi: 10.1056/NEJMsa042657. [DOI] [PubMed] [Google Scholar]
- 22.Paltiel AD, Weinstein MC, Kimmel AD, et al. Expanded screening for HIV in the United States--an analysis of cost-effectiveness. N Engl J Med. 2005;352(6):586–595. doi: 10.1056/NEJMsa042088. [DOI] [PubMed] [Google Scholar]
- 23.Walensky RP, Freedberg KA, Weinstein MC, Paltiel AD. Cost-effectiveness of HIV testing and treatment in the United States. Clin Infect Dis. 2007;45 Suppl 4:S248–S254. doi: 10.1086/522546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paltiel AD, Walensky RP, Schackman BR, et al. Expanded HIV screening in the United States: effect on clinical outcomes, HIV transmission, and costs. Ann Intern Med. 2006;145(11):797–806. doi: 10.7326/0003-4819-145-11-200612050-00004. [DOI] [PubMed] [Google Scholar]
- 25.Owens DK, Sundaram V, Lazzeroni LC, et al. Prevalence of HIV infection among inpatients and outpatients in Department of Veterans Affairs health care systems: implications for screening programs for HIV. Am J Public Health. 2007;97(12):2173–2178. doi: 10.2105/AJPH.2007.110700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanders GD, Bayoumi AM, Holodniy M, Owens DK. Cost-effectiveness of HIV screening in patients older than 55 years of age. Ann Intern Med. 2008;148(12):889–903. doi: 10.7326/0003-4819-148-12-200806170-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanders GD, Anaya HD, Asch S, et al. Cost-effectiveness of strategies to improve HIV testing and receipt of results: economic analysis of a randomized controlled trial. J Gen Intern Med. 2010 doi: 10.1007/s11606-010-1265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.U.S. Department of Health and Human Services Panel on Clinical Practices for Treatment of HIV Infection. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2009 doi: 10.1310/hct.2000.1.1.008. [DOI] [PubMed]
- 29.Centers for Disease Control and Prevention (CDC) CDC's HIV Prevention Progress in the United States. [Accessed August 1, 2010];2010 http://www.cdc.gov/hiv/resources/factsheets/PDF/cdcprev.pdf.
- 30.Centers for Disease Control and Prevention (CDC) Late HIV testing - 34 states, 1996–2005. MMWR Morb Mortal Wkly Rep. 2009;58(24):661–665. [PubMed]
- 31.Long EF, Brandeau ML, Owens DK. Potential population health outcomes and expenditures of HIV vaccination strategies in the United States. Vaccine. 2009;27(39):5402–5410. doi: 10.1016/j.vaccine.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 33.Bacon O, Lum P, Hahn J, et al. Commercial sex work and risk of HIV infection among young drug-injecting men who have sex with men in San Francisco. Sex Transm Dis. 2006;33(4):228–234. doi: 10.1097/01.olq.0000204914.91923.ad. [DOI] [PubMed] [Google Scholar]
- 34.Brisson M, Boily MC, Masse BR, Adrien A, Leaune V. Highlights of the sexual activity of the heterosexual population in the province of Quebec. Sex Transm Infect. 1999;75(5):296–299. doi: 10.1136/sti.75.5.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fryar CD, Hirsch R, Porter KS, Kottiri B, Brody DJ, Louis T. Drug use and sexual behaviors reported by adults: United States, 1999–2002. Adv Data. 2007;(384):1–14. [PubMed] [Google Scholar]
- 36.Johnson AM, Mercer CH, Erens B, et al. Sexual behaviour in Britain: partnerships, practices, and HIV risk behaviours. Lancet. 2001;358(9296):1835–1842. doi: 10.1016/S0140-6736(01)06883-0. [DOI] [PubMed] [Google Scholar]
- 37.Pathela P, Hajat A, Schillinger J, Blank S, Sell R, Mostashari F. Discordance between sexual behavior and self-reported sexual identity: a population-based survey of New York City men. Ann Intern Med. 2006;145(6):416–425. doi: 10.7326/0003-4819-145-6-200609190-00005. [DOI] [PubMed] [Google Scholar]
- 38.Davis JO, Smith TW. (National Opinion Research Center) General Social Surveys (GSS), 1972–2008. 2009 [Google Scholar]
- 39.Tyndall MW, Patrick D, Spittal P, Li K, O'Shaughnessy MV, Schechter MT. Risky sexual behaviours among injection drugs users with high HIV prevalence: implications for STD control. Sex Transm Infect. 2002;78 Suppl 1:i170–i175. doi: 10.1136/sti.78.suppl_1.i170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harawa NT, Greenland S, Bingham TA, et al. Associations of race/ethnicity with HIV prevalence and HIV-related behaviors among young men who have sex with men in 7 urban centers in the United States. J Acquir Immune Defic Syndr. 2004;35(5):526–536. doi: 10.1097/00126334-200404150-00011. [DOI] [PubMed] [Google Scholar]
- 41.Kral AH, Lorvick J, Ciccarone D, et al. HIV prevalence and risk behaviors among men who have sex with men and inject drugs in San Francisco. J Urban Health. 2005;82(1) Suppl 1:i43–i50. doi: 10.1093/jurban/jti023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacKellar DA, Valleroy LA, Behel S, et al. Unintentional HIV exposures from young men who have sex with men who disclose being HIV-negative. AIDS. 2006;20(12):1637–1644. doi: 10.1097/01.aids.0000238410.67700.d1. [DOI] [PubMed] [Google Scholar]
- 43.Rietmeijer CA, Wolitski RJ, Fishbein M, Corby NH, Cohn DL. Sex hustling, injection drug use, and non-gay identification by men who have sex with men. Associations with high-risk sexual behaviors and condom use. Sex Transm Dis. 1998;25(7):353–360. doi: 10.1097/00007435-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 44.Spittal PM, Craib KJ, Wood E, et al. Risk factors for elevated HIV incidence rates among female injection drug users in Vancouver. CMAJ. 2002;166(7):894–899. [PMC free article] [PubMed] [Google Scholar]
- 45.Abbas UL, Anderson RM, Mellors JW. Potential impact of antiretroviral therapy on HIV-1 transmission and AIDS mortality in resource-limited settings. J Acquir Immune Defic Syndr. 2006;41(5):632–641. doi: 10.1097/01.qai.0000194234.31078.bf. [DOI] [PubMed] [Google Scholar]
- 46.Downs AM, de Vincenzi I. Probability of heterosexual transmission of HIV: relationship to the number of unprotected sexual contacts. European Study Group in Heterosexual Transmission of HIV. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;11:388–395. doi: 10.1097/00042560-199604010-00010. [DOI] [PubMed] [Google Scholar]
- 47.Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. J Infect Dis. 2008;198:687–693. doi: 10.1086/590501. [DOI] [PubMed] [Google Scholar]
- 48.Kaplan EH. Modeling HIV infectivity: must sex acts be counted? J Acquir Immune Defic Syndr. 1990;3:55–61. [PubMed] [Google Scholar]
- 49.Mastro TD, de Vincenzi I. Probabilities of sexual HIV-1 transmission. AIDS. 1996;10 Suppl A:75–82. doi: 10.1097/00002030-199601001-00011. [DOI] [PubMed] [Google Scholar]
- 50.Nicolosi A, Correa Leite ML, Musicco M, Arici C, Gavazzeni G, Lazzarin A. The efficiency of male-to-female and female-to-male sexual transmission of the human immunodeficiency virus: a study of 730 stable couples. Italian Study Group on HIV Heterosexual Transmission. Epidemiology. 1994;5:570–575. doi: 10.1097/00001648-199411000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Padian NS, Shiboski SC, Glass SO, Vittinghoff E. Heterosexual transmission of human immunodeficiency virus (HIV) in northern California: results from a ten-year study. Am J Epidemiol. 1997;146:350–357. doi: 10.1093/oxfordjournals.aje.a009276. [DOI] [PubMed] [Google Scholar]
- 52.Wawer MJ, Gray RH, Sewankambo NK, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 53.Caceres CF, van Griensven GJ. Male homosexual transmission of HIV-1. AIDS. 1994;8:1051–1061. doi: 10.1097/00002030-199408000-00004. [DOI] [PubMed] [Google Scholar]
- 54.Jacquez JA, Koopman JS, Simon CP, Longini IM. Role of the primary infection in epidemics of HIV infection in gay cohorts. J Acquir Immune Defic Syndr. 1994;7:1169–1184. [PubMed] [Google Scholar]
- 55.Vittinghoff E, Douglas J, Judson F, McKirnan D, MacQueen K, Buchbinder SP. Per-contact risk of human immunodeficiency virus transmission between male sexual partners. Am J Epidemiol. 1999;150:306–311. doi: 10.1093/oxfordjournals.aje.a010003. [DOI] [PubMed] [Google Scholar]
- 56.Long EF, Brandeau ML, Galvin CM, et al. Effectiveness and cost-effectiveness of strategies to expand antiretroviral therapy in St. Petersburg, Russia. AIDS. 2006;20(17):2207–2215. doi: 10.1097/QAD.0b013e328010c7d0. [DOI] [PubMed] [Google Scholar]
- 57.Zaric GS, Barnett PG, Brandeau ML. HIV transmission and the cost-effectiveness of methadone maintenance. Am J Public Health. 2000;90(7):1100–1111. doi: 10.2105/ajph.90.7.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taylor A, Hutchinson S, Lingappa J, et al. Severe illness and death among injecting drug users in Scotland: a case-control study. Epidemiol Infect. 2005;133(2):193–204. doi: 10.1017/s0950268804003504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harris ZK. Efficient allocation of resources to prevent HIV infection among injection drug users: the Prevention Point Philadelphia (PPP) needle exchange program. Health Econ. 2006;15(2):147–158. doi: 10.1002/hec.1021. [DOI] [PubMed] [Google Scholar]
- 60.Wall SD, Olcott EW, Gerberding JL. AIDS risk and risk reduction in the radiology department. AJR Am J Roentgenol. 1991;157(5):911–917. doi: 10.2214/ajr.157.5.1927808. [DOI] [PubMed] [Google Scholar]
- 61.Kaplan EH, Heimer R. A model-based estimate of HIV infectivity via needle sharing. J Acquir Immune Defic Syndr. 1992;5(11):1116–1118. [PubMed] [Google Scholar]
- 62.Cleary PD, Van Devanter N, Rogers TF, et al. Behavior changes after notification of HIV infection. Am J Public Health. 1991;81(12):1586–1590. doi: 10.2105/ajph.81.12.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Higgins DL, Galavotti C, O'Reilly KR, et al. Evidence for the effects of HIV antibody counseling and testing on risk behaviors. JAMA. 1991;266(17):2419–2429. [PubMed] [Google Scholar]
- 64.Honiden S, Sundaram V, Nease RF, et al. The effect of diagnosis with HIV infection on health-related quality of Life. Qual Life Res. 2006;15(1):69–82. doi: 10.1007/s11136-005-8485-x. [DOI] [PubMed] [Google Scholar]
- 65.Tengs TO, Lin TH. A meta-analysis of utility estimates for HIV/AIDS. Med Decis Making. 2002;22(6):475–481. doi: 10.1177/0272989X02238300. [DOI] [PubMed] [Google Scholar]
- 66.Joyce VR, Barnett PG, Bayoumi AM, et al. Health-related quality of life in a randomized trial of antiretroviral therapy for advanced HIV disease. J Acquir Immune Defic Syndr. 2009;50(1):27–36. doi: 10.1097/QAI.0b013e31818ce6f3. [DOI] [PubMed] [Google Scholar]
- 67.Schackman BR, Goldie SJ, Freedberg KA, Losina E, Brazier J, Weinstein MC. Comparison of health state utilities using community and patient preference weights derived from a survey of patients with HIV/AIDS. Med Decis Making. 2002;22:27–38. doi: 10.1177/0272989X0202200103. [DOI] [PubMed] [Google Scholar]
- 68.Vernazza P, Hirschel B, Bernasconi E, Flepp M. HIV transmission under highly active antiretroviral therapy. Lancet. 2008;372(9652):1806–1807. doi: 10.1016/S0140-6736(08)61753-5. author reply 1807. [DOI] [PubMed] [Google Scholar]
- 69.Wilson DP, Law MG, Grulich AE, Cooper DA, Kaldor JM. Relation between HIV viral load and infectiousness: a model-based analysis. Lancet. 2008;372(9635):314–320. doi: 10.1016/S0140-6736(08)61115-0. [DOI] [PubMed] [Google Scholar]
- 70.McCormick AW, Walensky RP, Lipsitch M, et al. The effect of antiretroviral therapy on secondary transmission of HIV among men who have sex with men. Clin Infect Dis. 2007;44(8):1115–1122. doi: 10.1086/512816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cohen MS, Gay C, Kashuba AD, Blower S, Paxton L. Narrative review: antiretroviral therapy to prevent the sexual transmission of HIV-1. Ann Intern Med. 2007;146(8):591–601. doi: 10.7326/0003-4819-146-8-200704170-00010. [DOI] [PubMed] [Google Scholar]
- 72.Joint United Nations Programme on HIV/AIDS (UNAIDS) 2008 Report on the Global AIDS Epidemic. [Accessed August 1, 2010];2008 http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/2008_Global_report.asp.
- 73.CensusScope. United States Age Distribution. [Accessed August 1, 2010];2000 http://www.censusscope.org/us/chart_age.html.
- 74.Evans JL, Hahn JA, Page-Shafer K, et al. Gender differences in sexual and injection risk behavior among active young injection drug users in San Francisco (the UFO Study) J Urban Health. 2003;80(1):137–146. doi: 10.1093/jurban/jtg137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Friedman SR, Tempalski B, Cooper H, et al. Estimating numbers of injecting drug users in metropolitan areas for structural analyses of community vulnerability and for assessing relative degrees of service provision for injecting drug users. J Urban Health. 2004;81(3):377–400. doi: 10.1093/jurban/jth125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Centers for Disease Control and Prevention (CDC) HIV and AIDS among Gay and Bisexual Men. [Accessed August 1, 2010];2010 http://www.cdc.gov/nchhstp/newsroom/docs/FastFacts-MSM-FINAL508COMP.pdf.
- 77.Centers for Disease Control and Prevention (CDC) HIV/AIDS Surveillance Report. [Accessed August 1, 2010];2006 Volume 18 http://www.cdc.gov/hiv/topics/surveillance/resources/reports/2006report/default.htm.
- 78.Arias E, Rostron BL, Tejada-Vera B. United States Life Tables, 2005. Natl Vital Stat Rep. 2010;58(10) [PubMed] [Google Scholar]
- 79.Siegfried N, Muller M, Deeks JJ, Volmink J. Male circumcision for prevention of heterosexual acquisition of HIV in men. Cochrane Database Syst Rev. 2009;(2) doi: 10.1002/14651858.CD003362.pub2. CD003362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2(11):e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Desai K, Boily MC, Garnett GP, Masse BR, Moses S, Bailey RC. The role of sexually transmitted infections in male circumcision effectiveness against HIV--insights from clinical trial simulation. Emerg Themes Epidemiol. 2006;3:19. doi: 10.1186/1742-7622-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Centers for Disease Control and Prevention (CDC) Persons tested for HIV--United States, 2006. MMWR Morb Mortal Wkly Rep. 2008;57(31):845–849. [PubMed]
- 83.Teshale EH, Kamimoto L, Harris N, Li J, Wang H, McKenna MT. Estimated Number of HIV-infected Persons Eligible for and Receiving HIV Antiretroviral Therapy, 2003--United States. 12th Conference on Retroviruses and Opportunistic Infections; February 22–25, 2005; Boston, Massachusetts. (Abstract #167) [Google Scholar]
- 84.Fryback DG, Dasbach EJ, Klein R, et al. The Beaver Dam Health Outcomes Study: initial catalog of health-state quality factors. Med Decis Making. 1993;13(2):89–102. doi: 10.1177/0272989X9301300202. [DOI] [PubMed] [Google Scholar]
- 85.Holtgrave DR, Pinkerton SD. Updates of cost of illness and quality of life estimates for use in economic evaluations of HIV prevention programs. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16(1):54–62. doi: 10.1097/00042560-199709010-00009. [DOI] [PubMed] [Google Scholar]
- 86.Bozzette SA, Joyce G, McCaffrey DF, et al. Expenditures for the care of HIV-infected patients in the era of highly active antiretroviral therapy. N Engl J Med. 2001;344(11):817–823. doi: 10.1056/NEJM200103153441107. [DOI] [PubMed] [Google Scholar]
- 87.Schackman BR, Gebo KA, Walensky RP, et al. The lifetime cost of current human immunodeficiency virus care in the United States. Med Care. 2006;44(11):990–997. doi: 10.1097/01.mlr.0000228021.89490.2a. [DOI] [PubMed] [Google Scholar]
- 88.Hutchinson AB, Farnham PG, Dean HD, et al. The economic burden of HIV in the United States in the era of highly active antiretroviral therapy: evidence of continuing racial and ethnic differences. J Acquir Immune Defic Syndr. 2006;43(4):451–457. doi: 10.1097/01.qai.0000243090.32866.4e. [DOI] [PubMed] [Google Scholar]
- 89.World Health Organization (WHO) Global Health Observatory. [Accessed August 1, 2010];2009 http://www.who.int/gho/en/
- 90.Centers for Medicaid and Medicare Services. Medicaid Fee Schedule. 2009
- 91.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360(18):1815–1826. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Freedberg KA, Losina E, Weinstein MC, et al. The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med. 2001;344(11):824–831. doi: 10.1056/NEJM200103153441108. [DOI] [PubMed] [Google Scholar]
- 93.Bendavid E, Wood R, Katzenstein DA, Bayoumi AM, Owens DK. Expanding antiretroviral options in resource-limited settings--a cost-effectiveness analysis. J Acquir Immune Defic Syndr. 2009;52(1):106–113. doi: 10.1097/QAI.0b013e3181a4f9c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Goldie SJ, Yazdanpanah Y, Losina E, et al. Cost-effectiveness of HIV treatment in resource-poor settings--the case of Cote d'Ivoire. N Engl J Med. 2006;355(11):1141–1153. doi: 10.1056/NEJMsa060247. [DOI] [PubMed] [Google Scholar]
- 95.Anaya HD, Hoang T, Golden JF, et al. Improving HIV screening and receipt of results by nurse-initiated streamlined counseling and rapid testing. J Gen Intern Med. 2008;23(6):800–807. doi: 10.1007/s11606-008-0617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zetola NM, Bernstein K, Ahrens K, et al. Using surveillance data to monitor entry into care of newly diagnosed HIV-infected persons: San Francisco, 2006–2007. BMC Public Health. 2009;9:17. doi: 10.1186/1471-2458-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hoerger TJ, Harris R, Hicks KA, Donahue K, Sorensen S, Engelgau M. Screening for type 2 diabetes mellitus: a cost-effectiveness analysis. Ann Intern Med. 2004;140(9):689–699. doi: 10.7326/0003-4819-140-9-200405040-00008. [DOI] [PubMed] [Google Scholar]
- 98.Plevritis SK, Kurian AW, Sigal BM, et al. Cost-effectiveness of screening BRCA1/2 mutation carriers with breast magnetic resonance imaging. JAMA. 2006;295(20):2374–2384. doi: 10.1001/jama.295.20.2374. [DOI] [PubMed] [Google Scholar]
- 99.Hollingsworth TD, Anderson RM, Fraser C. Has the role of primary HIV been overstated?. 13th Conference on Retroviruses and Opportunistic Infections; February 5–8, 2006; Denver, Colorado. (Abstract #913) [Google Scholar]
- 100.Kelly JA, Morin SF, Remien RH, et al. Lessons learned about behavioral science and acute/early HIV infection. The NIMH Multisite Acute HIV Infection Study: V. AIDS Behav. 2009;13:1068–1074. doi: 10.1007/s10461-009-9579-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kerndt PR, Dubrow R, Aynalem G, et al. Strategies used in the detection of acute/early HIV infections. The NIMH Multisite Acute HIV Infection Study: I. AIDS Behav. 2009;13:1037–1045. doi: 10.1007/s10461-009-9580-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pinkerton SD. How many sexually-acquired HIV infections in the USA are due to acute-phase HIV transmission? AIDS. 2007;21(12):1625–1629. doi: 10.1097/QAD.0b013e32826fb6a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Prabhu VS, Hutchinson AB, Farnham PG, Sansom SL. Sexually acquired HIV infections in the United States due to acute-phase HIV transmission: an update. AIDS. 2009;23(13):1792–1794. doi: 10.1097/QAD.0b013e32832e7d04. [DOI] [PubMed] [Google Scholar]
- 104.Walensky RP, Weinstein MC, Kimmel AD, et al. Routine human immunodeficiency virus testing: an economic evaluation of current guidelines. Am J Med. 2005;118(3):292–300. doi: 10.1016/j.amjmed.2004.07.055. [DOI] [PubMed] [Google Scholar]