Abstract
The phytochemical resveratrol has undergone extensive preclinical investigation for its putative cancer chemopreventive properties. Low systemic availability of the parent compound due to rapid and extensive metabolism, may confound its usefulness as a potential agent to prevent malignancies in organs remote from the site of absorption. Micronization allows increased drug absorption, thus increasing availability. Here we describe a pilot study of SRT501, micronized resveratrol, given at 5.0 g daily for 14 days, to patients with colorectal cancer and hepatic metastases scheduled to undergo hepatectomy. The purpose of the study was to assess the safety, pharmacokinetics and pharmacodynamics of the formulation. SRT501 was found to be well tolerated. Mean plasma resveratrol levels following a single dose of SRT501 administration were 1942±1422 ng/mL, exceeding those published for equivalent doses of non-micronized resveratrol by 3.6-fold. Resveratrol was detectable in hepatic tissue following SRT501 administration (up to 2287 ng/g). Cleaved caspase-3, a marker of apoptosis, was significantly increased by 39% in malignant hepatic tissue following SRT501 treatment, compared to tissue from the placebo-treated patients. SRT501 warrants further clinical exploration to assess its potential clinical utility.
Keywords: Resveratrol, SRT501, colorectal cancer, pharmacokinetics, pharmacodynamics
Introduction
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a phytoalexin produced by certain plants when damaged by pathogens such as bacteria or fungi. It occurs in the skin of red grapes and in peanuts, and has been investigated extensively with respect to its putative cancer chemopreventive properties. Resveratrol is thought to mediate, at least in part, the purported disease-preventing actions of red wine and is also considered to prevent cardiovascular disease and morbidity associated with obesity and old age [1-3]. The numerous pharmacological properties of resveratrol and the suggestion that it may be a calorie-restriction mimetic which can prolong life, has engendered considerable interest by the lay press. An abundance of data obtained in cells in vitro and in pre-clinical models has highlighted a plethora of potential mechanisms by which resveratrol may prevent malignancies, predominantly those germane to cell survival and proliferation [4] including p53-dependant apoptosis [5] and cell cycle arrest [6]. However, robust clinical data remain scarce. Recent clinical pilot studies of resveratrol, in which it was administered at single or repeat doses of up to 5.0 g daily, focussed on its safety, pharmacokinetic (PK) properties and the analysis of potential efficacy biomarkers [4, 7-13]. PK profiles of resveratrol in healthy volunteers revealed rapid and extensive metabolism to resveratrol-4′-O-glucuronide, resveratrol-3′-O-glucuronide and resveratrol-3-O-sulfate following oral administration of either a single [8] or repeated daily doses [9] of 0.5 to 5.0 g. Despite detection of relatively high resveratrol concentrations in the colorectal tissue of patients, its systemic availability is severely reduced by its avid metabolism. Consequently, although resveratrol has demonstrated activity against a wide variety of malignancies in vitro (including bowel, breast, bladder, prostate, liver and thyroid) and in vivo (reviewed in [3, 14-16]), it is likely that poor bioavailability may limit efficacy at sites distant to the GI tract. Despite this, there is evidence to suggest that resveratrol elicits chemopreventive and therapeutic effects in pre-clinical models of liver cancer (reviewed in [17]), alluding to potential for efficacy against hepatocarcinogenesis and malignancies that preferentially metastasise to the liver.
Due to the poor aqueous solubility exhibited by resveratrol, digestive absorption is greatly influenced by drug dissolution rate. In an effort to increase absorption across the gastro-intestinal tract and thus systemically available parent compound, there has been considerable interest in the pharmaceutical manipulation of resveratrol. Decreasing the particle size of such chemicals can improve their rate of dissolution and thus their absorption [18]. Therefore, the aim of this clinical study was to investigate whether consumption of SRT501, a micronized resveratrol formulation designed by Sirtris, a GSK Company (Cambridge, MA, USA) is safe and generates measurable and pharmacologically active levels of parent agent in the circulation and in the liver. There is currently no clinical data available that determines resveratrol concentrations in human tissues other than the colon. To this end patients with colorectal cancer earmarked to undergo resection of liver metastases were recruited into a pilot study. They ingested either placebo or SRT501 on 14 consecutive days prior to surgery, and levels of resveratrol were quantified in blood and hepatic resection tissue. Potential pharmacodynamic effects of SRT501 were investigated by comparing the expression/activation of candidate protein biomarkers intrinsically associated with cell survival and apoptosis in the circulation and tissue of patients who received the agent and those who were on placebo.
Methods
Resveratrol formulation
Microparticular resveratrol of particle size <5 μm (SRT501) was manufactured by Sirtris Pharmaceuticals, a GSK Company. Particle size reduction is believed to enhance the bioavailability of resveratrol primarily due to the increase in surface area and improved suspension properties. A sachet containing SRT501 (5.0 g) was mixed with an aliquot (4 mL) of docusate sodium solution (docusate sodium, citric acid, colorant, glycerin, flavoring, sodium citrate, sodium saccharin and sorbitol) made up to 20 mL in distilled water forming a uniform suspension, and ingested by patients.
Patients
The pilot study, designed and sponsored by Sirtris, was conducted at the University Hospitals of Leicester (UHL) NHS-Trust (UHL 10507, trial registry: NCT00920803). It was reviewed and approved by the Leicestershire, Northamptonshire and Rutland Research Ethics Committee (UK) and conducted in accordance with applicable guidelines on Good Clinical Practices. Nine subjects (18 years or older) presenting with confirmed stage IV colorectal cancer and hepatic metastases, who had not received therapeutic intervention for their cancer within 6 weeks of study commencement and had a life expectancy of >3 months, were recruited into the study. All patients were due to undergo resection of liver metastases. Participants had to be physically capable of complying with the protocol and had a normal ECG and no history of HIV or hepatitis B/C.
Patient were asked to refrain from large quantities of resveratrol-containing foods and drinks such as peanuts, grapes, mulberries and alcohol within 48 h of scheduled PK collection days, and the day of surgical resection.
Study design
The study was a phase I, randomised (2:1), double-blind clinical trial. Six subjects were randomised to receive SRT501, and three placebo (titanium dioxide). Interventions were provided in clinical kits as a powder and reconstituted as described above. Patients ingested the formulation pre-operatively daily for a minimum of 10 and a maximum of 21 days, dependent upon surgical scheduling. Plasma samples for PK assessment were obtained on days 1 and 2, and on the day of surgical resection. Plasma for PD assessment was obtained pre-dose, immediately prior to, and during surgery. Diseased and normal adjacent hepatic tissue was resected 6-7 h after the last dose. Participants completed a daily adverse event (AE) diary which was assessed using the NCI Common Toxicity Criteria v3. Of the nine patients recruited, seven completed the study, one died from post-operative complications, and one ceased administration early due to AEs (diarrhoea). PK samples were obtained from all nine subjects. The primary objectives of the study were to determine safety and tolerability of SRT501 and resveratrol pharmacokinetics. The secondary objectives were to explore potential pharmacodynamic changes elicited by SRT501 in blood and tissue.
Sample collection
Blood samples were collected prior to administration on day 1, for PK at 0.25, 0.5, 1, 2, 4, 8, 24 h post the first dose, and prior to, and during surgery. Plasma (sodium heparin tubes) and serum were obtained and stored at −80 °C until analysis. Tissues were harvested from resected material, part of which was formalin-fixed and paraffin-embedded, and part immediately frozen and stored at −80 °C until analysis.
Sample preparation and analysis
Quantitation of resveratrol in plasma and tissues was conducted by Charles River Laboratories, Montreal, Canada. The methodology complied with the US Food and Drug Administration (21CFR part 58) Good Laboratory Practice regulations, using an in-house validated liquid chromatography tandem-mass spectrometry (LC-MS/MS) method. In brief, 50 μL plasma samples were extracted using acidified methanol (ascorbic acid:methanol:formic acid, 50:50:0.025) (5 μL) containing resveratrol-13C6 as an internal standard. Samples were then centrifuged (14000 rpm/0 °C/10 min), and the supernatant aliquoted into a 96-well collection plate for analysis. Separation, quantitation and characterisation of resveratrol was achieved using a Symbiosis Pharma System (XLC method) in conjunction with an MDS Sciex API 3000 mass spectrometer. A Waters YMC ODS-AQ column (3 μm, 4.6 × 50 mm) was linked to a Peek pre-column 0.5 μm filter. The eluant consisted of a binary mobile phase (A; water:formic acid, 100:0.025 (v/v), B; methanol:acetonitrile:formic acid, 20:80:0.025 (v/v/v)). The elution gradient was A85% B15% to A5% B95% for 4 min. Resveratrol was characterised by electrospray mass spectrometry (negative ionisation mode) with ion spray voltage −4500 V, declustering potential −25 V, focusing potential −30 V, entrance potential −12 V, collision energy −27 V, collision exit potential 40 V and temperature 550 °C. Lower limit of quantitation (LLOQ) was 5 ng/mL and upper limit of quantitation (ULOQ) 1000 ng/mL.
Tissue samples were minced on ice and ~100 mg was used for analysis. Acidified methanol (ascorbic acid:methanol:formic acid, 50:50:0.025) (5 μL) containing resveratrol-13C6 as an internal standard, 100 μL matrix green beads and 500 μL ethanol was added to each sample, the mixture vortexed and left on ice (10 min). Samples were homogenised (Fastprep, 2.4-5.5 m/s, 20 sec), left on ice (10 min) and the homogenisation repeated prior to centrifugation (14000 rpm/0 °C/10 min). An aliquot (100 μL) of the supernatant was added to 100 μL water:formic acid (100:0.25). Separation, quantitation and characterisation of resveratrol was achieved using an Agilent 1100 chromatograph with an MDX Sciex API 5000 mass spectrometer. A Waters YMC ODS-AQ column (3 μm, 4.6 × 50 mm) was linked to a Peek pre-column 0.5 μm filter. The eluant was as for the plasma analysis; gradient elution was over 6.5 min from A70% B30% to A0% B100%, then back to A70% B30%. MS conditions were as above except: ion spray voltage −4500 V, declustering potential −120 V, entrance potential −10 V, collision energy −27 V, collision exit potential −10 V and temperature 700 °C. For tissue samples, LLOQ was set at 2.5 ng/g and ULOQ at 500 ng/g. Data collection was performed using Analyst version 1.4.2. (MDX Sciex).
Analysis of cell proliferation and apoptosis
Formalin-fixed tissues were analyzed for markers of proliferation and apoptosis using the NovoLink Polymer Detection System (Novocastra Laboratories, Newcastle-upon-Tyne, UK) in conjunction with anti-Ki-67 (Dako, Cambridgeshire, UK) and anti-cleaved-caspase-3 (Cell Signalling Technology, MA, USA) antibodies. In brief, paraffin-embedded sections were de-waxed at 65 °C and re-hydrated through a series of alcohol washes. Antigen retrieval was via microwaving in either Tris-EDTA (pH 9.0) for Ki-67 or citrate buffer (pH 6.0) for cleaved-caspase-3. Sections were visualized at x40 magnification using a Leica DC300 inverted light microscope and camera system. Images were processed using Adobe Photoshop v7.0.1. Positive staining cells were scored on 10 random fields of view on each slide, and expressed as percent positively stained cells. All analyses were performed blinded.
PGE2, VEGF and IGF-1 analysis
ELISA assays were undertaken to assess VEGF (Invitrogen, Paisley, UK) levels in serum, PGE2 (Cayman Chemicals, Cambridge UK) levels in plasma and IGF-1 (R&D Systems, Oxford, UK) levels in liver tissue extracts. Assays were performed according to the manufacturers’ instructions and ELISA plates analysed using a Fluostar Optima plate reader (BMG Labtech, Aylesbury, UK)
Statistical analysis
Statistical analyses for pharmacokinetic data included linear regression with 1/concentration weighting and descriptive statistics; arithmetic means and standard deviations, accuracy and precision were calculated using Watson Laboratory Information Management System (LIMS) (version 7.2.0.02) and Microsoft Excel (version 2000/2003).
Statistical analyses for pharmacodynamic data were undertaken using SPSS 16.0 for Windows. Means were compared via one way, one sided ANOVA followed by Tukey’s post hoc test. A p value of less than 0.05 was deemed significant.
Results
Safety of SRT-501
Nine patients were recruited into the study (for demographics see table 1). Six individuals ingested 5.0 g of SRT501, and three received placebo, daily for approximately 14 days prior to surgery. One patient on SRT501 ceased dosing on day 13. AEs possibly or probably attributable to agent intake are shown in table 2. They were primarily of a gastrointestinal nature, including nausea and diarrhoea, and mild in grade (grade 1 NCI CTC v3.0). Other AEs included chills, lethargy, rash, skin irritation and vascular flushing, which resolved without sequelae, with the exception of one case of lethargy in the placebo group, which was ongoing at follow-up. One patient developed post-operative peritonitis and liver failure, and died during follow-up. The Principle Investigator deemed this serious adverse event (SAE) unrelated to study drug.
Table 1.
Demographics for patients randomised to 5.0 g/day SRT501 and placebo
| SRT501 (n=6) |
Placebo (n=3) |
|
|---|---|---|
| Age (years) | 68.5(±10.8) | 64.3(±6.35) |
| Gender | M: 5 | M: 1 |
| F: 1 | F: 2 | |
| BMI | 28.10(±4.35) | 26.87(±1.61) |
| Race (100%) |
Caucasian (100%) | Caucasian |
| Days on study | 13.3(±1.03) | 14.0(±0.00) |
| Cumulative dose per subject (g) | 66.7(±5.16) | 70.0(±0.00) |
BMI: body mass index; F: female; M: male.
Table 2.
Adverse event listings deemed to be possibly or probably related to SRT501/placebo intervention.
| Adverse event | SRT501 | Placebo | ||
|---|---|---|---|---|
| (n=6) | (n=3) | |||
| N° of events N° of patients | N° of events N° of patients | |||
| Gastrointestinal disorders: | 12 | 5 | 2 | 1 |
| Anal pruritus | 1 | 1 | 0 | 0 |
| Diarrhoea | 7 | 5 | 2 | 1 |
| Nausea | 4 | 1 | 0 | 0 |
| General disorders: | 1 | 1 | 0 | 0 |
| Chills | 1 | 1 | 0 | 0 |
| Nervous system disorders: | 1 | 1 | 1 | 1 |
| Lethargy | 0 | 0 | 1 | 1 |
| Peripheral neuropathy | 1 | 1 | 0 | 0 |
| Skin/tissue disorders: | 2 | 2 | 0 | 0 |
| Rash | 1 | 1 | 0 | 0 |
| Skin irritation | 1 | 1 | 0 | 0 |
| Vascular disorders: | 1 | 1 | 0 | 0 |
| Flushing | 1 | 1 | 0 | 0 |
Resveratrol pharmacokinetics and concentration in liver tissue
Patients’ plasma was analysed by HPLC-MS/MS for resveratrol. Resveratrol concentrations were below the lower limits of quantitation (LLOQ) for all samples from subjects receiving placebo, and measurable in patients who received SRT501. Pharmacokinetic parameters for resveratrol are shown in table 3, and plasma concentration versus time curves for individual subjects in figure 1. Cmax levels were reached 2.8 h post-dose, and the mean maximum plasma concentration was 1942 ng/mL (8.51 nmol/mL). The mean plasma elimination half-life was just over 1h.
Table 3.
Descriptive statistics for resveratrol pharmacokinetics from plasma of patients receiving SRT501. All samples from patients randomised to placebo group were below limit of quantitation at all time points (data not shown).
| Cmax (ng/mL) | tmax (hr) | t1/2 (hr) | AUC (0-24hr) (ng/hr/mL) | |
|---|---|---|---|---|
| n | 6 | 6 | 3 | 3 |
| Geometric mean | 1942.00 | 2.80 | 1.06 | 6327.40 |
| SD | 1422.62 | 1.10 | 0.39 | 2247.20 |
| Range | 896 – 4890 | 2.0 – 4.0 | 0.81 – 1.54 | 5030 - 9140 |
Cmax: maximal plasma concentration; tmax: time of maximal plasma concentration; t1/2 : apparent first order elimination half-life; AUC: area under the plasma concentration versus time curve from time 0 to 24 hours.
Figure 1.
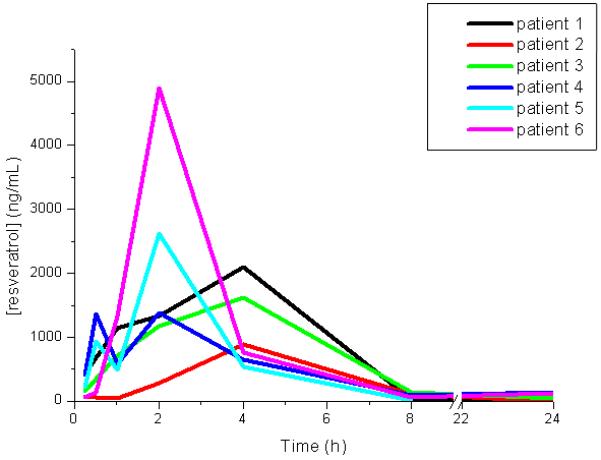
Plasma concentration of resveratrol in individual patients who received one dose (5.0 g) of SRT501. Analysis was by HPLC/MS-MS, for details see Methods.
Resveratrol was quantified in tumor and normal adjacent hepatic tissues. Levels of resveratrol were below the LLOQ in all subjects on placebo and one of the six patients on SRT501. Mean resveratrol levels in the remaining five patients receiving SRT501 were 1098±1393 ng/g (4.81 nmol/g, range 52-2834 ng/g) and 420±341 ng/g (1.84 nmol/g, range 46-914 ng/g) in tumor and normal tissue, respectively.
Effect of SRT501 on pharmacodynamics
Potential effects of SRT501 on processes relevant to cell survival and apoptosis were measured in plasma and tissue. There was no difference between patients who received placebo or SRT501 in terms of plasma/serum levels of prostaglandin E2 (PGE-2) and vascular endothelial growth factor (VEGF) (not shown). Tissue samples were analyzed by immunohistochemistry, ELISA or western blot for SRT501-induced changes to levels of IGF-1, Ki67, phospho Akt (ser473), Akt1, phospho-GSK3, GSK3, phospho ERK, ERK, phospho-JNK, JNK, beta-catenin, survivin, BCL2, Bax or PARP. In all cases there were no significant differences between placebo and SRT501 (data not shown). Apoptosis, as reflected by immunohistochemistry for cleaved caspase 3 in tumor tissue, was significantly increased by 39% (to 1.44% total apoptotic cells, p=0.038) in patients on SRT501 compared to those taking placebo (figure 2).
Figure 2.
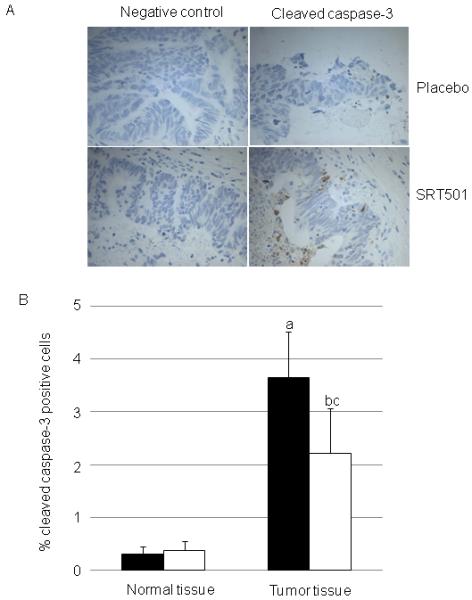
Apoptosis reflected by cleaved caspase-3 (A, representative photomicrographs, B, quantitation of photomicrographs) in hepatic normal and tumor tissue of patients who consumed SRT501 (N=6, black bars) or placebo (N=3, white bars) daily for 14 days. Values are the mean+/−SD. (a) represents significant difference between normal and tumour tissue for SRT501 group. (b) represents significant difference between normal and tumor tissue for placebo group. (c) represents significant difference between SRT501 and placebo tumor tissue. (P=<0.05, oneway ANOVA followed by Tukey’s post hoc test).
Discussion
This trial represents the first description of pharmacokinetic parameters for resveratrol after ingestion of SRT501, micronized resveratrol, in subjects with colorectal cancer. The dose of resveratrol employed (5.0 g) is equivalent to one of the dose levels at which non-micronized resveratrol was recently administered to healthy volunteers [9]. In the healthy volunteer study, ten subjects on 5.0 g of the non-micronized resveratrol reported twenty AEs possibly or probably related to ingestion of resveratrol capsules, which compares with seventeen AEs reported for six patients who received SRT501. The two formulations therefore seem to cause a similar incidence of AEs, although in the previous study non-micronized resveratrol was administered for a longer duration (29 days) than SRT501. All AEs reported following SRT501 administration were mild, whilst two of the ten healthy volunteers on non-micronized resveratrol, presented with moderate and one with severe gastrointestinal AEs [9]. This comparison provides the possibility that SRT501 formulated as a suspension may be better tolerated than non-micronized resveratrol given as ten 0.5 g capsules per day. Since micronized resveratrol was expected to afford higher systemic concentrations [19], this finding also suggests that ingestion of a large number of capsules or the presence of non-absorbed resveratrol in the GI tract may be responsible for the adverse effects, rather than the actual plasma concentrations achieved.
The pharmacokinetic analysis revealed that the mean plasma Cmax for resveratrol measured after ingestion of SRT501 (mean 1942 ng/mL , CV:73.3%) was 3.6-fold higher than that achieved following a single dose of non-micronized resveratrol in healthy volunteers (mean 538.8 ng/mL (CV:72.5%) [8], consistent with the superior bioavailability of resveratrol when administered as SRT501 compared to non-micronized agent. The time taken to reach the Cmax for resveratrol was 2.8 h in the case of SRT501 compared to 1.5 h for non-micronized resveratrol [8].
Concentrations of resveratrol achieved in hepatic metastases after administration of SRT501 (0.22-12.4 μM) were of the order of magnitude observed to elicit pharmacological effect in human colorectal cancer cells in vitro and in pre-clinical models in vivo. This interpretation is corroborated by the finding that SRT501 caused a small but significant increase in cleaved caspase-3 immuno-reactivity in tumor tissue, when compared to equivalent tissue from subjects on placebo. Whilst we were not able to confirm these effects via other pharmacodynamic markers, this may be due to low sensitivity of the techniques used. Mechanisms by which low concentrations of resveratrol have been observed to induce apoptotic and anti-proliferative effect in vitro include induction of Fas redistribution and its association with the death-inducing signalling (DISC) complex [20], and via inhibition of wnt signalling and subsequent decrease in nuclear β-catenin localisation [21]. In a pre-clinical model of diethylnitrosamine-initiated hepatocarcinogenesis, significant resveratrol-mediated apoptosis likely resulted from increased Bax expression and consequent increase in the Bax:Bcl2 ratio [22]. Whilst tissue resveratrol levels were not determined in this study, extrapolation of data from rodent PK studies [23, 24] would suggest hepatic resveratrol concentrations necessary for apoptosis induction, to be only moderately higher than the maximum achieved in the SRT501 study described here.
This is the first demonstration that resveratrol can reach potentially active concentrations in human tissues that are distant to the GI tract.
In summary, the results of this pilot study of SRT501 allow three conclusions: i. its daily consumption for 14 days seems to be well tolerated in colorectal cancer patients, ii. Cmax for SRT501 was higher than reported for equivalent dose of non-micronised resveratrol, iii. its ingestion furnished measurable resveratrol levels in a tissue distant to the GI tract (in particular the liver), and these concentrations were accompanied by a significant pharmacological effect.
Micronized resveratrol, as exemplified by SRT501, warrants further clinical exploration in larger cohorts of subjects, in order to test the hypothesis that resveratrol may be of use as a cancer chemopreventive or chemotherapeutic agent.
Acknowledgments
Grant support: This work was supported by Cancer Research UK on a programme grant [C325/A6691], by Cancer Research UK in conjunction with the UK Department of Health on an Experimental Cancer Medicine Centre grant [C325/A7241] and by Sirtris, A GSK company.
Financial Support: This work was supported by Cancer Research UK on a programme grant [C325/A6691], by Cancer Research UK in conjunction with the UK Department of Health on an Experimental Cancer Medicine Centre grant [C325/A7241] and by Sirtris, A GSK company.
Footnotes
Conflict of interest: This study was sponsored and partially funded by Sirtris, A GSK Company.
References
- [1].Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, et al. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS One. 2008;3:e2264. doi: 10.1371/journal.pone.0002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- [3].A Bishayee. Cancer prevention and treatment with resveratrol: from rodent studies to clinical trials. Cancer Prev Res (Phila) 2009;2:409–18. doi: 10.1158/1940-6207.CAPR-08-0160. [DOI] [PubMed] [Google Scholar]
- [4].Shankar S, Singh G, Srivastava RK. Chemoprevention by resveratrol: molecular mechanisms and therapeutic potential. Front Biosci. 2007;12:4839–54. doi: 10.2741/2432. [DOI] [PubMed] [Google Scholar]
- [5].She QB, Bode AM, Ma WY, Chen NY, Dong Z. Resveratrol-induced activation of p53 and apoptosis is mediated by extracellular-signal-regulated protein kinases and p38 kinase. Cancer Res. 2001;61:1604–10. [PubMed] [Google Scholar]
- [6].Joe AK, Liu H, Suzui M, Vural ME, Xiao D, Weinstein IB. Resveratrol induces growth inhibition, S-phase arrest, apoptosis, and changes in biomarker expression in several human cancer cell lines. Clin Cancer Res. 2002;8:893–903. [PubMed] [Google Scholar]
- [7].Almeida L, Vaz-da-Silva M, Falcao A, Soares E, Costa R, Loureiro AI, et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol Nutr Food Res. 2009;53(Suppl 1):S7–15. doi: 10.1002/mnfr.200800177. [DOI] [PubMed] [Google Scholar]
- [8].Boocock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007;16:1246–52. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- [9].Brown VA, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, et al. Repeat Dose Study of the Cancer Chemopreventive Agent Resveratrol in Healthy Volunteers: Safety, Pharmacokinetics, and Effect on the Insulin-like Growth Factor Axis. Cancer Res. 2010;70:9003–11. doi: 10.1158/0008-5472.CAN-10-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].la Porte C, Voduc N, Zhang G, Seguin I, Tardiff D, Singhal N, et al. Steady-State pharmacokinetics and tolerability of trans-resveratrol 2000 mg twice daily with food, quercetin and alcohol (ethanol) in healthy human subjects. Clin Pharmacokinet. 2009;49:449–54. doi: 10.2165/11531820-000000000-00000. [DOI] [PubMed] [Google Scholar]
- [11].Nunes T, Almeida L, Rocha JF, Falcao A, Fernandes-Lopes C, Loureiro AI, et al. Pharmacokinetics of trans-resveratrol following repeated administration in healthy elderly and young subjects. J Clin Pharmacol. 2009;49:1477–82. doi: 10.1177/0091270009339191. [DOI] [PubMed] [Google Scholar]
- [12].Patel KR, Brown VA, Jones DJ, Britton RG, Hemingway D, Miller AS, et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010;70:7392–9. doi: 10.1158/0008-5472.CAN-10-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Walle T, Hsieh F, DeLegge MH, Oatis JE, Jr., Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–82. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- [14].Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 2004;24:2783–840. [PubMed] [Google Scholar]
- [15].Athar M, Back JH, Tang X, Kim KH, Kopelovich L, Bickers DR, et al. Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol. 2007;224:274–83. doi: 10.1016/j.taap.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Aziz MH, Kumar R, Ahmad N. Cancer chemoprevention by resveratrol: in vitro and in vivo studies and the underlying mechanisms (review) Int J Oncol. 2003;23:17–28. [PubMed] [Google Scholar]
- [17].Bishayee A, Politis T, Darvesh AS. Resveratrol in the chemoprevention and treatment of hepatocellular carcinoma. Cancer Treat Rev. 36:43–53. doi: 10.1016/j.ctrv.2009.10.002. [DOI] [PubMed] [Google Scholar]
- [18].Hintz R, Johnson K. The effect of particle size distribution on dissolution rate and oral absorption. International Journal of Pharmaceutics. 1989;51:8. [Google Scholar]
- [19].Elliott PJ, Jirousek M. Sirtuins: novel targets for metabolic disease. Curr Opin Investig Drugs. 2008;9:371–8. [PubMed] [Google Scholar]
- [20].Delmas D, Rebe C, Lacour S, Filomenko R, Athias A, Gambert P, et al. Resveratrol-induced apoptosis is associated with Fas redistribution in the rafts and the formation of a death-inducing signaling complex in colon cancer cells. J Biol Chem. 2003;278:41482–90. doi: 10.1074/jbc.M304896200. [DOI] [PubMed] [Google Scholar]
- [21].Hope C, Planutis K, Planutiene M, Moyer MP, Johal KS, Woo J, et al. Low concentrations of resveratrol inhibit Wnt signal throughput in colon-derived cells: implications for colon cancer prevention. Mol Nutr Food Res. 2008;52(Suppl 1):S52–61. doi: 10.1002/mnfr.200700448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bishayee A, Dhir N. Resveratrol-mediated chemoprevention of diethylnitrosamine-initiated hepatocarcinogenesis: inhibition of cell proliferation and induction of apoptosis. Chem Biol Interact. 2009;179:131–44. doi: 10.1016/j.cbi.2008.11.015. [DOI] [PubMed] [Google Scholar]
- [23].Sale S, Verschoyle RD, Boocock D, Jones DJ, Wilsher N, Ruparelia KC, et al. Pharmacokinetics in mice and growth-inhibitory properties of the putative cancer chemopreventive agent resveratrol and the synthetic analogue trans 3,4,5,4′-tetramethoxystilbene. Br J Cancer. 2004;90:736–44. doi: 10.1038/sj.bjc.6601568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Vitrac X, Desmouliere A, Brouillaud B, Krisa S, Deffieux G, Barthe N, et al. Distribution of [14C]-trans-resveratrol, a cancer chemopreventive polyphenol, in mouse tissues after oral administration. Life Sci. 2003;72:2219–33. doi: 10.1016/s0024-3205(03)00096-1. [DOI] [PubMed] [Google Scholar]


