Abstract
Purpose
Ocular mucous membrane pemphigoid (OcMMP) is a sight-threatening autoimmune disease in which referral to specialists units for further management is a common practise. This study aims to describe referral patterns, disease phenotype and management strategies in patients who present with either early or established disease to two large tertiary care hospitals in the United Kingdom.
Patients and Methods
In all, 54 consecutive patients with a documented history of OcMMP were followed for 24 months. Two groups were defined: (i) early-onset disease (EOD:<3years, n=26, 51 eyes) and (ii) established disease (EstD:>5years, n=24, 48 eyes). Data were captured at first clinic visit, and at 12 and 24 months follow-up. Information regarding duration, activity and stage of disease, visual acuity (VA), therapeutic strategies and clinical outcome were analysed.
Results
Patients with EOD were younger and had more severe conjunctival inflammation (76% of inflamed eyes) than the EstD group, who had poorer VA (26.7%=VA<3/60, P<0.01) and more advanced disease. Although 40% of patients were on existing immunosuppression, 48% required initiation or switch to more potent immunotherapy. In all, 28% (14) were referred back to the originating hospitals for continued care. Although inflammation had resolved in 78% (60/77) at 12 months, persistence of inflammation and progression did not differ between the two phenotypes. Importantly, 42% demonstrated disease progression in the absence of clinically detectable inflammation.
Conclusions
These data highlight that irrespective of OcMMP phenotype, initiation or escalation of potent immunosuppression is required at tertiary hospitals. Moreover, the conjunctival scarring progresses even when the eye remains clinically quiescent. Early referral to tertiary centres is recommended to optimise immunosuppression and limit long-term ocular damage.
Keywords: cicatrising conjunctivitis, conjunctival scarring, progression, immunosuppression
Introduction
Mucous membrane pemphigoid (MMP) is a potentially fatal autoimmune disease1 with a mortality usually secondary to aero-digestive tract stricture formation, quoted as 0.029 per 100 000 in the United States during 1992–2002.2 Although the condition is associated with skin and mucous membrane involvement including the oral cavity, oesophagus, trachea and genitals,3 ocular manifestations of MMP (OcMMP) are defined as ‘high risk' and can be blinding.3 Management strategies are aimed at early diagnosis together with the prevention of both life- and sight-threatening complications through the removal of factors that precipitate inflammation, careful immunomodulation and/or surgical intervention.
The course of ocular disease is variable, and determining disease activity and progression represents a major challenge.4 Patients are often diagnosed at an advanced stage of disease or with an acutely inflamed eye,5, 6 which if left untreated, may result in an acceleration of disease progression that can be unresponsive to pharmacological manipulation.7, 8 In order to delay or arrest this process, early intervention with systemic immunosuppression is required,3, 4, 5, 9, 10, 11, 12 usually by adopting either a validated stepladder approach based on the severity of disease activity,10, 13 or the primary use of oral cyclophosphamide,11 both inducing long-lasting remission.
In the United Kingdom, streams of hospital referral are to tertiary care services specialising in the management of OcMMP, from ophthalmologists practicing in secondary care, directly from the primary care services such as general (family) practitioners or optometrists, and from dermatologists/oral medicine specialists referring patients for screening with a diagnosis of MMP at extra-ocular sites. Although the possibility of a referral bias of patients with a more severe phenotype is recognised,11 there is limited information regarding the clinical features of patients with early or established disease who present to specialised services, whether these patients require continued tertiary care, or are discharged back to their referring unit.
Amongst our patient cohorts, we have noted that delaying referral of patients with OcMMP to our specialist hospitals seemed to augment a clinical phenotype that is refractory or only partially responsive to therapeutic intervention. The aim of this study, therefore, was to characterise referral patterns and disease phenotype (including activity, staging and progression) in patients with OcMMP who present either early or late according to duration of symptoms, to the two largest tertiary specialist hospitals in the United Kingdom, and the strategies employed to manage these patients.
Materials and Methods
Study population
A total of 54 consecutive patients with a documented history of OcMMP referred to dedicated ocular surface disease clinics over a 3-year period at the two largest specialist referral centres in the United Kingdom, Moorfields Eye Hospital (MEH, London, UK) and the Birmingham and Midland Eye Centre (BMEC, Birmingham, UK) were identified from electronic databases and followed for 24 months. Patients were stratified according to duration of symptoms, where symptoms were defined as redness, tearing, burning, decreased vision or foreign body sensation.14 The frequency distribution of the duration of disease defined two groups straddling either side of the median (1460 days (4 years); Supplementary Figure 1). Group1 (n=26, 51 eyes) consisted of patients with <1095 days (<3 years) history and was termed the ‘early-onset' disease (EOD) group, whereas group 2 (n=24, 48 eyes) comprised patients with >1825 days (>5 years) history and was termed the ‘established' disease (EstD) group. Four patients had a duration of symptoms that fell on the median (4 years), and these patients were excluded from further analysis. The study was conducted following ethical approval and conformed to the tenets of the Declaration of Helsinki.
Diagnosis
Diagnosis of OcMMP was based on clinical findings characteristic for the disease, namely progressive conjunctival cicatrisation in the absence of other causes of conjunctival scarring. If patients did not had a previous positive tissue biopsy, a confirmatory perilesional conjunctival and/or oral mucosal biopsy for direct immunofluorescence was undertaken. A positive result was defined as linear deposition of immunoglobulin G, A or complement (C3) along the basement membrane zone.3 If typical clinical characteristics were evident, a negative result did not exclude the diagnosis10, 14, 15, 16 because of the recognition of a subgroup of ocular patients who have ocular features consistent with OcMMP but have a negative biopsy.15, 17 In accordance with the first international consensus, a positive indirect immunofluorescence was not an essential requirement for diagnosis.3
Study design
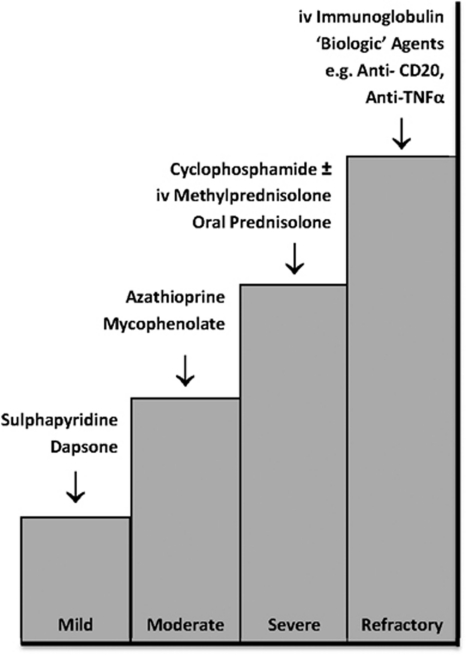
Data were captured at presentation, immediately following first consultation, at 12 and 24 months for both the EOD and EstD groups. Visual acuity (VA) was classified as good (6/6–6/18), or in accordance with the WHO definitions of ‘visual impairment' (<6/18–6/60), ‘severe visual impairment' (<6/60) and ‘blind' (<3/60). Disease activity was based upon the degree of conjunctival inflammation: absent, mild, moderate or severe (including inflammation in all four quadrants, limbitis and/or conjunctival ulceration).18 Stage of disease and progression was determined by using the staging systems described by Mondino and Brown (I, 0–25% II, 25–50% III, 50–75% IV, 75–100% loss of inferior fornix)7 and Foster (I, subconjunctival scarring and fibrosis; II, fornix foreshortening of any degree; III, presence of any degree of symblepharon; IV, end-stage cicatricial pemphigoid).19 Progression was defined as an advance in either Mondino or Foster staging criteria. Immunosuppression strategies used a ‘step–ladder' approach as previously described (Figure 1).13 Information regarding surgical intervention was also recorded.
Figure 1.
Immunosuppression strategies (based on Rauz et al13). A step–ladder approach to treatment with agents having the fewest side effects to those that have the greatest side effects is adopted according to disease activity (mild, moderate or severe), which is used to guide therapy. Dapsone (25–50 mg twice a day) or sulphapyridine (500 mg twice a day) can be used for mild inflammation; azathioprine (1–2.5 mg/kg/day) or mycophenolate mofetil (500–1000 mg twice a day if intolerant to azathioprine) may be added or substituted for persistent disease. Severe inflammatory disease is treated with cyclophosphamide (1–2 mg/kg/day) and adjuvant prednisolone (1 mg/kg/day with or without supplementary loading doses of 1 g intravenous methylprednisolone preceding oral therapy) for up to 3 months until the optimal effects of cyclophosphamide have taken effect. Patients with refractory disease are managed through intravenous immunoglobulin or ‘biological' agents such as anti-CD 20 (rituximab) or anti-TNFα therapy.
Statistical analysis
Statistical analysis was by SPSS 16.0 for Macintosh and 14.0 for Windows (SPSS, Chicago, IL, USA; 2006), and Prism version 5.0 for Macintosh (GraphPad Software, CA, USA; 2008) using Fishers-exact test, McNemar's test and Kendall's τ-b for rank correlations. Continuous variables were analysed by non-parametric tests (Mann–Whitney U-test). Data were collected on all eyes and comparisons were undertaken between the worst affected eye for cross-sectional analysis of inflammation/fibrosis and the better-seeing eye for VA. When determining disease progression, comparisons were undertaken between patients (either one or both eyes). In order to determine whether changes seen at differing time points were significant rather than as a result of a change in the cohort (eg, because of the patients being discharged back to the referring hospital or missing data), longitudinal analysis of the same eye was undertaken. Owing to the referral of patients back to the originating physician, the sample sizes at the three time points differ and percentages rather than absolute counts are therefore reported.
Results
Demographic information and referral patterns
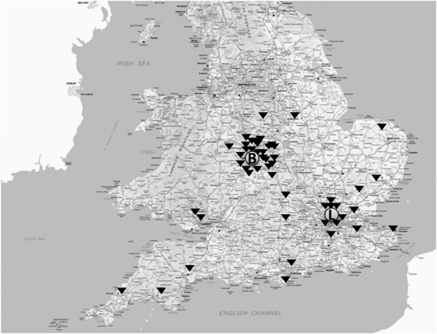
The geographical origin of our patient cohort is illustrated in Figure 2, where the furthest referral was from Newquay in Cornwall to MEH (238 miles). Associated patient demographics and subgroup stratification (EOD vs EstD) is detailed in Table 1. The EOD group was younger than the EstD group (62 (32–82) vs 69 (39–91) years (median, range; P=0.02)). In all, 19 patients (37 eyes) from the EOD and 16 patients (32 eyes) from the EstD were followed for the full 24 months. Of the 15 patients, not reviewed at 24 months, 1 patient in the EstD group died before 12 months follow-up and 14 (28%) were referred back to the referring hospital. Of these, 11 had no clinically detectable inflammation at their last visit before discharge from the tertiary centre, 2 had mild inflammation, which continued to be monitored and treated at the local referring hospital, and 1 patient repeatedly failed to attend for follow-up despite recall. The remaining cohort (EOD 19; EstD 16) consisted of patients with more severe ocular disease.
Figure 2.
Map containing ordnance survey data (© Crown copyright and database right 2010) showing the combined geographical distribution of referrals (▾) to the two tertiary referral hospitals: Moorfields Eye Hospital, London, UK (circled, L) and the Birmingham and Midland Eye Centre, Birmingham, UK (circled, B). The furthest referral was for Newquay, Cornwall to Moorfields (238 miles).
Table 1. Patient demographics and characteristics.
| All patients | Early-onset disease | Established disease | P-value | |
|---|---|---|---|---|
| Total no. of patients | 50 | 26 | 24 | — |
| Total no. of eyes | 99 | 51 | 48 | — |
| Male: female (% female) | 23:27 (54) | 11:15 (58) | 12:12 (50) | — |
| Median age (years; range) | 67 (32–91) | 62 (32–82) | 69 (39–91) | P=0.02 |
| Median duration of symptoms (years; range) | 3 (0–41) | 1.5 (0–3) | 14 (5–41) | P<0.0001 |
| Patient follow-up (eyes) | ||||
| 12 months | 43 (85) | 23 (45) | 20 (40) | — |
| 24 months | 35 (69) | 19 (37) | 16 (32) | — |
| No. of patients discharged back to referring hospitala | ||||
| Total number discharged | 14 | 7 | 7 | — |
| 12 months follow-up | 4 | 1 | 3 | — |
| 24 months follow-up | 10 | 6 | 4 | — |
| Biopsyb | ||||
| DIF positive | 87.2% (34/39) | 92% (23/25) | 78.6% (11/14) | P=0.33 |
| IIF positivec | 34.8% (8/23) | 42.9 % (6/14) | 2.2% (2/9) | P=0.4 |
| Extraocular mucocutaneous involvement | ||||
| All mucocutaneous tissues | 52% (26/50) | 62% (16/26) | 42% (10/24) | P=0.26 |
| Skin | 18% (9/50) | 7.7% (2/26) | 29.2% (7/24) | — |
| Oral | 40% (20/50) | 57.7% (15/26) | 20.8% (5/24) | P=0.01 |
| Visual acuityd | ||||
| Normal: 6/6 to >6/18 | 80.6% (29/36) | 95.2% (20/21) | 60% (9/15) | P=0.007 |
| Visual impairment: <6/18 to 6/60 | 8.3% (3/36) | 4.8% (1/21) | 13.3% (2/15) | |
| Severe visual impairment: 6/60 to 3/60 | 0% (0/36) | 0% (0/21) | 0% (0/15) | |
| Blind: <3/60 | 11.1% (4/36) | 0% (0/21) | 26.7% (4/15) | |
| Excluded due to other causesd | 28% (14/50) | 19% (5/26) | 38% (9/24) | |
Abbreviations: DIF, direct immunofluorescence; IIF, indirect immunofluorescence.
Follow-up: one patient from the established disease group died before 12 months follow-up, and one from the established disease group failed to attend between 12 and 24 months, and was referred back to their local hospital for continuing care.
Ten patients in total did not undergo a conjunctival biopsy (seven patients with advanced age (>80 years) and immunosuppression was systemically contraindicated and the remaining three patients had end-stage disease (defined as Mondino/Foster stage 4)). Data were missing for one individual and this patient was excluded from analysis.
All patients who were IIF were also DIF positive. There were no patients who were IIF positive in the absence of positive DIF studies.
Visual acutiy represents a comparison of visual acuity in the better-seeing eye, after exclusion of other causes of reduced vision such as cataract, glaucoma, age-related macular degeneration and diabetic retinopathy (n=14, early-onset disease 5; established disease 9).
The early-onset disease group consisted of a younger cohort of patients with increased frequency of oral mucous membrane pemphigoid. DIF and IIF refer to the proportion of patients who demonstrated the linear deposition of immunoglobulin G, A or complement (C3) along the basement membrane zone or had measurable titres of immunoglobulin in the serum, respectively. Comparisons were undertaken with Fishers-exact test, Kendall's τ-b for rank correlations and continuous variables were analysed by nonparametric tests (Mann–Whitney U-test). Significant P-values are in bold text.
Biopsies
A total of 87.2% (34/39) of patients, who underwent a biopsy, were direct immunofluorescence (DIF) positive. By contrast, indirect immunofluorescence studies were positive in only 34.8% (8/23) of tested individuals, all of whom were also DIF positive (Table 1). Although five (12.8%) patients were biopsy negative, these patients had clinical features consistent with OcMMP in the absence of other causes of progressive conjunctival scarring. Ten patients in total did not undergo a conjunctival biopsy: seven patients were of advanced age (>80 years) with co-morbidities in which systemic immunosuppression was contraindicated; and the remaining three patients had end-stage disease (defined as Mondino/Foster stage 4) in which the sensitivity of a positive DIF conjunctival biopsy is low due to physical destruction of the basement membrane zone architecture.15
Extra-ocular features
Extra-ocular mucocutaneous involvement was present in 52% (26/50) of patients at presentation (62% (16/26) of the EOD group; 42% (10/24) of EstD group; P=0.257; Table 1). A total of 18% (9/50) patients had a history of skin involvement and this was more frequently reported in the EstD group (29.2% (7/24)) than the EOD group (7.7% (2/26)). Conversely, oral involvement was more common in the EOD group (57.7% (15/26)) compared with 20.8% (5/24) in the EstD group (P=0.01).
Visual acuity
After excluding other causes of reduced vision such as cataract, age-related macular degeneration, glaucoma and diabetic retinopathy (n=14, EOD 5; EstD 9) at presentation, 95% (20/21) of patients in the EOD group and 60% (19/15) in the EstD group had a Snellen VA of between 6/6 and 6/18 in the better-seeing eye. Only patients in the EstD group were severely visually impaired (<3/60, 26.7% (4/15)) and overall VA was significantly worse for the EstD group (P<0.01; Kendal τ-b; Table 1).
Inflammation
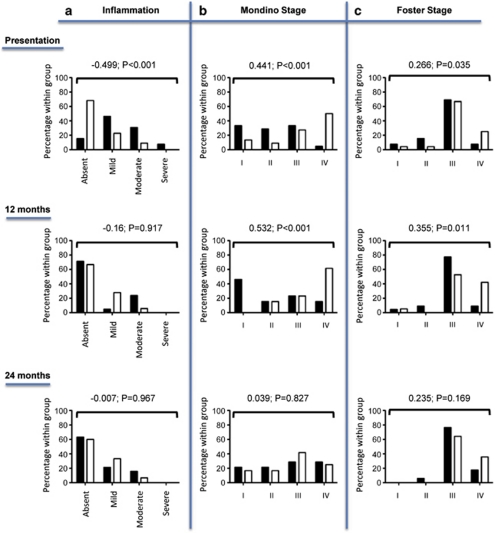
At presentation, 53% (50/94) of all eyes had clinical evidence of conjunctival inflammation where the majority (76% (38/50 eyes)) were in the EOD group (P<0.001) when comparing the worst affected eye (Figure 3a). Patients with moderate/severe inflammation were also more likely to have EOD. By 12 months follow-up (Figure 3a), inflammation had resolved in 78% (60/77) of all eyes (EOD=83% (35/42) vs EstD=71% (25/35), P=0.917) and there were no patients with residual severe conjunctival inflammation. These data were endorsed by McNemar's longitudinal analysis, showing a significant reduction in inflammation in the EOD (P<0.001) compared with the EstD group (P=1.0).
Figure 3.
Cross-sectional analysis of clinically detected conjunctival inflammation (a) and ocular staging using Mondino (b) and Foster (c) systems in the worst eye at presentation, 12 months and 24 months follow-up in the EOD (▪) and EstD groups (□). Differences in the extent of conjunctival inflammation and stage of disease were compared between the two groups by rank correlation using Kendal's τ-b. At 12 months follow-up, inflammation had resolved in the majority of eyes within both groups, and there were no patients with severe inflammation. By 24 months, 30% of the remaining patients at the tertiary centres had residual inflammation not responsive to treatment. Note that patients in the EstD had more advanced stage of disease compared with the EOD throughout the follow-up period, but there was no difference in the progression rate (worsening of clinical stage of disease) between the two groups. NB 14 patients had been referred back to their original hospital by 24 months and 1 had died. These patients have been excluded from the analysis thereby accounting for the apparent increase in the percentage of patients at stage 1 and decrease in the percentage of patients at stage 4 disease during the 12 and 24 months according to the Mondino staging system.
A recalcitrant group of patients with persistent inflammation not responsive or only partially responsive to treatment was identified in 29.9% (20/67) of eyes examined at 24 months. Interestingly, the persistence of inflammation was independent of group phenotype (EOD=27% (10/37) vs EstD=33.3% (10/30), P=0.967; Figure 3a).
Stage of disease and progression
Eyes in the EstD group had more severe conjunctival fibrosis at presentation (Figures 3b and c) gauged by staging systems described by both Mondino (stage IV: EOD=5% (2/40) vs EstD=39.5% (17/45), P<0.001) and Foster (stage IV: EOD=3.9% (2/51) vs EstD=13% (16/46), P<0.035). At 12 months, the EstD group demonstrated significantly advanced stage of disease, irrespective of staging system used (Figures 3b and c), despite a total of 20.8% of all eyes having progressed according to both Mondino and Foster systems.
There was no significant difference when comparing progression (defined by worsening of clinical stage of disease in at least one eye) amongst patients in both the groups, neither between presentation and at 12 months (Mondino: EOD=33.3% (4/12) vs EstD=23.1% (3/13), P=0.67; Foster: EOD=18.2% (4/22) vs EstD=38.9% (7/18) P=0.173), nor during the subsequent 12 to 24 months follow-up period (Mondino: EOD=53.8% (4/22) vs EstD=16.7% (2/12), P=0.10; Foster: EOD=23.5% (4/17) vs EstD=28.6% (4/14) P=1.0; Fishers exact test).
Progression and the presence of conjunctival inflammation
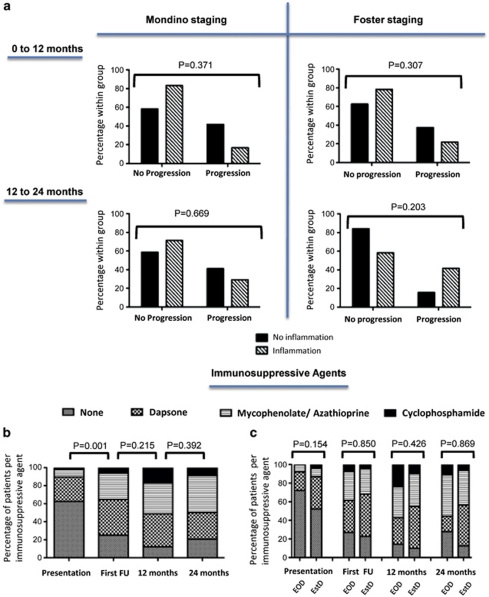
Differences in the rates of progression (defined by an advance in Mondino or Foster staging) were stratified according to the presence or absence of clinically identifiable conjunctival inflammation in at least one eye. There was no significant difference observed between the first and second 12 months follow-up periods (data not shown). Moreover, despite the absence of clinically detectable inflammation, progression of disease occurred in 42% of patients according to the Mondino system (Figure 4a, left panel), and 16 and 38% of patients according to the Foster (Figure 4a, right panel) system for each of the 12 months follow-up periods, respectively.
Figure 4.
Progression rates, defined by worsening of either Mondino or Foster clinical staging of MMP, in the presence or absence of clinically detectable conjunctival inflammation are shown in the upper composite (a). Note there was no significant difference in progression between eyes with clinically detectable inflammation or those that were seemingly uninflamed (Fishers exact test). The percentage of patients requiring immunosuppression at presentation, following the first follow-up (FU) clinic visit, 12 months and 24 months follow-up time points are shown in the lower b and c. Immunosuppression strategies were ranked according to the hierarchy described by Rauz et al.13 Overall, a significant initiation or escalation in ‘strategic-step' was required at the first FU visit (b; McNemar's test), but this did not significantly differ when the early onset (EoD) and established disease (EstD) groups were compared (c; Kendal's τ-b). By 12 months follow-up, five patients stabilised on immunosuppression and were discharged back to their originating hospitals, and similarly a further 10 between the 12 and 24 months follow-up.
Surgical intervention
At initial presentation to the specialist units, 32% (16/50) of patients had previously undergone eyelid or fornix reconstructive surgery by the referring hospital with the majority of cases being performed in the EstD group (P<0.01; Supplementary Table 1). Although both groups required oculoplastic surgical intervention at the tertiary hospitals, this did not differ between the two groups, nor during the first and second 12 months follow-up periods.
Immunosuppression strategies
In all, 40% (20/50) of all patients were on immunosuppression at the time of referral. After first consultation, 36% (18/50) required initiation and 12% (6/50) a switch to a more potent immunomodulatory treatment representing a significant overall ‘step-up' on the step ladder approach (P=0.001; Figure 4b). During the first 12 months follow-up period, a further 30% (13/43) of patients required ‘step-up' treatment (P=0.215; Figure 4b) equating to a total of 88% (38/43) of patients requiring initiation or changes in immunosuppression at presentation or during the first year of follow-up. By 24 months, immunosuppression could be withdrawn in only one patient but no further escalation in therapy was required. In all, 28% (14) patients had stabilised and were discharged to the referring unit for immunosuppression monitoring (Table 1). There was no statistical difference between the requirements for immunosuppression for each of the patient groups at each of the time points (Figure 4c).
The most commonly used drug by the referring unit was Dapsone (26%, n=13) followed by either azathioprine or mycophenolate (8%, n=4) with only one patient on cyclophosphamide (2%, n=1). The majority of these patients were commenced on azathioprine or mycophenolate (10%, n=5) or switched to these drugs from dapsone (10%, n=5). Two further patients required oral cyclophosphamide to control inflammation. By 12 months, an additional seven patients had initiated cyclophosphamide therapy and this was either because of the presence of exuberant inflammation (n=3) not adequately responding to less potent agents (two requiring intravenous (i.v.) methylprednisolone) or there was a requirement for an increase in immuno-modulation before ocular or eyelid reconstructive surgery. Resolution of inflammation occurred in two patients who were ‘stepped down' to less potent agents. By 24 months, oral cyclophosphamide was withdrawn in three patients (because of completing the maximum safe duration of therapy of approximately 14 months, that is, a cumulative dose (oral or i.v.) of <20 g. The majority (40%) of patients were maintained on either mycophenolate or azathioprine. There was no statistical difference in the immunosuppressive agents used between the EOD and EstD groups. i.v. immunoglobulin or biological agents were not administered during the course of this study.
Adverse reactions to immunosuppression
Only 6 of the 38 patients that required immunosuppression suffered from adverse effects. Adverse events included one episode of anaemia following dapsone; two patients reported headaches after the use of azathioprine and one had induction of hepatic enzymes; three patients developed lymphopaenia while taking cyclophosphamide, including one patient who developed respiratory failure secondary to a combined cytomegalovirus and Pneumocystis carinii pneumonitis, which resolved following admission to the intensive care unit and treatment with i.v. ganciclovir and oral cotrimoxazole.
Discussion
OcMMP is a bilateral sight-threatening disorder characterised by progressive conjunctival cicatrisation associated with corneal vascularisation and scarring. The true incidence is not known although the outcome of a recent British Ophthalmological Surveillance Unit study suggests a minimum United Kingdom incidence of 0.7 per 1000 000 population with a regional variance exemplified by 1.1 per million in Greater London and 1.8 per million in the West Midlands20 (Radford et al, unpublished data). MMP usually presents between 30 and 90 years of age, with a peak age of onset after 70 years.3, 7, 19 Although disease progression is more aggressive in younger patients,13 the disease is lifelong causing chronic discomfort, with 75% of patients requiring immunosuppression to control inflammation and limit disease progression.8 The presence of extra-ocular manifestations of MMP in approximately half of our patients is consistent with other studies,5, 10 although higher rates have been reported.17
In this series, two disease phenotypes of OcMMP were statistically defined: (i) those with EOD who were characterised as having less advanced disease stage but significantly greater conjunctival inflammation, and (ii) those patients with EstD who had less clinically identifiable inflammation but more advanced stage of disease. Although 40% of the patient cohort were on existing systemic immunosuppression, the majority of patents required initiation or escalation in systemic immunosuppression following either their first clinic visit, or during the first year of follow-up in order to control inflammation, facilitate corrective eyelid surgery or prevent further progression in already advanced disease states. Despite these measures, 20.8% of eyes demonstrated disease progression during the first 12 months and another 20.8% between 12 and 24 months; and this progression was independent of the EOD or EstD clinical phenotypes. These results indicate that OcMMP may progress at any stage of disease,7, 8 and more importantly, progression rates amongst eyes that are clinically inflamed and those that are not do not differ. These data endorse previously reported literature,4, 10, 21 and signify a molecular, fibrotic process independent of inflammation, which can be seen clinically.
Accurately identifying early disease and documenting progression presents difficulties. The staging systems currently used are reliant on subjective assessment of conjunctival fibrosis and obliteration of the inferior fornix, with judgment of progression open to individual interpretation. Information regarding horizontal obliteration of the fornix by symblephara is not routinely documented, precluding use of the proposed and improved staging system described by Tauber.22 In addition, there is no standardised method for measuring and documenting disease progression of the upper fornix, when the disease is clearly not confined to the inferior conjunctival surface. Subsequent to this study, we have designed and adopted the use of a validated bespoke Fornix depth measurer to routine clinical practise to enable quantification of upper and lower forniceal obliteration and to monitor disease progression.23
The difficulty lies not only in identifying early disease and determining which patients may progress, but also recognising when this is happening. ‘Activity' and ‘damage' indices have been validated and accepted for a number of autoimmune conditions including systemic lupus erythematosis and primary Sjögren's syndrome.24, 25, 26, 27 These indices facilitate not only comparison of clinical cohorts worldwide, but also inform clinical trials specifically those targeting therapeutic intervention. As such, we suggest that clearer strategies for discriminating MMP disease ‘activity' and ‘damage' are necessary to afford a uniform language and understanding when describing OcMMP phenotypes, before molecular targeting and evaluation of novel therapeutic approaches through randomised controlled trials can be considered.
These difficulties in determining activity and progression highlight the challenge in directing appropriate therapy. The issue of suboptimal therapeutic immuno-modulation of disease course has been widely described.4, 5, 9, 10, 11, 28 We highlight a recalcitrant group of patients with persistent mild or moderate inflammation in keeping with the findings of others.10, 11, 13 Unfortunately, there is also a disease subset that either is completely refractory to conventional immunosuppression or relapses despite initial success.11 A few isolated case reports indicate that ‘biological' agents, such as rituximab (anti-CD20) or infliximab (anti-TNFα), may be beneficial in some of these patients, but as randomised trials are lacking, funding for such treatment in the United Kingdom prohibits regular use.29, 30, 31, 32, 33, 34 These data re-emphasise the fact that the pathogenesis of OcMMP is not resolved and strengthen the case for further study of clinically involved and seemingly uninvolved mucous membranes.35, 36, 37
The reasons for a delay in presentation in our EstD group are not clear. It is possible that the clinical features or the severity of the disease may not have been recognised until late, as early symptoms may have been insidious and non-specific.9, 38 A variable duration of disease and course have been described by others,11 but the true definition of early disease in the context of OcMMP is not known. This may well lie under the 3 year duration of symptoms statistically defined in our cohort, and this is particularly relevant if disease activity or progression is initially either subtle or sub-clinical. Experience in other more common autoimmune diseases, such as rheumatoid arthritis, point to the clinician actively pursuing identification of early disease to enable early therapeutic intervention in order to limit tissue damage.39 In the light of the potential for disease progression in both early or late onset OcMMP disease forms, irrespective of whether inflammation is clinically detected or not, it may be prudent for ophthalmologists to take precedence from the rheumatological concepts for capturing early disease, and adopt a similar approach.
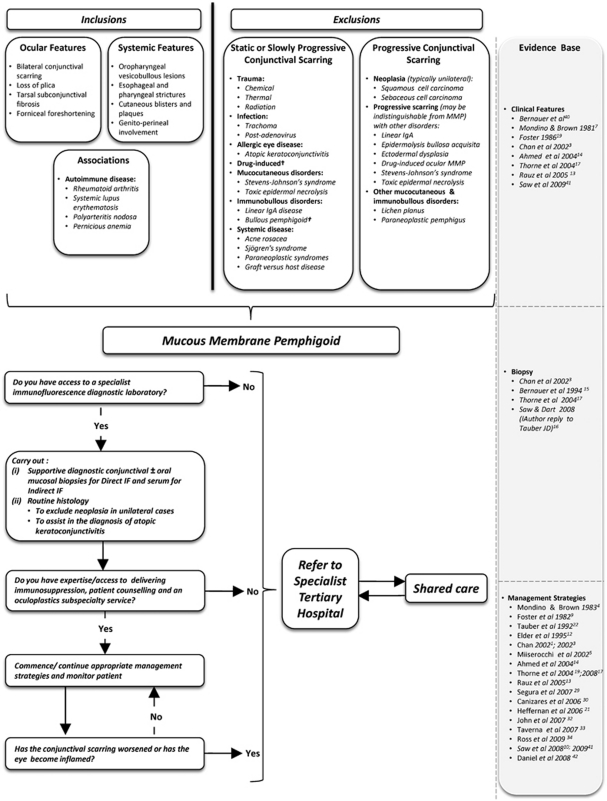
Many of our patients travelled long distances to our centres and this may represent a barrier to early tertiary care of this rare disease, resulting in initiation of suboptimal immunomodulation and/or surgery. Stringent efforts to identify features characteristic of OcMMP are necessary in order to avoid missing an early diagnosis. Where local diagnosis or management is not possible, or where the identification of high risk features including severe refractory inflammation or evidence of progression is manifest, prompt referral of cases with OcMMP to specialised tertiary centres is essential for optimisation of immunosuppression aimed at limiting long-term tissue damage. Furthermore, this may include implementation of shared care pathways or stabilisation of disease before discharge back to local referring centres, as evident in many (28%) of our patients. We therefore propose a referral algorithm as a putative model to help educate these decisions and prompt early referral (Figure 5).
Figure 5.
An algorithm highlighting clues to the diagnosis of OcMMP (ocular features, systemic involvement, autoimmune disease associations), together with differential diagnoses for conjunctival scarring subdivided into ‘static or slowly progressive' or ‘progressive' aetiologies is shown. A putative model for early referral to tertiary care hospitals is also suggested. †, A subset develop autoantibody-positive progressive conjunctival scarring similar to MMP; IF, immunofluorescence; MMP, mucous membrane pemphigoid.
Although our study is limited by its retrospective nature and bias in our study population towards a more severe clinical phenotype, a high proportion (48%) of patients required initiation or a switch to more potent immunosuppression following referral to our tertiary centres. In addition, 28% of patients were eventually returned to their local referring unit for monitoring after stabilisation of disease. Most importantly, however, up to 42% of patients (irrespective of disease phenotype, early or late) continued to demonstrate progressive conjunctival scarring in the absence of clinically detectable inflammation. A greater understanding of disease pathology is required to facilitate earlier recognition of disease, improved activity and damage scores, and more accurate therapeutic targeting, specifically for patients recalcitrant to existing immunomodulatory therapy.

Acknowledgments
We thank Peter J McDonnell FRCP FRCOphth, Consultant Ophthalmologist (Birmingham and Midland Eye Centre, UK), for reviewing the manuscript. This study was supported by the Wellcome Trust, UK (GPW, SR), Moorfields Eye Hospital Unrestricted Grant (JKGD, CR), the Academic Unit of Ophthalmology is supported by the Birmingham Eye Foundation (Registered (UK) Charity 257549).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Eye website (http://www.nature.com/eye)
Supplementary Material
References
- Chan LS. Mucous membrane pemphigoid. Clin Dermatol. 2001;19:703–711. doi: 10.1016/s0738-081x(00)00196-6. [DOI] [PubMed] [Google Scholar]
- Risser J, Lewis K, Weinstock MA. Mortality of bullous skin disorders from 1979 through 2002 in the United States. Arch Dermatol. 2009;145 (9:1005–1008. doi: 10.1001/archdermatol.2009.205. [DOI] [PubMed] [Google Scholar]
- Chan LS, Ahmed AR, Anhalt GJ, Bernauer W, Cooper KD, Elder MJ, et al. The first international consensus on mucous membrane pemphigoid: definition, diagnostic criteria, pathogenic factors, medical treatment, and prognostic indicators. Arch Dermatol. 2002;138:370–379. doi: 10.1001/archderm.138.3.370. [DOI] [PubMed] [Google Scholar]
- Mondino BJ, Brown SI. Immunosuppressive therapy in ocular cicatricial pemphigoid. Am J Ophthalmol. 1983;96:453–459. doi: 10.1016/s0002-9394(14)77908-5. [DOI] [PubMed] [Google Scholar]
- Miserocchi E, Baltatzis S, Roque MR, Ahmed AR, Foster CS. The effect of treatment and its related side effects in patients with severe ocular cicatricial pemphigoid. Ophthalmology. 2002;109:111–118. doi: 10.1016/s0161-6420(01)00863-6. [DOI] [PubMed] [Google Scholar]
- Rogers RS, III, Seehafer JR, Perry HO. Treatment of cicatricial (benign mucous membrane) pemphigoid with dapsone. J Am Acad Dermatol. 1982;6:215–223. doi: 10.1016/s0190-9622(82)70014-3. [DOI] [PubMed] [Google Scholar]
- Mondino BJ, Brown SI. Ocular cicatricial pemphigoid. Ophthalmology. 1981;88:95–100. doi: 10.1016/s0161-6420(81)35069-6. [DOI] [PubMed] [Google Scholar]
- Elder MJ, Bernauer W, Leonard J, Dart JK. Progression of disease in ocular cicatricial pemphigoid. Br J Ophthalmol. 1996;80:292–296. doi: 10.1136/bjo.80.4.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster CS, Wilson LA, Ekins MB. Immunosuppressive therapy for progressive ocular cicatricial pemphigoid. Ophthalmology. 1982;89:340–353. doi: 10.1016/s0161-6420(82)34791-0. [DOI] [PubMed] [Google Scholar]
- Saw VP, Dart JK, Rauz S, Ramsey A, Bunce C, Xing W, et al. Immunosuppressive therapy for ocular mucous membrane pemphigoid strategies and outcomes Ophthalmology 2008115253–261.e1. [DOI] [PubMed] [Google Scholar]
- Thorne JE, Woreta FA, Jabs DA, Anhalt GJ.Treatment of ocular mucous membrane pemphigoid with immunosuppressive drug therapy Ophthalmology 20081152146–2152.e1. [DOI] [PubMed] [Google Scholar]
- Elder MJ, Lightman S, Dart JK. Role of cyclophosphamide and high dose steroid in ocular cicatricial pemphigoid. Br J Ophthalmol. 1995;79:264–266. doi: 10.1136/bjo.79.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauz S, Maddison PG, Dart JK. Evaluation of mucous membrane pemphigoid with ocular involvement in young patients. Ophthalmology. 2005;112:1268–1274. doi: 10.1016/j.ophtha.2005.01.039. [DOI] [PubMed] [Google Scholar]
- Ahmed M, Zein G, Khawaja F, Foster CS. Ocular cicatricial pemphigoid: pathogenesis, diagnosis and treatment. Prog Retin Eye Res. 2004;23:579–592. doi: 10.1016/j.preteyeres.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Bernauer W, Elder MJ, Leonard JN, Wright P, Dart JK. The value of biopsies in the evaluation of chronic progressive conjunctival cicatrisation. Graefes Arch Clin Exp Ophthalmol. 1994;232:533–537. doi: 10.1007/BF00181996. [DOI] [PubMed] [Google Scholar]
- Tauber J.Ocular cicatricial pemphigoid Ophthalmology 20081151639–1640.author reply 40-41. [DOI] [PubMed] [Google Scholar]
- Thorne JE, Anhalt GJ, Jabs DA. Mucous membrane pemphigoid and pseudopemphigoid. Ophthalmology. 2004;111:45–52. doi: 10.1016/j.ophtha.2003.03.001. [DOI] [PubMed] [Google Scholar]
- Elder MJ, Bernauer W.Monitoring of activity and progression in cicatrising conjunctivitisIn: Bernauer W, Dart JKG, Elder MJ (eds).Developments in Ophthalmology, vol 28. Cicatrising Conjunctivitis Karger: Basel, Switzerland; 1997111–122. [DOI] [PubMed] [Google Scholar]
- Foster CS. Cicatricial pemphigoid. Trans Am Ophthalmol Soc. 1986;84:527–663. [PMC free article] [PubMed] [Google Scholar]
- Radford CF, Saw VP, Rauz S, Dart JKG.Incidence and presentation of ocular mucous membrane pemphigoid in the UK. Invest Ophthalmol Vis Sci 201051E-Abstract 3769.
- Elder MJ.The role of cytokines in chronic progressive conjunctival cicatrisationIn: Bernauer W, Dart JKG, Elder MJ (eds). Developments in Ophthalmology, vol 28. Cicatrising Conjunctivitis Karger: Basel, Switzerland; 1997159–175. [DOI] [PubMed] [Google Scholar]
- Tauber J, Jabbur N, Foster CS. Improved detection of disease progression in ocular cicatricial pemphigoid. Cornea. 1992;11:446–451. doi: 10.1097/00003226-199209000-00015. [DOI] [PubMed] [Google Scholar]
- Williams GP, Saw VP, Saeed T, Evans ST, Cottrell P, Curnow SJ, et al. Validation of a Fornix depth measurer—a putative tool for the assessment of progressive cicatrising Conjunctivitis. Br J Ophthalmol. 2011;95 6:842–847. doi: 10.1136/bjo.2010.188011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee CS, Farewell V, Isenberg DA, Rahman A, Teh LS, Griffiths B, et al. British Isles Lupus Assessment Group 2004 index is valid for assessment of disease activity in systemic lupus erythematosus. Arthritis Rheum. 2007;56:4113–4119. doi: 10.1002/art.23130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladman D, Goldsmith C, Urowitz M, Bacon P, Fortin P, Ginzler E, et al. The Sytemic Lupus International Collaborating Clinics/American College of Rheumatology (SLICC/ACR) Damage Index for systemic lupus erythematosus international comparison. J Rheumatol. 2000;27:373–376. [PubMed] [Google Scholar]
- Bowman SJ, Sutcliffe N, Isenberg DA, Goldblatt F, Adler M, Price E, et al. Sjögren's Systemic Clinical Activity Index (SCAI)--a systemic disease activity measure for use in clinical trials in primary Sjögren's syndrome. Rheumatology (Oxford) 2007;46 (12:1845–1851. doi: 10.1093/rheumatology/kem280. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Sutcliffe N, Isenberg DA, Price E, Goldblatt F, Adler M, et al. The Sjögren's Syndrome Damage Index--a damage index for use in clinical trials and observational studies in primary Sjögren's syndrome. Rheumatology (Oxford) 2008;47 (8:1193–1198. doi: 10.1093/rheumatology/ken164. [DOI] [PubMed] [Google Scholar]
- Tauber J, Sainz de la Maza M, Foster CS. Systemic chemotherapy for ocular cicatricial pemphigoid. Cornea. 1991;10:185–195. doi: 10.1097/00003226-199105000-00001. [DOI] [PubMed] [Google Scholar]
- Segura S, Iranzo P, Martínez-de Pablo I, Mascaró JM, Alsina M, Herrero J, et al. High-dose intravenous immunoglobulins for the treatment of autoimmune mucocutaneous blistering diseases: evaluation of its use in 19 cases. J Am Acad Dermatol. 2007;56 (6:960–967. doi: 10.1016/j.jaad.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Canizares MJ, Smith DI, Conners MS, Maverick KJ, Heffernan MP. Successful treatment of mucous membrane pemphigoid with etanercept in 3 patients. Arch Dermatol. 2006;142 (11:1457–1461. doi: 10.1001/archderm.142.11.1457. [DOI] [PubMed] [Google Scholar]
- Heffernan MP, Bentley DD. Successful treatment of mucous membrane pemphigoid with infliximab. Arch Dermatol. 2006;142 (10:1268–1270. doi: 10.1001/archderm.142.10.1268. [DOI] [PubMed] [Google Scholar]
- John H, Whallett A, Quinlan M. Successful biologic treatment of ocular mucous membrane pemphigoid with anti-TNF-alpha. Eye (Lond) 2007;21:1434–1435. doi: 10.1038/sj.eye.6702948. [DOI] [PubMed] [Google Scholar]
- Taverna JA, Lerner A, Bhawan J, Demierre MF. Successful adjuvant treatment of recalcitrant mucous membrane pemphigoid with anti-CD20 antibody rituximab. J Drugs Dermatol. 2007;6 (7:731–732. [PubMed] [Google Scholar]
- Ross AH, Jaycock P, Cook SD, Dick AD, Tole DM.The use of rituximab in refractory mucous membrane pemphigoid with severe ocular involvement Br J Ophthalmol 200993421–422.548. [DOI] [PubMed] [Google Scholar]
- Bernauer W, Wright P, Dart JK, Leonard JN, Lightman S. The conjunctiva in acute and chronic mucous membrane pemphigoid. An immunohistochemical analysis. Ophthalmology. 1993;100:339–346. doi: 10.1016/s0161-6420(93)31644-1. [DOI] [PubMed] [Google Scholar]
- Rice BA, Foster CS. Immunopathology of cicatricial pemphigoid affecting the conjunctiva. Ophthalmology. 1990;97:1476–1483. doi: 10.1016/s0161-6420(90)32402-8. [DOI] [PubMed] [Google Scholar]
- Sacks EH, Jakobiec FA, Wieczorek R, Donnenfeld E, Perry H, Knowles DM., Jr Immunophenotypic analysis of the inflammatory infiltrate in ocular cicatricial pemphigoid. Further evidence for a T cell-mediated disease. Ophthalmology. 1989;96:236–243. doi: 10.1016/s0161-6420(89)32922-8. [DOI] [PubMed] [Google Scholar]
- Bernauer W, Itin PH, Kirtschig G.Cicatricial pemphigoidIn: Bernauer W, Dart JKG, Elder MJ (eds).Developments in Ophthalmology, vol 28. Cicatrising Conjunctivitis Karger: Basel, Switzerland; 199746–63. [DOI] [PubMed] [Google Scholar]
- Sheppard J, Kumar K, Buckley CD, Shaw KL, Raza K. ‘I just thought it was normal aches and pains': a qualitative study of decision-making processes in patients with early rheumatoid arthritis. Rheumatology (Oxford) 2008;47 (10:1577–1582. doi: 10.1093/rheumatology/ken304. [DOI] [PubMed] [Google Scholar]
- Bernauer W, Elder MJ, Dart JK.Introduction to cicatrising conjunctivitisIn: Bernauer W, Dart JKG, Elder MJ (eds).Developments in Ophthalmology, vol 28. Cicatrising Conjunctivitis Karger: Basel, Switzerland; 19971–10. [DOI] [PubMed] [Google Scholar]
- Saw VP, Dart JK. Ocular mucous membrane pemphigoid: diagnosis and management strategies. Ocul Surf. 2008;6 (3:128–142. doi: 10.1016/s1542-0124(12)70281-1. [DOI] [PubMed] [Google Scholar]
- Daniel E, Thorne JE. Recent advances in mucous membrane pemphigoid. Curr Opin Ophthalmol. 2008;19 (4:292–297. doi: 10.1097/ICU.0b013e328302cc61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.