Abstract
Testosterone is an important hormone for both bone gain and maintenance in men. Hypogonadal men have accelerated bone turnover and increased fracture risk. In these men, administration of testosterone inhibits bone resorption and maintains bone mass. Testosterone, however, is converted into estradiol via aromatization in many tissues including male bone. The importance of estrogen receptor alpha activation as well of aromatization of androgens into estrogens was highlighted by a number of cases of men suffering from an inactivating mutation in the estrogen receptor alpha or in the aromatase enzyme. All these men typically had low bone mass, high bone turnover and open epiphyses. In line with these findings, cohort studies have confirmed that estradiol contributes to the maintenance of bone mass after reaching peak bone mass, with an association between estradiol and fractures in elderly men. Recent studies in knock-out mice have increased our understanding of the role of androgens and estrogens in different bone compartments. Estrogen receptor activation, but not androgen receptor activation, is involved in the regulation of male longitudinal appendicular skeletal growth in mice. Both the androgen and the estrogen receptor can independently mediate the cancellous bone-sparing effects of sex steroids in male mice. Selective KO studies of the androgen receptor in osteoblasts in male mice suggest that the osteoblast in the target cell for androgen receptor mediated maintenance of trabecular bone volume and coordination of bone matrix synthesis and mineralization. Taken together, both human and animal studies suggest that testosterone has a dual mode of action on different bone surfaces with involvement of both the androgen and estrogen receptor.
1. Introduction
The major circulating androgen in men is testosterone. Testosterone, like adrenal androgens, is a C19 steroid synthesized from cholesterol. Both gonadal and adrenal testosterone can be converted into estrogens (C18 steroids) by the P 450 aromatase, encoded by CYP 19, which is present in many peripheral tissues, including bone. Bone cells express androgen receptor (AR) as well as estrogen receptor-α (ERα) and –β (ERβ) [1]. Therefore, androgen action on male bone may be explained by AR activation or, alternatively, activation of ERα and –β (Figure 1).
Figure 1.
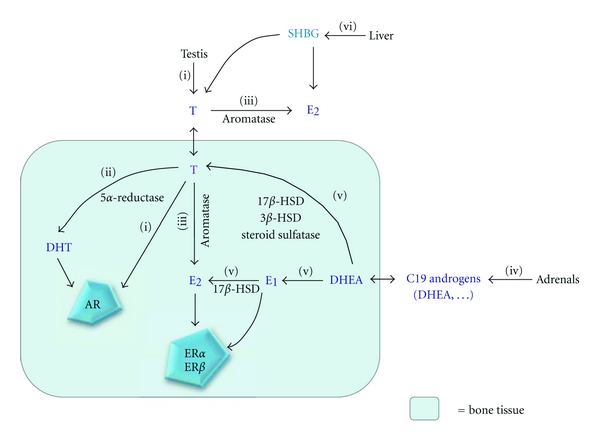
Metabolism of sex steroids. (i) Testosterone (T), that is secreted by the testes, can directly act on its receptor, the androgen receptor (AR), present in bone cells. (ii) T can also be converted locally to dihydrotestosterone (DHT) by 5α-reductase. (iii) In addition, T can undergo aromatization to 17β-estradiol (E2) by aromatase. E2acts on one or both estrogen receptors (ERα or ERβ). (iv) The adrenals secrete C19 androgens including dehydroepiandrosterone (DHEA) that can also be converted to (v) estrone (E1) and E2by aromatase, 17β-hydroxysteroid dehydrogenase (17β-HSD), and 3β-HSD or to T by 17β-HSD and/or 3β-HSD. (vi) In men and women, T and E2are predominantly bound to sex hormone binding globulin (SHBG), synthesized by the liver.
2. Evidence from Human Studies
The importance of estrogen receptor alpha activation as well as of aromatization of androgens into estrogens was highlighted by a number of cases of men suffering from an inactivating mutation in the estrogen receptor alpha or in the aromatase enzyme [2–4].
Table 1 shows the age of diagnosis, bone phenotype, and effect of estrogen treatment (if appropriate) in these men. All these men had low bone mass as measured by DEXA, high bone turnover, and open epiphyses and the distal radius despite normal to elevated testosterone concentrations. Estrogen treatment resulted in closure of the epiphyses, increased bone density, and reduced bone turnover. One patient diagnosed at much younger age was followed by peripheral computerized tomography during estrogen treatment over a period of three years. During estrogen treatment, the cortical area expanded as a result of periosteal expansion. There was no effect on trabecular bone density suggesting that the main action of estrogen on male growing bone is on the cortical and not the cancellous compartment [5]. However, it has been suggested that trabecular bone may require higher levels for its regulation. For cortical bone, estrogen deficiency is the major cause of age-related bone loss [6]. It is clear, therefore, that estrogen is important for epiphyseal closure as well as periosteal bone formation and hence cortical bone mineral acquisition during male growth. Observations in a single patient suffering from an inactivating mutation in the androgen receptor suggest that estrogen may also increase mineral apposition at the endocortical surface [7]. During adult life, estrogens mediate endosteal bone apposition and volumetric bone density, without marked influence on periosteal bone apposition. The finding of a bone size intermediate between male and female supports a role for testosterone as an essential mediator for periosteal bone expansion, but not as the sole stimulus for bone expansion during growth, supporting the concept that estrogen is also involved in bone maintenance after growth in men.
Table 1.
Clinical parameters of men with aromatase deficiency or estrogen resistance.
| Aromatase deficiency | Estrogen resistance | |
|---|---|---|
| Age of diagnosis | Newborn, 24–38 yrs | 28.5 yrs |
| Bone phenotype | (i) Persistent linear growth | (i) Continuing linear growth into adulthood |
| (ii) Unfused epiphyseal cartilages | (ii) Unfused epiphyses | |
| (iii) Delayed bone age | (iii) Delayed bone age | |
| (iv) Osteopenia/osteoporosis | (iv) Osteoporosis | |
| (v) Eunuchoid proportion of the skeleton | (v) Progressive genu valgum | |
| (vi) Progressively worsening of bilateral genu vagum | (vi) Eunuchoid proportions of the skeleton | |
| Hormonal analysis | (i) Serum estradiol below the range of detection | (i) High concentration of serum estradiol, estrone, FSH, LH |
| (ii) Gonadotropins and circulating testosterone ranging from normal to elevated | (ii) Normal serum testosterone | |
| (iii) Impaired glucose metabolism | ||
| (iv) Insulin resistance | ||
| Effect of estrogen treatment | (i) Complete epiphyseal closure | No changes |
| (ii) Spinal BMD increase | ||
| (iii) Skeletal maturation |
Table 2 shows cohort studies in men in which both estradiol and testosterone were measured, with either tandem mass spectrometry (LC-MS-MS) or with immunoassay, and in relation to bone loss and fractures [8–12]. Most but not all (Dubbo Osteoporosis Epidemiology study) of these studies showed an association between estradiol and fractures in elderly men [14].
Table 2.
Overview of cohort studies in men.
| Average age of men | Number of men | Study duration | LC-MS-MS | Immunoassay | Main result | |
|---|---|---|---|---|---|---|
| Rancho Bernardo study [8] | 66 | 352 | 12 yr | x | (i) E: strong correlation with fractures (ii) T: no correlation with fractures | |
| Dubbo study [9] | >60 | 609 | 16 yr | x | (i) T: no correlation with fractures (ii) E: strong correlation with fractures |
|
| Tromso [10] | 50–84 | 1364 | 8.4 yr | x | T, E, SHBG: no correlation with fractures | |
| Rotterdam study [11] | 67.7 ± 6.8 | 178 | 6.5 yr | x | T, E, SHBG: no correlation with fractures | |
| Framingham study [12] | 71 | 793 | 18 yr | x | (i) E: strong correlation with fractures (ii) T + E: strong correlation with fractures | |
| MrOS study [13] | 75 | 2639 | 3.3 yr | x | E, SHBG: strong correlation with fractures |
E: estrogen; T: testosterone; SHBG: sex hormone binding globulin.
While serum estradiol and testosterone are inversely related to fracture risk in older men, serum-sex-hormone binding globulin (SHBG) shows a positive relationship. Low serum estradiol, low serum testosterone, and high SHBG predict clinical vertebral fractures, nonvertebral osteoporosis fractures, and hip fractures. For estradiol, a threshold effect has been documented, below which estradiol is related to fracture risk [13]. Low estradiol induces bone resorption independently of testosterone, presumably reflecting a net increase in endocortical bone resorption [15, 16].
The effect of estrogen on bone resorption was illustrated by administration of an aromatase inhibitor to men receiving a GnRH agonist in combination with testosterone, resulting in increased bone resorption markers (deoxypyridinoline, N-telopeptide of type I collagen). Bone resorption markers increased significantly in the absence of both testosterone and estrogen and were unchanged in men receiving both hormones. Estrogen prevented the increase in the bone resorption markers, whereas testosterone had no significant effect. By contrast, serum osteocalcin, a bone formation marker, decreased in the absence of both hormones, and both estrogen and testosterone maintained osteocalcin levels. Taken together, according to this one study in men, estrogen may be important in regulating bone resorption, whereas estrogen and testosterone may both be important in maintaining bone formation [17]. Another study in younger men, however, concluded that both androgens and estrogens play independent and fundamental roles in regulating bone resorption. In this study, young men (20–44 yrs) were divided in 3 groups. The first group received only a GnRH analog, the second group a GnRH analog plus testosterone, and the third group a GnRH analog plus an aromatase inhibitor. Bone resorption markers increased in the group who received a GnRH analog alone and the group who received a GnRH analog plus an aromatase inhibitor. Bone formation markers increased more in the first group than in the second group. Overall, these findings suggest that both estrogens and androgens play independent and fundamental roles in regulating bone resorption in men. This study also suggests that androgens may play an important role in the regulation of bone formation in men [18]. Genetic polymorphism in the Cyp 19 or in ESR genes, encoding for aromatase and estrogen receptor, respectively, may further mediate the risk for bone loss induced by low estradiol in men [19].
In humans, in contrast to rodents, circulating testosterone and estradiol are bound to sex-hormone-binding globulin, with testosterone more tightly bound than estradiol. The role of free (not bound to any protein) or bioavailable (not bound to SHBG) versus total testosterone remains controversial [20]. SHBG, which increases with age, has been associated with bone loss in elderly men [21]. However, other studies in younger men have shown a positive rather than a negative association between peak bone mass and SHBG [22–24]. However, the exact role and contribution of SHBG in bone gain and loss in men remains to be clarified.
Compared to estradiol levels (see Table 1), testosterone concentrations are less consistently associated with bone loss/fractures in men, except for very low levels in hypogonadal men (especially men following chemical and surgical castration) who show a significant increase in bone turnover, bone loss, and fracture risk [25]. In these men, testosterone inhibits bone resorption and maintains bone mass [26] whereas its effect in elderly men with borderline low testosterone or low normal testosterone concentrations is more controversial [27].
With selective estrogen receptor modulators (SERM's), very few data are currently available in men. In one study in men receiving a GnRH agonist for prostate cancer, raloxifene increased bone mineral density of the hip and, to a lesser degree, the spine. In this study, Raloxifene reduced serum concentrations of aminoterminal propeptide of type I collagen, a marker of bone formation and also tended to reduce urinary excretion of deoxypyridinoline, a marker of bone resorption [28]. Short-term administration (12 weeks) of an aromatase inhibitor in elderly men was found to increase testosterone and reduce estrogen levels but with hardly any effect on bone metabolism [29]. This lack of an effect may be due to the concomitant increase in testosterone production, the relative modest effect on estradiol production, or a combination of both factors. However when the aromatase inhibitor was administered for a longer term (12 months) there was a decrease in bone mineral density [30]. Therefore, aromatase inhibition does not improve skeletal health in aging men with low or normal testosterone levels. With selective androgen receptor modulators (SARMs), capable of selectively stimulating the androgen receptor in bone and muscle but not in prostate, no clinical bone data are available in men [31].
3. Evidence from Animal Studies
To further investigate the relative importance of androgen receptor-mediated testosterone actions compared to estradiol effects, an increasing number of animal experiments have been published, in particular in the orchidectomized rodent, a well-characterized model for hypogonadal osteoporosis. Following orchidectomy, bone resorption increases at cancellous and endocortical surfaces and results in reduced cancellous and cortical bone volume. Periosteal bone formation during growth is decreased in orchidectomized rodents as well and further lowers bone strength [1]. A number of animal experiments have investigated the bone phenotypic changes induced by androgens, nonaromatisable androgens and estrogens in orchidectomized rodents (mice, rat and growing/non-growing). Table 3 shows the relative effects of androgens [32–34], non-aromatisable androgens [32–36], estrogens [33, 36, 37] on bone turnover, bone density, and periosteal bone formation in male orchidectomized rats.
Table 3.
Relative effects of testosterone, dihydrotestosterone, estradiol, and selective estrogen receptor modulator on body weight, appendicular skeletal growth, cancellous bone mass, and on cortical bone area.
| Body weight gain | Appendicular skeletal growth | Cancellous bone mass | Cortical bone area | |
|---|---|---|---|---|
| Orch + T | =,↑ | =, ↑ | ↑ | ↑ |
| Orch + DHT | =,↓ | =,↑ | ↑ | =, ↑ |
| Orch + E2 | =, ↓ | = | ↑ | =, ↑ |
| Orch + SERM | ↓ | NA | ↑ | ↑ |
Orch: orchidectomy; T: testosterone; DHT: dihydrotestosterone; E2: estradiol; SERM: selective estrogen receptor modulator.
From these studies, it is clear that non-aromatisable androgens can also stimulate periosteal bone formation and inhibit cancellous bone, although less than testosterone, and that estradiol exerts potent effects on different bone surfaces [36]. However, what is not always clear is to what extent the effects of these hormones are pharmacological or physiological and, if physiological, to what extent the effects can be extrapolated to the human condition, in a context of higher estradiol concentrations than in mice [38].
Other animal experiments have investigated the bone phenotype of transgenic male animals with KO of AR (ArKO), ER alpha (ERKO), beta (BERKO), or both (DERKO), and this in combination with orchidectomy with or without replacement with androgens and estrogens. The latter is needed because the ArKO and ERKO models may have an impact on respective concentrations of androgens/estrogens [26, 39] (Table 4). Overall, available evidence from these studies suggests that, ER activation, not AR activation, is involved in the regulation of male longitudinal appendicular skeletal growth in mice. ERα and AR but not ERβ enhance cortical radial bone growth. The AR, not Erβ, is required for the maintenance of cancellous bone mass. AR and ERα, but not ERβ, can independently mediate the cancellous bone-sparing effects of sex steroids in male mice [26].
Table 4.
Summary of the skeletal phenotypes in mice with different sex steroid-related gene inactivations.
| Cancellous bone | ||||||
|---|---|---|---|---|---|---|
| Longitudinal skeletal growth | Cortical bone area | Intact mice | Effect of E in Orch | Effect of T in Orch | Effect of DHT in Orch | |
| BERKO | 0 | 0 | 0 | Yes | ND | Yes |
| ERKO | − | − | + | No | Yes | Yes |
| DERKO | − | − | + | No | ND | Yes |
| ArKO | − | − | − | Yes | ND | ND |
| Tfm | ? | ? | − | Yes | Partial | ND |
+: Increased; −: decreased; 0: no effect; conflicting results; ND: not determined; Orch: orchidectomy; E: treatment with physiological levels of estrogen; T: treatment with physiological of testosterone; DHT: treatment with physiological levels of 5α-dihydrotestosterone.
These studies have greatly increased our understanding of the role of estrogen receptor and androgen receptors in male skeletal growth and maintenance in male rodents. Both AR and ERα are involved in male skeletal growth and maintenance, supporting a dual mode of action for testosterone, either directly on the AR or indirectly on the ERα through aromatization.
In summary, both AR and ERα activation appear to stimulate periosteal bone formation and cortical bone growth. ERα is also involved in longitudinal bone formation but its action on periosteal surface as well as growth plate may be mediated indirectly by the GH-IGF-I axis [40]. On trabecular bone surfaces, AR activation may be most critical, at least in mice, as elegantly illustrated with a double KO AR-ER in comparison with either AR or ERα disruption alone. Combined AR and ERα inactivation further reduced cortical bone and muscle mass and AR activation was found to be solely responsible for the development and maintenance of male trabecular bone mass. However, both AR and ERα activation appeared to be essential to optimize the acquisition of cortical bone and muscle mass [41]. Erβ, on the other hand, seems not to be relevant for bone growth and maintenance in male mice [42]. To further document the target cell of AR and ER in mice, the AR was recently selectively knockedout in bone cells by cre-lox technology. In two studies (Table 5), the osteoblast was targeted which resulted in a trabecular and no cortical phenotype, suggesting that the osteoblast is the target cell for androgen-receptor-mediated maintenance of trabecular bone volume and coordination of bone matrix synthesis and mineralization [43, 44].
Table 5.
Overview bone parameters in osteoblast-specific AR knockout mice.
| Tb. N (/mm) | Tb. Th (μm) | BV/TV (%) | Osteoid surface (%BS) | OCL surface (%BS) | MAR (μm/day) | BFR (μm2/μm/day) |
|
|---|---|---|---|---|---|---|---|
| Col 2.3-cre AR KO | ↓ | ↑ | ↓ | = | = | = | = |
| Osteocalcin-cre KO | ↓ | ↓ | ↓ | ↑ | = | = | = |
Tb. N: trabecular number; Tb. Th: trabecular thickness; BV/TV: trabecular bone volume; OCL: osteoclast; MAR: mineral apposition rate; BFR: bone formation rate.
This assumption was further supported by a coculture experiment were the in vitro osteoclastogenesis was assessed using osteoclast precursor cells from bone marrow and calvaria osteoblasts from male WT and ARKO mice. When the AR was absent in osteoclast precursor cells, osteoclastogenesis was unaffected [45]. Osteoclastogenesis, in response to 1α,25(OH)2D3 after activation by RANKL and M-CSF (macrophage-colony stimulating factor), also seemed unaffected in AR-deficient osteoclasts. However, AR inactivation in osteoblasts potentiated osteoblastic functions that promote osteoclastogenesis in the presence of inducers. RANKL turned out to be upregulated in these AR-deficient osteoblasts, suggesting that the suppressive function of AR on RANKL gene expression mediates the protective effects of androgens on bone remodelling through inhibition of bone resorption. It would seem therefore that intact AR function is required for the suppressive effects of androgens on the osteoclastogenesis supporting activity of osteoblasts, but not osteoclasts [45].
In conclusion, both human and animal experiments suggest that testosterone has a dual mode of action on different bone surfaces. Activation of both ERα and AR seems to be involved.
Conflict of Interests
The authors have no conflict of interests.
Acknowledgments
S. Boonen is senior clinical investigator of the Fund for Scientific Research (FWO-Vlaanderen) and holder of the Leuven University Chair in Gerontology and Geriatrics. This work was supported by Grant G.0488.08 from the Fund for Scientific Research (FWO-Vlaanderen) to S. Boonen and Research Grants OT-05-53 and OT-09-035 from the Catholic University Leuven to D. Vanderschueren. D. Vanderschueren is a senior clinical investigator of the Leuven University Hospital Clinical Research Fund.
References
- 1.Vanderschueren D, Vandenput L, Boonen S, Lindberg MK, Bouillon R, Ohlsson C. Androgens and bone. Endocrine Reviews. 2004;25(3):389–425. doi: 10.1210/er.2003-0003. [DOI] [PubMed] [Google Scholar]
- 2.Smith EP, Specker B, Bachrach BE, et al. Impact on bone of an estrogen receptor-α gene loss of function mutation. Journal of Clinical Endocrinology and Metabolism. 2008;93(8):3088–3096. doi: 10.1210/jc.2007-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lanfranco F, Zirilli L, Baldi M, et al. A novel mutation in the human aromatase gene: insights on the relationship among serum estradiol, longitudinal growth and bone mineral density in an adult man under estrogen replacement treatment. Bone. 2008;43(3):628–635. doi: 10.1016/j.bone.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Zirilli L, Rochira V, Diazzi C, Caffagni G, Carani C. Human models of aromatase deficiency. Journal of Steroid Biochemistry and Molecular Biology. 2008;109(3–5):212–218. doi: 10.1016/j.jsbmb.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 5.Bouillon R, Bex M, Vanderschueren D, Boonen S. Estrogens are essential for male pubertal periosteal bone expansion. Journal of Clinical Endocrinology and Metabolism. 2004;89(12):6025–6029. doi: 10.1210/jc.2004-0602. [DOI] [PubMed] [Google Scholar]
- 6.Khosla S, Melton LJ, III, Riggs BL. The unitary model for estrogen deficiency and the pathogenesis of osteoporosis: is a revision needed? Journal of Bone and Mineral Research. 2011;26(3):441–451. doi: 10.1002/jbmr.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taes Y, Lapauw B, Vandewalle S, et al. Estrogen-specific action on bone geometry and volumetric bone density: longitudinal observations in an adult with complete androgen insensitivity. Bone. 2009;45(2):392–397. doi: 10.1016/j.bone.2009.04.198. [DOI] [PubMed] [Google Scholar]
- 8.Barrett-Connor E, Mueller JE, Von Mühlen DG, Laughlin GA, Schneider DL, Sartoris DJ. Low levels of estradiol are associated with vertebral fractures in older men, but not women: the Rancho Bernardo study. Journal of Clinical Endocrinology and Metabolism. 2000;85(1):219–223. doi: 10.1210/jcem.85.1.6327. [DOI] [PubMed] [Google Scholar]
- 9.Meier C, Nguyen TV, Handelsman DJ, et al. Endogenous sex hormones and incident fracture risk in older men: the Dubbo Osteoporosis Epidemiology study. Archives of Internal Medicine. 2008;168(1):47–54. doi: 10.1001/archinternmed.2007.2. [DOI] [PubMed] [Google Scholar]
- 10.Bjornerem A, Ahmed LA, Joakimsen RM, et al. A prospective study of sex steroids, sex hormone-binding globulin, and non-vertebral fractures in women and men: the Tromso study. European Journal of Endocrinology. 2007;157(1):119–125. doi: 10.1530/EJE-07-0032. [DOI] [PubMed] [Google Scholar]
- 11.Goderie-Plomp HW, Van Der Klift M, De Ronde W, Hofman A, De Jong FH, Pols HAP. Endogenous sex hormones, sex hormone-binding globulin, and the risk of incident vertebral fractures in elderly men and women: the Rotterdam study. Journal of Clinical Endocrinology and Metabolism. 2004;89(7):3261–3269. doi: 10.1210/jc.2002-022041. [DOI] [PubMed] [Google Scholar]
- 12.Amin S, Zhang Y, Felson DT, et al. Estradiol, testosterone, and the risk for hip fractures in elderly men from the framingham study. American Journal of Medicine. 2006;119(5):426–433. doi: 10.1016/j.amjmed.2005.10.048. [DOI] [PubMed] [Google Scholar]
- 13.Mellström D, Vandenput L, Mallmin H, et al. Older men with low serum estradiol and high serum SHBG have an increased risk of fractures. Journal of Bone and Mineral Research. 2008;23(10):1552–1560. doi: 10.1359/jbmr.080518. [DOI] [PubMed] [Google Scholar]
- 14.Gennari L, Khosla S, Bilezikian JP. Estrogen and fracture risk in men. Journal of Bone and Mineral Research. 2008;23(10):1548–1551. doi: 10.1359/jbmr.0810c. [DOI] [PubMed] [Google Scholar]
- 15.Szulc P, Claustrat B, Munoz F, Marchand F, Delmas PD. Assessment of the role of 17β-oestradiol in bone metabolism in men: does the assay technique matter? The MINOS study. Clinical Endocrinology. 2004;61(4):447–457. doi: 10.1111/j.1365-2265.2004.02117.x. [DOI] [PubMed] [Google Scholar]
- 16.Khosla S, Joseph Melton L, Lawrence Riggs B. Clinical review 144: estrogen and the male skeleton. Journal of Clinical Endocrinology and Metabolism. 2002;87(4):1443–1450. doi: 10.1210/jcem.87.4.8417. [DOI] [PubMed] [Google Scholar]
- 17.Falahati-Nini A, Riggs BL, Atkinson EJ, O’Fallon WM, Eastell R, Khosla S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. Journal of Clinical Investigation. 2000;106(12):1553–1560. doi: 10.1172/JCI10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leder BZ, LeBlanc KM, Schoenfeld DA, Eastell R, Finkelstein JS. Differential effects of androgens and estrogens on bone turnover in normal men. Journal of Clinical Endocrinology and Metabolism. 2003;88(1):204–210. doi: 10.1210/jc.2002-021036. [DOI] [PubMed] [Google Scholar]
- 19.Limer KL, Pye SR, Thomson W, et al. Genetic variation in sex hormone genes influences heel ultrasound parameters in middle-aged and elderly men: results from the European Male Aging study (EMAS) Journal of Bone and Mineral Research. 2009;24(2):314–323. doi: 10.1359/jbmr.080912. [DOI] [PubMed] [Google Scholar]
- 20.Ly LP, Handelsman DJ. Empirical estimation of free testosterone from testosterone and sex hormone-binding globulin immunoassays. European Journal of Endocrinology. 2005;152(3):471–478. doi: 10.1530/eje.1.01844. [DOI] [PubMed] [Google Scholar]
- 21.LeBlanc ES, Nielson CM, Marshall LM, et al. The effects of serum testosterone, estradiol, and sex hormone binding globulin levels on fracture risk in older men. Journal of Clinical Endocrinology and Metabolism. 2009;94(9):3337–3346. doi: 10.1210/jc.2009-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lorentzon M, Swanson C, Andersson N, Mellström D, Ohlsson C. Free testosterone is a positive, whereas free estradiol is a negative, predictor of cortical bone size in young Swedish men: the GOOD study. Journal of Bone and Mineral Research. 2005;20(8):1334–1341. doi: 10.1359/JBMR.050404. [DOI] [PubMed] [Google Scholar]
- 23.Vanbillemont G, Lapauw B, Bogaert V, et al. Sex hormone-binding globulin as an independent determinant of cortical bone status in men at the age of peak bone mass. Journal of Clinical Endocrinology and Metabolism. 2010;95(4):1579–1586. doi: 10.1210/jc.2009-2189. [DOI] [PubMed] [Google Scholar]
- 24.Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocrine Reviews. 2008;29(4):441–464. doi: 10.1210/er.2008-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daniell HW, Dunn SR, Ferguson DW, Lomas G, Niazi Z, Stratte PT. Progressive osteoporosis during androgen deprivation therapy for prostate cancer. Journal of Urology. 2000;163(1):181–186. [PubMed] [Google Scholar]
- 26.Vanderschueren D, Vandenput L, Boonen S, Lindberg MK, Bouillon R, Ohlsson C. Androgens and bone. Endocrine Reviews. 2004;25(3):389–425. doi: 10.1210/er.2003-0003. [DOI] [PubMed] [Google Scholar]
- 27.Isidori AM, Giannetta E, Greco EA, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clinical Endocrinology. 2005;63(3):280–293. doi: 10.1111/j.1365-2265.2005.02339.x. [DOI] [PubMed] [Google Scholar]
- 28.Smith MR, Fallon MA, Lee H, Finkelstein JS. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. Journal of Clinical Endocrinology and Metabolism. 2004;89(8):3841–3846. doi: 10.1210/jc.2003-032058. [DOI] [PubMed] [Google Scholar]
- 29.Leder BZ, Finkelstein JS. Effect of aromatase inhibition on bone metabolism in elderly hypogonadal men. Osteoporosis International. 2005;16(12):1487–1494. doi: 10.1007/s00198-005-1890-8. [DOI] [PubMed] [Google Scholar]
- 30.Burnett-Bowie SAM, McKay EA, Lee H, Leder BZ. Effects of aromatase inhibition on bone mineral density and bone turnover in older men with low testosterone levels. Journal of Clinical Endocrinology and Metabolism. 2009;94(12):4785–4792. doi: 10.1210/jc.2009-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang J, Wu Z, Wu D, et al. Pharmacokinetics, biodistribution and metabolism of a novel selective androgen receptor modulator designed for prostate cancer imaging. International Journal of Oncology. 2010;36(1):213–222. [PubMed] [Google Scholar]
- 32.Wakley GK, Schutte HD, Hannon KS, Turner RT. Androgen treatment prevents loss of cancellous bone in the orchidectomized rat. Journal of Bone and Mineral Research. 1991;6(4):325–330. doi: 10.1002/jbmr.5650060403. [DOI] [PubMed] [Google Scholar]
- 33.Vanderschueren D, Van Herck E, Suiker AMH, Visser WJ, Schot LPC, Bouillon R. Bone and mineral metabolism in aged male rats: short and long term effects of androgen deficiency. Endocrinology. 1992;130(5):2906–2916. doi: 10.1210/endo.130.5.1572302. [DOI] [PubMed] [Google Scholar]
- 34.Prakasam G, Yeh JK, Chen MM, Castro-Magana M, Liang CT, Aloia JF. Effects of growth hormone and testosterone on cortical bone formation and bone density in aged orchiectomized rats. Bone. 1999;24(5):491–497. doi: 10.1016/s8756-3282(99)00018-6. [DOI] [PubMed] [Google Scholar]
- 35.Turner RT, Wakley GK, Hannon KS. Differential effects of androgens on cortical bone histomorphometry in gonadectomized male and female rats. Journal of Orthopaedic Research. 1990;8(4):612–617. doi: 10.1002/jor.1100080418. [DOI] [PubMed] [Google Scholar]
- 36.Vandenput L, Boonen S, Van Herck E, Swinnen JV, Bouillon R, Vanderschueren D. Evidence from the aged orchidectomized male rat model that 17β-estradiol is a more effective bone-sparing and anabolic agent than 5α-dihydrotestosterone. Journal of Bone and Mineral Research. 2002;17(11):2080–2086. doi: 10.1359/jbmr.2002.17.11.2080. [DOI] [PubMed] [Google Scholar]
- 37.Gaumet-Meunier N, Coxam V, Robins S, et al. Gonadal steroids and bone metabolism in young castrated male rats. Calcified Tissue International. 2000;66(6):470–475. doi: 10.1007/s002230010094. [DOI] [PubMed] [Google Scholar]
- 38.Mödder UIL, Riggs BL, Spelsberg TC, et al. Dose-response of estrogen on bone versus the uterus in ovariectomized mice. European Journal of Endocrinology. 2004;151(4):503–510. doi: 10.1530/eje.0.1510503. [DOI] [PubMed] [Google Scholar]
- 39.Frenkel B, Hong A, Baniwal SK, et al. Regulation of adult bone turnover by sex steroids. Journal of Cellular Physiology. 2010;224(2):305–310. doi: 10.1002/jcp.22159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olson LE, Ohlsson C, Mohan S. The role of GH/IGF-I-mediated mechanisms in sex differences in cortical bone size in mice. Calcified Tissue International. 2010;88(1):1–8. doi: 10.1007/s00223-010-9436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Callewaert F, Venken K, Ophoff J, et al. Differential regulation of bone and body composition in male mice with combined inactivation of androgen and estrogen receptor-α. FASEB Journal. 2009;23(1):232–240. doi: 10.1096/fj.08-113456. [DOI] [PubMed] [Google Scholar]
- 42.Sims NA, Dupont S, Krust A, et al. Deletion of estrogen receptors reveals a regulatory role for estrogen receptors-β in bone remodeling in females but not in males. Bone. 2002;30(1):18–25. doi: 10.1016/s8756-3282(01)00643-3. [DOI] [PubMed] [Google Scholar]
- 43.Notini AJ, McManus JF, Moore A, et al. Osteoblast deletion of exon 3 of the androgen receptor gene results in trabecular bone loss in adult male mice. Journal of Bone and Mineral Research. 2007;22(3):347–356. doi: 10.1359/jbmr.061117. [DOI] [PubMed] [Google Scholar]
- 44.Chiang C, Chiu M, Moore AJ, et al. Mineralization and bone resorption are regulated by the androgen receptor in male mice. Journal of Bone and Mineral Research. 2009;24(4):621–631. doi: 10.1359/jbmr.081217. [DOI] [PubMed] [Google Scholar]
- 45.Kawano H, Sato T, Yamada T, et al. Suppressive function of androgen receptor in bone resorption. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(16):9416–9421. doi: 10.1073/pnas.1533500100. [DOI] [PMC free article] [PubMed] [Google Scholar]


