Abstract
Background:
A decoction prepared with barks of Adenanthera pavonina and Thespesia populnea is a herbal formulation which has been prescribed in Sri Lanka in the treatment of cancer patients for many years. This study was designed to investigate its phytochemical and antioxidant properties.
Materials and Methods:
The total phenolics and flavonoids were determined using Folin–Ciocalteau and aluminum chloride colorimetric methods, respectively. Gallic acid content in the decoction was determined using high-performance liquid chromatography. The antioxidant properties were evaluated by 1, 1-diphenyl-2-picrylhydrazyl (DPPH) radical assay, nitric oxide scavenging assay, deoxyribose method, and the reducing power of the decoction.
Results:
The concentration of total phenols, flavonoids, and gallic acid of the decoction were 34.13 ± 3.54 w/w % gallic acid equivalents, 41.37 ± 0.57 w/w % of (-)-Epigallocatechin gallate equivalents, and 0.58 ± 0.24 mg/g, respectively. The EC50 for DPPH, nitric oxide scavenging, and deoxyribose assays were 7.24 ± 0.50, 14.02 ± 0.66, and 53.21 ± 2.82 μg/ml, respectively. Reducing power of the decoction increased with the concentration.
Conclusion:
These investigations suggested that the decoction prepared with A. pavonina and T. populnea can be a potential source of nutraceuticals with antioxidant activity.
Keywords: Adenanthera pavonina and Thespesia populnea, antioxidant activity, high-performance liquid chromatography, phenolic content, traditional medicine
INTRODUCTION
Plant secondary metabolites are promising nutraceuticals for control of various disorders including cancer.[1] The decoction investigated in this study contained a mixture of Adenanthera pavonina bark and Thespesia populnea bark, and has been prescribed to cancer patients for many years in Sri Lanka. A. pavonina L. has shown antiseptic and anti-inflammatory activities and it is used to treat many diseases.[2–4] The different solvent extracts of T. populnea bark have many beneficial health effects, including cholesterol lowering, anticholinesterase, anti-inflammatory, and antioxidant activities.[5,6] Plant phenolic compounds are associated with antioxidative action in biological systems, acting as scavengers of free radicals, thus preventing the oxidative damages and thereby limiting the risk of various diseases such as cancer.[7,8] Three such poly herbal formulations used for cancer therapy have been reported and the highest antioxidant activity was observed with two decoctions which had Terminalia bellerica, Terminalia chebula, Phyllanthus emblica, and detoxified Commiphora mukul[9] as common constituents. No scientific investigations have been reported with the decoction prepared from A. pavonina and T. populnea, and this study was conducted to evaluate the antioxidant activities and phytochemicals present in the aqueous extract of the decoction.
MATERIALS AND METHODS
Chemicals and equipment
Gallic acid, Folin–Ciocalteau reagent, trichloroacetic acid, N-1naphthylethylene diamine dihydrochloride, 2-deoxy-D-ribose, and ethylenediaminetetraacetic acid (EDTA) were purchased from Sigma Chemicals Co. (P.O. Box 14508, St. Louis, MO 63178 USA). 1,1-diphenyl-2-picrylhydrazyl (DPPH), (-)-epigallocatechin gallate (EGCG), aluminium chloride, and sulfanilamide were purchased from Fluka (Fluka chemie GmbH, CH-9471 Buchs). Ascorbic acid and hydrogen peroxide were purchased from BDH Chemicals (BDH Chemicals Ltd. Poole, England). Sodium nitroprusside (SNP) was purchased from Qualigens (A Division of GlaxoSmith Kline Pharmaceuticals Ltd). Ferric chloride, potassium ferricyanide, and sodium nitrite were purchased from Riedel –De Haen Ag, Seelze- Hannover- Germany. Shimatdzu Biospec 1601 UV visible spectrophotometer (Shimatdzu, Japan) was used to measure the absorbance. SHIMADZU High-Performance Liquid Chromatographic system equipped with LC10As solvent delivery system, SPD-10AVP UV visible detector, DGU-14A degasser, and Chromatopac CR8A recorder was used to determine the Gallic acid content by high-performance liquid chromatography (HPLC).
Plant materials
The barks of T. populnea and A. pavonina were collected from Matara and Colombo districts of Sri Lanka, respectively. All plant materials were identified and confirmed by Department of Botany, Bandaranayake Memorial Ayurveda Research Institute, Nawinna, Colombo, Sri Lanka, and voucher specimens were retained at the above premises.
Preparation of the decoction
Equal amount of barks (30 g) of T. populnea L. and A. pavonina L. were used to prepare the decoction according to the traditional method. All the plant ingredients were made to fine powder using a clean kitchen blender. The contents were boiled with 1 600 ml of water in a clay pot with a lid. Boiling was continued until the volume was reduced to 200 ml (1/8th of the original volume). The water extracts were decanted, filtered, and centrifuged at 1 000 rpm for 5 minutes. The supernatant was retained. The pellet was sonicated with a minimum quantity of deionized water and centrifuged at 2 500 rpm for 8 minutes. Both supernatants were pooled and then freeze dried. Three sets of decoctions were prepared separately and lyophilized as described above. The freeze-dried samples were stored at -20°C in sterile tubes until further use. The yield was calculated as a percentage of the total raw material weight (n = 3).
Determination of total phenolic content
Total phenolic content of the decoction were determined by the Folin-Ciocalteau method.[10] Each lyophilized decoction (n = 3) prepared with T. populnea and A. pavonina were analyzed in triplicates for phenolic content. Folin-Ciocalteau reagent (1N; 250 μl) was added to the decoction (500 μl) at three different concentrations (25, 50, and 100 μg/ml). The mixture was allowed to stand for 2 minutes at room temperature. Sodium carbonate solution (10%; 1.25 ml) was then added to the contents and incubated for 45 minutes at room temperature. The absorbance of the resulting solution was measured at 760 nm. A calibration curve with gallic acid was used as the reference to determine the phenolic content of the decoction. The phenolic content of the test samples were expressed as w/w% gallic acid equivalents.
Determination of flavonoid content
The flavonoid content of the decoction was determined by a colorimetric method as described by Zhischen et al.[11] Different concentration of the decoction was prepared by diluting the stock solution (4 000 μg/ml) with deionized water. Each sample (100 μl) was diluted with distilled water (200 μl). Sodium nitrite (5%; 30 μl) was added to the samples and then at 5 minutes, aluminium chloride (10%; 30 μl) and at 6 minutes, sodium hydroxide (1M; 200 μl) were added to the mixture. Finally, 400 μl of deionized water was added. The absorbance was recorded at 510 nm. EGCG was used as the standard to calculate the flavonoid content using a calibration curve and the values were expressed as w/w% EGCG equivalents. The analysis was performed in triplicates.
Quantification of gallic acid by high-performance liquid chromatography
The gallic acid content in the decoction was determined using a mobile phase constituted with acetonitrile (8%) in acetic acid (1%) at a flow rate of 1.2 ml/min. The internal standard was β-hydroxyethyl-theophylline (100 μg/ml). The separation was performed on a C18 column (3.9 × 300 mm) and the eluents were detected at 260 nm. Different concentrations of the decoction were prepared from each freeze-dried sample for analysis. The calibration curve was constructed using gallic acid prepared in deionized water to determine the gallic acid content present in the decoction. The test sample or the standards of gallic acid (100 μl) were mixed with β-hydroxyethyl-theophylline (100 μl; 100 μg/ml) and centrifuged (4 000 rpm; 5 minutes). The supernatant (25 μl) was injected on to the column and detected at 270 nm wavelength. The gallic acid content in the decoction was determined by using the calibration curve plotted with concentration of gallic acid against the Peak Area Ratio (PA Ratio). PA ratio was calculated using the ratio of the peak area of gallic acid to that of internal standard.
Gallic acid content = (PA Ratio of sample/PA Ratio of standard) × Concentration (GA Standard)
1, 1- diphenyl- 2- picrylhydrazyl radical scavenging activity
DPPH radical scavenging method, described by Blois,[12] was used to assay the free radical scavenging activity of the decoction with slight modifications. DPPH reagent prepared in absolute ethanol (100 μM; 950 μl) was added to different concentrations (3.13, 6.25, 12.5, 25, 50, 100, and 200 μg/ml) of the test sample (50 μl) and the mixture was allowed to stand for 30 minutes in the dark. The absorbance of the resulting solution was measured at 517 nm. Different concentrations of L-Ascorbic acid (3.13 - 200 μg/ml) were used as the standard antioxidant. The control was prepared by adding deionized water (50 μl) to DPPH reagent (100 μM; 950 μl) and the analysis was followed as described above. The results were expressed as percentage inhibition (I %) using the equation below:
I% = (Abscontrol - Abssample)/Abscontrol × 100
Abscontrol is the absorbance of the control reaction with deionized water (50 μl) without the decoction and Abssample is the absorbance in the presence of the sample. The effective concentration of the sample required to scavenge DPPH radical by 50% (EC50) was obtained by linear regression analysis of dose response curve plotting between I % vs concentrations.
Nitric oxide radical scavenging activity
The nitric oxide radical scavenging activity was estimated by using Griess nitrite assay.[13] The reaction mixture contained SNP (10 mM; 2 ml), phosphate buffered saline (50 mM; pH, 7.4; 500 μl), and the test sample (500 μl) at different concentrations (20-100 μg/ml). The same reaction mixture without the decoction, instead, phosphate buffered saline served as the reagent blank. After incubation for 150 minutes at 25°C, Griess reagent (1% sulfanilamide, 2% phosphoric acid, and 0.1% N-1 naphthylethylene diamine dihydrochloride; 500 μl) was added to the mixture (500 μl). The pink chromophore formed was measured spectroscopically at 540 nm after an incubation period of 30 minutes at 25°C. The water extracts of the decoction had a color of dark brown to red which interfered with the resultant color of the pink chromophore. Hence, a blank for each concentration was prepared with test samples, phosphate buffered saline (PBS), and Griess reagent. Absorbance of the sample value was corrected for interfering substances by deducting the sample absorbance from the absorbance of the respective blank.
I % was calculated as shown below:
I% = (Abscontrol - Abssample)/ Abscontrol × 100
Abscontrol is the absorbance of control reaction with PBS without the decoction and Abssample is the absorbance (corrected value) in the presence of the sample. The effective concentration of the sample required to scavenge nitric oxide radical by 50% (EC50 ) was obtained by linear regression analysis of dose response curve plotting between percentage inhibitions vs concentrations.
Deoxyribose assay
The sugar deoxyribose on exposure to hydroxyl radicals, generated by Fenton reaction model system, degrades into fragments and generates a pink chromophore on heating with thiobarbituric acid (TBA) at low pH. The ability of the extracts to inhibit non-site-specific hydroxyl radical-mediated peroxidation was carried out according to Halliwell et al.,[14] with modifications. The reaction mixture contained 2-deoxy-D-ribose (2.8 mM; 100 μl), dissolved in potassium dihydrogen phosphate - dipotassium hydrogen phosphate (KH2PO4 - K2HPO4) buffer (pH, 7.4; 50 mM), test sample (500 μl) at different concentrations (200, 400, 500, 600, 700, 800, and 1 000 μg/ml) prepared from stock solution (1 mg/ml), a mixture (200 μl) prepared by dilution (1 : 1) with iron (III) chloride (200 μM) and EDTA (1.04 mM), hydrogen peroxide (1.0 mM; 100 μl), and ascorbic acid (1.0 mM; 100 μl). After an incubation period of 1 hour at 37°C, the deoxyribose degradation was measured by the TBA reaction. TBA (1% in 50 mM NaOH; 1 ml) and trichloroacetic acid (2.8%; 1 ml) were added to the reaction mixture and heated at 100°C for 20 minutes. The absorbance was read at 532 nm after cooling. The reagent blank contained buffer and deoxyribose. Different concentrations of gallic acid (25-150 μg/ml) were used as the standard antioxidant.
The I% was calculated as given below:
I% = (Abscontrol - Abssample)/Abscontrol × 100
The EC50 value was determined by constructing a dose response curve between I% and concentration of test samples or the standard. The values were presented as means of triplicate analyses.
Measurement of reducing power
The reducing power of the decoction was performed according to the method described by Dhalwal et al., with slight modifications.[15] Different concentrations of the decoction were prepared from the stock solution (1 mg/ml). The test sample (100 μl) was mixed with phosphate buffer (0.2M; pH, 6.6; 250 μl) and potassium ferricyanide (1%; 250 μl). The mixture was incubated at 50°C for 20 minutes. Trichloroacetic acid (1%; 250 μl) was then added and centrifuged at 5 000 g for 10 minutes. The supernatant was mixed with distilled water and iron (III) chloride (0.1%) at a ratio of 1 : 1 : 2 and the absorbance was read at 700 nm. Different concentrations of ascorbic acid (125-250 μg/ml) were used as the standard antioxidant to compare the reducing power with the decoction.
Calculations and statistics
Results are presented as mean ± standard deviation (Mean ± SD). A minimum of three independent experiments were carried out, unless otherwise specified. Linear regression analysis and statistical analysis were carried out using Microsoft Excel. Calibration curves of the standards were considered as linear if R2 >0.99. Linear segment of the sigmoid curves of the dose response curves were used to determine the EC50 values and R2 >0.95 was considered as linear in calculating EC50.
RESULTS
Extraction yield and phytochemical composition
The yield obtained for lyophilized samples of the decoction composed of A. pavonina and T. populnea was 3.6 ± 1.49 g. The mean ± SD of the total phenols was estimated as 34.14 ± 3.54 w/w % gallic acid equivalents and flavonoids as 42.40 ± 0.39% w/w EGCG equivalents [Table 1]. The gallic acid content in the decoction was quantified by HPLC as described earlier. Calibration curve between the PA ratio and the concentration was linear (y = 0.057x + 0.037; r2 = 0.995) over a range of 0 to 50 μg/ml. HPLC chromatograms of a standard mixture prepared in water and of the plant extract are depicted in Figure 1. The retention times for the gallic acid and for the internal standard were 3.3 and 4.2 minutes, respectively [Figure 1]. The gallic acid concentration and the mean ± SD of gallic acid content in the decoction was 0.58 ± 0.01 mg/g.
Table 1.
Summary of the phytochemical composition in the decoction containing Adenanthera pavonina L. and Thespesia populnea L.
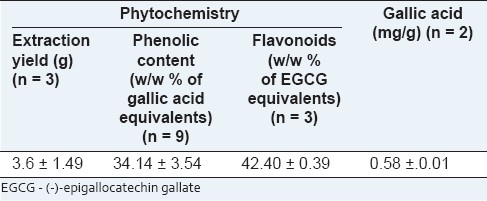
Figure 1.
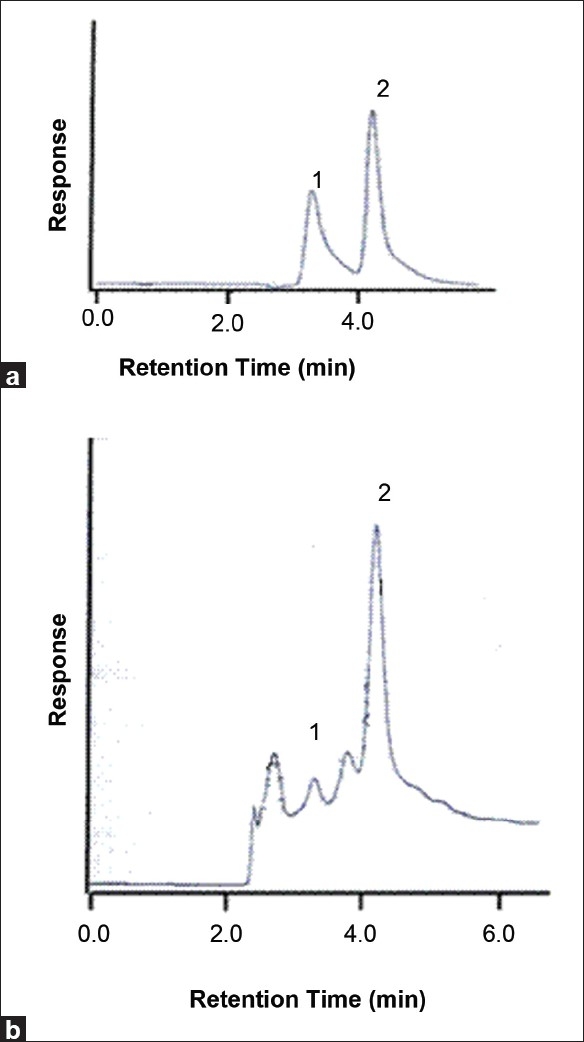
High-performance liquid chromatography chromatograms of gallic acid; (a) Chromatogram of standard solution of gallic acid (50 μg/ml) and the internal standard in water; (b) Chromatogram of the decoction (the peaks at 3.3 and 4.2 minutes represents the gallic acid (1) and β- hydroxyethyl-theophylline (2), respectively)
Antioxidant assays
The concentrations of the decoction required to reduce DPPH, nitric oxide, and hydroxyl radical formation by 50% (EC50) were determined using the dose response curve obtained between the I % and the concentration of the decoction [Figures 2–4], and the values were 7.24 ± 0.495, 14.02 ± 0.66, and 53.21 ± 2.82 μg/ml, respectively [Table 2]. The reducing power of the decoction was found to increase with increasing concentrations; however, the values remained lower compared with ascorbic acid [Figure 5].
Figure 2.
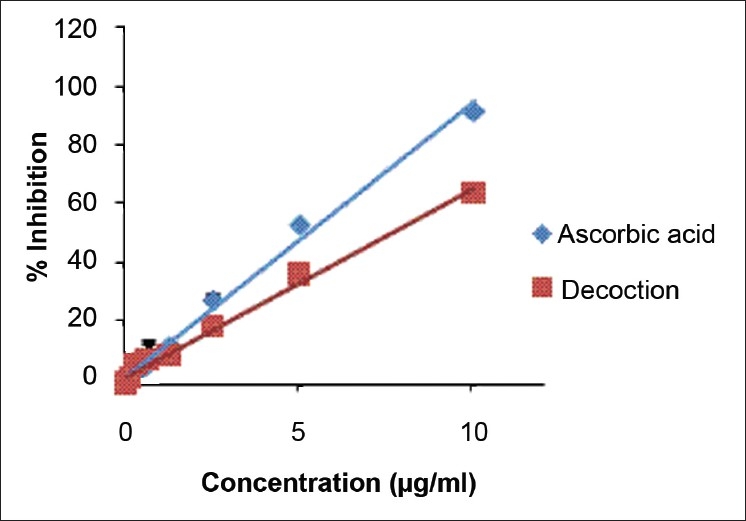
The dose response curve for percentage scavenging of 1, 1-diphenyl-2-picrylhydrazyl for L-Ascorbic acid and decoction. The results are presented as mean ± SD of nine independent experiments
Figure 4.
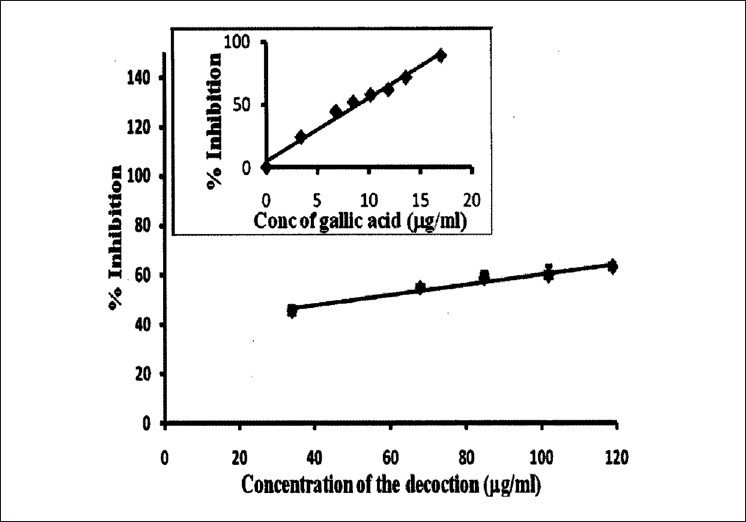
The dose response curve of gallic acid and the decoction for deoxyribose assay. The results are presented as mean ± SD of three independent experiments
Table 2.
Summary of antioxidant activity in the decoction containing Adenanthera pavonina L. and Thespesia populnea L.

Figure 5.
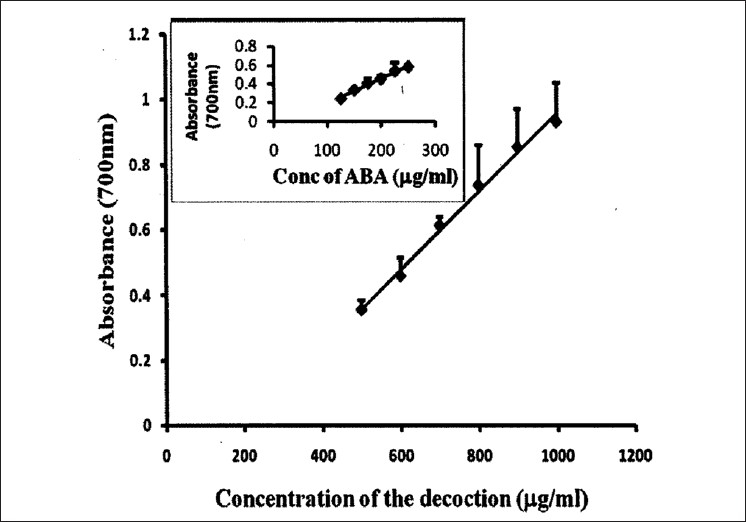
Total reduction capability of different concentrations of the decoction in comparison with ascorbic acid. The results are presented as mean ± SD of six independent experiments
Figure 3.
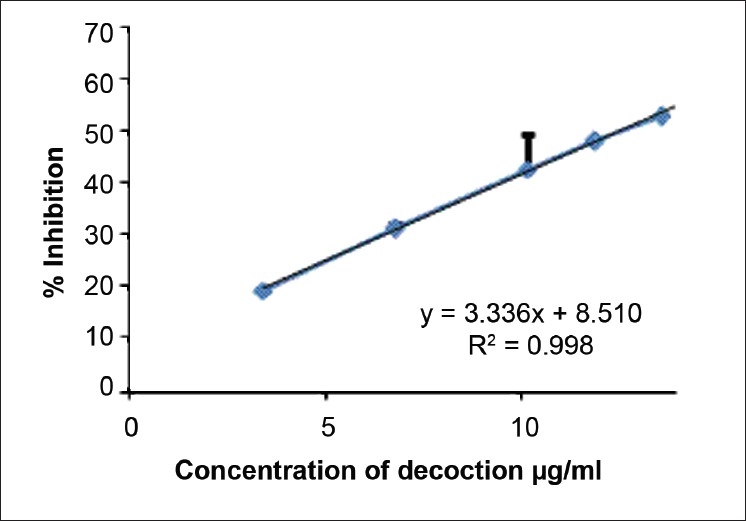
The dose response curve for the percentage of nitric oxide scavenging by the decoction. The results are presented as mean ± SD of six independent experiments
DISCUSSION
A. pavonina and T. populnea belong to the families of Mimosaceae and Malvaceae, respectively.[16] The decoction contained a higher total phenol content (34.14 ± 3.54 w/w % of gallic acid equivalents) compared with an ayurvedic formulation, Panchvalkala which contains the stem barks of Ficus benghalensis, Ficus glomerata, Ficus religiosa, Ficus virens, and T. populnea.[17] The phenolic content of the methanolic extracts of individual components of this formulation ranged from 3.5 to 10.8 w/w % of gallic acid equivalents and T. populnea showed a value of 10.11%.[17] A flavonoid content of 11.06% expressed in TAE (Tannic Acid Equivalent) was reported in the aqueous extract of Entada africana barks, a herb which belongs to the same family of A. pavonina (Mimosaceae).[18] A qualitative study indicated the presence of carbohydrate, protein, tannins, phenol, flavonoids, terpenes, saponins, and gums in the ethanolic and aqueous extract of T. populnea bark.[19] The decoction used in the present study which is composed of both T. populnea L. and A. pavonina L. showed a total flavonoid content of 42.40 ± 0.39 w/w % of EGCG equivalents. The gallic acid content in the decoction was quantified using HPLC method. Although the decoction showed high content of phenolics and flavonoids, the free gallic acid content was 0.58 ± 0.01 mg/g. Phenolic compounds and flavonoids have been reported to be associated with antioxidative action in biological systems, through acting as scavengers of singlet oxygen and free radicals based on the redox properties of their hydroxyl groups and their chemical structures.[20,21]
DPPH is the most extensively used assay to detect antioxidant activity of plants. Its violet color disappears in the presence of substance which can donate a hydrogen depending on the antioxidant activity.[22] The present study showed that the aqueous extract of the decoction was a potent scavenger of DPPH free radical and has an EC50 value of 7.24 ± 0.49 μg/ml. This value is comparable with the ascorbic acid and with two decoctions prepared using T. bellerica, T. chebula, P. emblica, and detoxified C. mukul, and in addition, Smilax china and Nigella sativa were present in the second decoction.[9] However, Anandjiwala et al. demonstrated a value of 12.08 μg/ml for methanolic extract of T. populnea for EC50 in DPPH assay.[17] Ramli et al. reported that the bark extract of A. pavonina has an EC50 value of 58.68 μg/ml.[23] However, this extract was obtained using petroleum ether, dichloromethane, and ethanol in contrary to our study where the water extract was being used. The methanolic extract of Mimosa pudica aerial parts, a herb of the family Mimosaceae, reported an IC50 of 296.92 μg/ml.[24] Another study carried out with the whole plant of M. pudica and its different parts showed that the DPPH scavenging activity of the ethanol extracts was in the order of leaf > the whole plant > seed > stem and the values were 0.35, 0.61, 1.07, and 1.07 mg/ml, respectively.[25]
Nitric oxide reacts with oxygen or with superoxides to form highly reactive compounds such as NO2, N2O4, N3O4, and NO3. These compounds are responsible for altering the structural and functional behavior of many cellular components.[26] Nitric oxide produced by SNP over 2-hour incubation at 25°C has been scavenged by the decoction. The decoction used in the present study contains strong nitric oxide scavenging activity with an EC50 of 14.02 ± 0.66 μg/ml as compared with Pyrus boissieriana, Cnidium officinale, and Ligusticum chuanxiong, which showed an EC50 of 650, 57.25, and 76.50 μg/ml, respectively.[27,28] This suggests that the decoction investigated in this study might be a potent and novel therapeutic agent for scavenging of NO and thereby minimizing of pathological conditions caused by excessive generation of NO.
The antioxidant strength of the decoction can be further probed by detection of hydroxyl radicals by deoxyribose assay. Increasing doses of decoction added to the reaction mixture removed the hydroxyl radicals from the sugar and protected it from degradation in a dose-dependent manner.[29] The hydroxyl scavenging ability of Hibiscus tiliaceus flowers, a herb belonging to Malvaceae family, was 70.25 ± 1.7% at 2 500 μg/ml aqueous extract.[30] The synergistic effects of T. populnea (family Malvaceae) and A. pavonina (family Mimosaceae) yielded an EC50 value of 53.21 ± 2.82 μg/ml, demonstrating that the decoction is a strong scavenger of OH· radicals, compared with the Hibiscus tiliaceus; however, the EC50 is higher than the positive control.
The reducing ability of a compound generally depend on the presence of reductants[31] which depends on its hydrogen donating ability.[32] The presence of reductants in the decoction causes the reduction of ferric/ferricyanide complex to the ferrous form in a dose-dependent manner; however, it had lesser reductive ability than the reference standard of ascorbic acid.
CONCLUSION
The results of this study clearly demonstrate the antioxidant capacity of the decoction containing A. pavonina L. and T. populnea L., and the potent antioxidant activity is justified by its high concentration of phenolic constituents and flavonoids. This study indicates that bioactive molecules present in the decoction can be used as a prototype for development of new drugs and/or as a source of antioxidant pharmaceutical raw material.
ACKNOWLEDGEMENTS
We acknowledge the research grant from the National Science Foundation, Sri Lanka, grant No. RG/2005/HS 17. Authors particularly thank Dr. N. Jayathilake for providing the recipe of the decoction and Ms. Sudeepa Sugathadasa, Department of Botany, Bandaranayake Memorial Ayurveda Research Institute, Nawinna, Colombo, Sri Lanka, for the identification of the plant material.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Hafidh RR, Abas F, Abdulamir AS, Jahanshiri F, AbuBakar F, Sekawi Z. A review: Cancer research of natural products in Asia. Int J Cancer Res. 2009;5:69–82. [Google Scholar]
- 2.Tan R. Saga seed tree Adenanthera pavonina L. 2001. [last cited on 2010 Jul]. Available from: http://www.naturia.per.sg/buloh/plants/saga_tree.htm .
- 3.Mayuren C, Ilavarasan R. Anti-inflammatory activity of ethanolic leaf extracts from Adenanthera pavonina (L) in Rats. Pharmacognosy. 2009;1:125–8. [Google Scholar]
- 4.Australian native hibiscus and hibiscus like species. 2002. [last cited on 2010 Jul]. Available from: http://www.hibiscus.org/species/tpopulnea.php.
- 5.Vasudevan M, Parle M. Pharmacological actions of Thespesia populnea relevant to Alzheimer's disease. Phytomedicine. 2006;13:677–87. doi: 10.1016/j.phymed.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Ilavarasan R, Vasudevan M, Anbazhagan S, Venkataraman S. Antioxidant activity of Thespesia populnea bark extracts against carbon tetrachloride-induced liver injury in rats. J Ethnopharmacol. 2003;87:227–30. doi: 10.1016/s0378-8741(03)00147-8. [DOI] [PubMed] [Google Scholar]
- 7.Rice-Evans CA, Miller NJ, Bolwell PG, Bramley PM, Pridham JB. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic Res. 1995;22:375–83. doi: 10.3109/10715769509145649. [DOI] [PubMed] [Google Scholar]
- 8.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 9.Perera MG, Soysa SS, Abeytunga DT, Ramesh DT. Antioxidant cytotoxic properties of three traditional decoctions used for the treatment of cancer in Sri Lanka. Pharmacogn Mag. 2008;4:172–81. [Google Scholar]
- 10.Makkar HP, Bluemmel M, Borowy NK, Becker K. Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J Sci Food Agric. 1993;61:161–5. [Google Scholar]
- 11.Zhishen J, Mengcheng T, Jianming W. The determination of flavonoids contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–9. [Google Scholar]
- 12.Blois MS. Antioxidant determination by the use of stable free radical. Nature. 1985;181:1199–200. [Google Scholar]
- 13.Rajasekhar MD, Ramesh Babu K, Vinay K, Sampath MR, Sameena SK, Apparao C. Antihyperglycemic and antioxidant activities of active fraction from the aqueous extract of Momordica cymbalaria fruits in Streptozotocin induced diabetic rats. Pharmacogn Res. 2009;1:352–8. [Google Scholar]
- 14.Halliwell B, Gutteridge J, Aruoma O. The deoxyribose method: A simple test tube assay for determination of rate constants for reaction of hydroxyl radicals. Anal Biochem. 1987;165:215–9. doi: 10.1016/0003-2697(87)90222-3. [DOI] [PubMed] [Google Scholar]
- 15.Dhalwal K, Deshpande YS, Purohit AP, Radam SS. Evaluation of antioxidant activity of Sida cordifolia. Pharm Biol. 2005;43:754–61. [Google Scholar]
- 16.Encyclopedia on Indian Medicinal Plants. ENVIS Centre on Medicinal Plants. [last cited on 2011 Feb 1]. Available from: http://envis.frlht.org/bot_search.php.
- 17.Anandjiwala S, Bagul MS, Parabia M, Rajani M. Evaluation of free radical scavenging activity of an ayurvedic formulation, Panchvalkala. Indian J Pharm Sci. 2008;70:31–5. doi: 10.4103/0250-474X.40328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tibiri A, Sawadogo RW, Ouedraogo N. Evaluation of Antioxidant Activity, Total Phenolic and Flavonoid Contents of Entada africana Guill. et Perr. (Mimosaceae) Organ Extracts. Res J Med Sci. 2010;4:81–7. [Google Scholar]
- 19.Parthasarathy R, Ilavarasan R, Nandanwar R. A study on preliminary phytochemical and diuretic activity of bark of Thespesia populnea. IJPSR. 2010;1:72–7. [Google Scholar]
- 20.Rice-Evans CA, Miller J, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–9. [Google Scholar]
- 21.Burda S, Oleszek W. Antioxidant and antiradical activities of flavonoids. J Agric Food Chem. 2001;49:2774–9. doi: 10.1021/jf001413m. [DOI] [PubMed] [Google Scholar]
- 22.Molyneux P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity.Songklanakarin. J Sci Technol. 2004;26:211–9. [Google Scholar]
- 23.Ramli S, Bunrathep S, Tansaringkarn T, Ruangrungsi N. Screening for free radical scavenging activity from ethanolic extract of mimosaceous plants endemic to Thailand. J Health Res. 2008;22:55–9. [Google Scholar]
- 24.Chowdhury SA, Islam MD, Rahaman M, Rahman MM, Rumzhum NN, Sultana R. Cytotoxicity, antimicrobial and antioxidant studies of the different plant parts of Mimosa pudica. Stamford J Pharm Sci. 2008;1:80–4. [Google Scholar]
- 25.Zhang J, Yuan K, Zhou W, Zhou J, Yang P. Studies on the active components and antioxidant activities of the extracts of Mimosa pudica Linn.from southern China. Pharmacogn Mag. 2011;7:35–9. doi: 10.4103/0973-1296.75899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saha MR, Jahangir R, Vhuiyan MM, Biva IJ. In vitro nitric oxide scavenging activity of ethanol leaf extracts of four Bangladeshi medicinal plants. Stamford J Pharm Sci. 2008;1:57–62. [Google Scholar]
- 27.Nabavi SM, Ebrahimzadeh MA, Nabavi SF, Fazelian M, Eslami B. In vitro antioxidant and free radical scavenging activity of diospyros lotus and pyrus boissieriana growing in Iran. Pharmacogn Mag. 2009;5:122–6. [Google Scholar]
- 28.Ramalingam M, Yong-Ki P. Free radical scavenging activities of Cnidium officinale Makino and Ligusticum chuanxiong Hort. methanolic extracts. Pharmacogn Mag. 2010;6:323–30. doi: 10.4103/0973-1296.71794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aruoma OI, Halliwell B. The iron-binding and hydroxyl radical scavenging action of anti-inflammatory drugs. Xenobiotica. 1988;18:459–70. doi: 10.3109/00498258809041682. [DOI] [PubMed] [Google Scholar]
- 30.Kumar S, Kumar D, Prakash O. Evaluation of antioxidant, phenolic and flavonoid contents of Hibiscus tiliaceus flowers. EJEAFChe. 2008;7:2863–71. [Google Scholar]
- 31.Gordon MH. The mechanism of the antioxidant action in vitro. In: Hudson BJ, editor. Food antioxidants. London: Elsevier; 1990. pp. 1–18. [Google Scholar]
- 32.Ozen T, Turkekul I. Antioxidant activities of Sarcodon imbricatum wildly grown in the black sea region of Turkey. Pharmacogn Mag. 2010;6:89–97. doi: 10.4103/0973-1296.62892. [DOI] [PMC free article] [PubMed] [Google Scholar]


