Abstract
Background:
Zingiber officinale Rosc. is not only an important medical plant in China, but also one of the most commonly used plant spices around the world. Early researches in Z. officinale Rosc. were focused on rapid propagation, germplasm preservation, and somatic embryogenesis, only a few reports focused on the generation of tetraploid ginger plants with colchicines treatment in vitro.
Materials and Methods:
The adventitious buds were submerged into different concentrations of colchicine water solution for different time to induce polyploid plants, and the induced buds were identified by root-tip chromosome determination and stomatal apparatus observation. Eighteen selected tetraploid lines were transferred to the field, and the leaf characteristics, rhizome yield, contents of volatile oil and gingerol were respectively evaluated to provide evidence of high-yield and good qualities of tetraploid ginger.
Results:
The induction rate reached as high as 33.3% of treated buds. More than 48 lines of autotetraploid plants were obtained. All tetraploid plants showed typical polyploidy characteristics. All of the 18 selected tetraploid lines possessed higher rhizome yield and overall productivity of volatile oil and gingerol than those of the control.
Conclusion:
Five elite lines have been selected for further selection and breeding new varieties for commercial production in agricultural production.
Keywords: Ginger, gingerol, tetraploid, volatile oil
INTRODUCTION
Zingiber officinale Rosc., belonging to Zingiberaceae family, is an economic plant cultivated in many tropical and subtropical countries. Ginger, the pungent rhizome of Z. officinale Rosc., is the most commonly used spices material around the world, especially in the Southeastern Asian countries. Ginger is also a typical traditional Chinese medicine that has been widely used for the treatment of indigestion, sore throats, rheumatism, and hypertension.[1–4] Ginger has been demonstrated to have various pharmacological activities, such as antiemetic, antiulcer, anti-inflammatory, antioxidant, antiplatelet, and anticancer activities.[5–8]
Early researches in Z. officinale Rosc. were focused on rapid propagation,[9–12] germplasm preservation,[13,14] and somatic embryogenesis.[15,16] Since Ramachandran obtained tetraploid ginger plants in vivo in 1982,[17] only a few reports focused on the generation of tetraploid ginger plants with colchicines treatment in vitro.[18] In this paper, we report a convenient and effective method in inducing polyploid plants by submerging adventitious buds from tissue culture into different concentrations of colchicine water solution for different time. To breeding superior varieties or cultivars of Z. officinale in agricultural production, the leaf characteristics, rhizome yield, volatile oil content, and gingerol content were evaluated respectively to provide evidence of high-yield and good qualities of tetraploid ginger.
MATERIALS AND METHODS
Plant material
Rhizomes of Z. officinale Rosc. (2× = 22) were obtained from Laiwu city, Shandong Province, China. The original plant was identified by the Department of Genetics and Breeding at the China Pharmaceutical University.
Material disinfection and adventitious bud induction
Rhizome buds of Z. officinale Rosc. (2× = 22) were collected and washed under running tap water for 20 minutes to remove the soil particles. After removing several leaves from the apical buds, the buds were sterilized in a solution of 0.1% (v/v) mercuric chloride (containing two to three drops of Tween 20/l) for 8 minutes. The buds were washed with sterile distilled water for 3 to 5 times and then transferred to a Petri dish containing a layer of sterile filter paper to remove excess surface water. The surface-sterilized buds were placed under an anatomical lens to remove the leaves, shoot tips (about 0.5 mm in height) were dissected and transferred to Murashige and Skoog (MS)[19] medium containing 3% (w/v) sucrose and 0.35% (w/v) agar powder (gel strength: 1 100 gcm-2) supplemented with 2.5 mg/l 6-benzylaminopurine (BAP) and 0.2 mg/l α-naphthaleneacetic acid (NAA). The inoculated shoot tips were kept in an illuminated incubator for a 16-hour photoperiod under a 1 200 lux light intensity and 25 ± 1°C to induce clump buds.
Induction of polyploid plantlets
To determine the optimum concentration of colchicine treatment for the induction of tetraploids, 30 buds regenerated from the shoot tips were submerged in five different concentrations of colchicine (0.0%, 0.1%, 0.2%, 0.3%, and 0.4% (w/v)) for 24 hours and transferred to the MS medium supplemented with 2.0 mg/l BAP and 0.2 mg/l NAA. The inoculated buds were cultured in an illuminated incubator under a 16-hour photoperiod of 1 200 lux light intensity at 25°C for 20 days. Alternatively, 30 buds were submerged into 0.2% (w/v) colchicine solution for 0, 6, 12, 18, 24, 30, 36, 42, or 48 hours. The treated buds were then transferred to MS media supplemented with 2.0 mg/l BAP and 0.2 mg/l NAA and cultured in an illuminated incubator under a 16-hour photoperiod of 1 200 lux light intensity at 25°C for 20 days. After that, all of the survival materials were transferred to the MS solid medium supplemented with 2.5 mg/l BAP and 0.2 mg/l NAA for 30 days. The seedling of the subculture materials were transferred to the rooting solid MS medium at half the macronutrient concentration supplemented with 0.4 mg/l NAA to induce roots for chromosome determination.
Chromosome determination
Root tips approximately 0.5 cm in length were excised and pretreated in the 0.2% (w/v) colchicine solution for 2.5 hours. After pretreatment, the root tips were transferred to Carnoy's fixative (containing 3 : 1 ethanol and glacial acetic acid) and stored at 3 to 5°C for 2 to 24 hours, rinsed with 95% (w/v) alcohol, 70% (w/v) alcohol, and distilled water three times, respectively, and then macerated for 12 minutes with 0.2 M HCl at 60°C. After soaking in distilled water for 30 minutes, the fixed root tips were stained with improved carbol fuchsin (1.8 g sorbitol dissolved in 10 ml carbol fuchsin, and then mixed with 45% v/v acetic acid, 90 ml). A photomicroscope (Olympus BX40, Japan) was used for chromosome determination. The chromosome count of each tetraploid line (4× = 44) was repeated for at least three generations of subculture.
The buds (approximately 3 cm in height) of each tetraploid line were excised and transferred to the rooting medium consisting of the solid MS medium at half the macronutrient concentration and supplemented with 0.4 mg/l NAA to induce roots. And, the rooted plantlets were transplanted into a seedling bed for evaluation of agriculture characteristics.
Stomatal measurements
An area about 0.1 cm2 on the upper epidermis of the leaf was peeled off and spread onto a glass microscope slide. A photomicroscope (Olympus BX40, Japan) was used to measure the stomatal apparatus length and width. Four leaves were chosen from the same part of each of five diploid plants and each of five tetraploid plants. Twenty stomatal apparatus were measured for each leaf.
The contents of volatile oil and gingerol of polyploid plants
Rhizomes of all obtained tetraploid plants (two years old) were harvested in November in the field and were used to determine the rhizome yield and contents of volatile oil and gingerol.
The measurement of volatile oil contents in rhizomes of all obtained tetraploid plants was conducted according to the guideline of China Pharmacopoeia (edition 2010). Three fresh rhizome samples (approximately 500 g in weight) from each tetraploid line were used to measure the volatile oil content.
The determination of gingerol contents in rhizomes of all obtained tetraploid plants were carried out according to the method established by Zhang et al.[20] 0.5 g accurately weighted fine-grinded powder (W, g) was placed in a flask, submerged with 100 ml ethanol, then extracted by ultrasonication (power, 250 W; frequency, 33 kHz) at 60°C for 30 minutes, cooled down to room temperature, weighted and compensated for the weight loss by ethanol, and then filtered through filter paper. 4 ml of subsequent filtrate diluted with ethanol to 25 ml (V1, ml) was used as sample solution. The absorbance (A) of sample solution was measured by UV-spectrophotometry (Beckman DU-600, USA) at 280 nm. Ethanol was used as blank control. Vanillin was used as the external standard to build the regression equation to calculate the corresponding concentration (C, μg/ml). The content of gingerol was calculated by the following equation and each sample was repeated for three times:
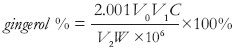
Here, V2 was the volume of test solution used at measuring, ml.
RESULTS
Effect of colchicine treatment on inducing tetraploid buds
After treatment with increasing colchicine concentration for 24 hours, the percentages of buds that died increased significantly with the colchicine concentration, while all of the untreated buds survived. When colchicine concentration was 0.1% (w/v), the death rate of buds was 10.0%, and as colchicine concentration increased to 0.4% (w/v), the death rate of buds increased as high as 83.3%. Chromosome determination was used to identify the tetraploids from the regenerated plants. According to the chromosome counts, the tetraploid induction rates were also influenced by the colchicine concentration, the induction rate increased in the first three concentrations, but decreased when the colchicine concentration reached 0.3% (w/v). The highest induction rate was found at the concentration of 0.2% (w/v), while the lowest induction rate was found at the concentration of 0.4% (w/v), 26.7%, and 3.3%. These results indicated that the optimum concentration of colchicine treatment for tetraploid induction was 0.2% (w/v) [Table 1].
Table 1.
The influence of different colchicine concentration on polyploid induction in Zingiber officinale
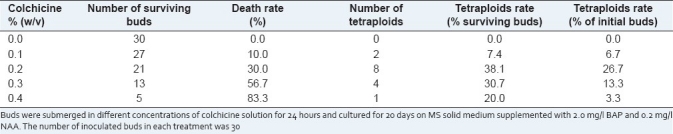
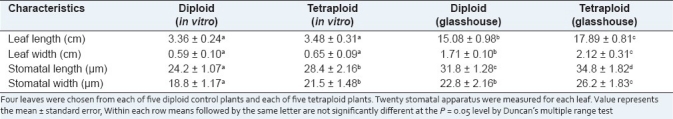
To evaluate the effect of treated time on the tetraploid induction, the buds were submerged in 0.2% (w/v) colchicine solution for different time. The effect of treated time on the tetraploid induction is shown in Table 2. The results showed that with the treated time increasing, the number of survival buds decreased significantly. Twenty-eight buds survived at the treated time of 6 hours, but this survival number was decreased to three when the treated time increased to 48 hours. The results in Table 2 also showed that the tetraploid induction rate was affected by the treated time in 0.2% (w/v) colchicine solution. According to the chromosome count, immersing buds in the 0.2% (w/v) colchicine solution for 6, 12, 18, 24, 30, 36, 42, and 48 hours was effective in induction of polyploid buds, and the induction rate increased gradually when the treated time reached 30 hours. The percentage of polyploid buds was 33.3% when immersing in 0.2% (w/v) colchicine for 30 hours. This is by far the highest induction ratio in our experiments. Chromosome count revealed that the tetraploid plantlets had 44 chromosomes (4× = 44) [Figure 1a and b], and 48 tetraploid plantlets were obtained in our experiments.
Table 2.
The effect of different treated time of 0.2% (w/v) colchicine solution on polyploid induction in Zingiber officinale
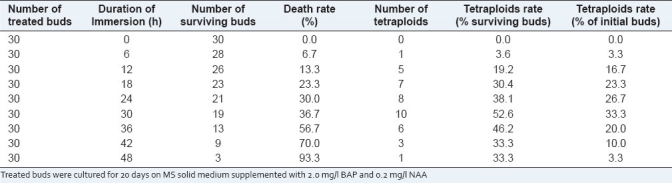
Figure 1.
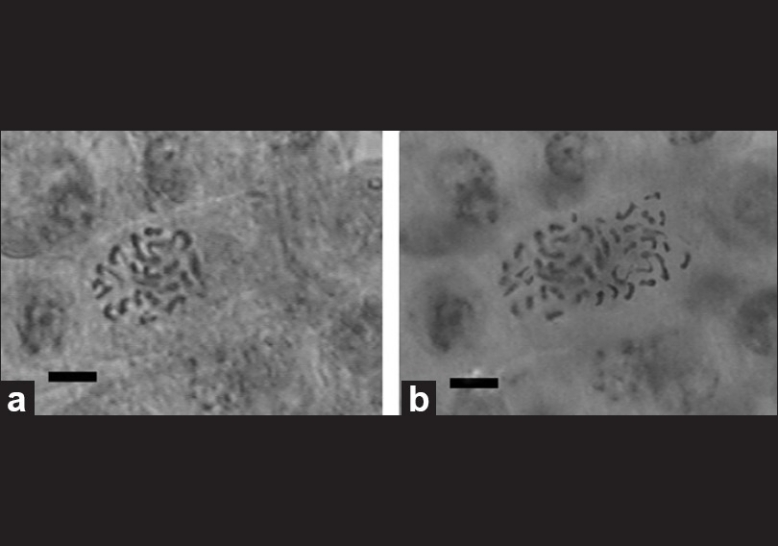
(a) The chromosome of diploid plant, 2n = 2× = 44 (bar: 2.14 × 10-4 cm); (b)The chromosome of tetraploid plant, 2n = 4× = 44 (bar: 2.14 × 10-4 cm)
Morphological differences between diploid and tetraploid
In order to determine whether the morphological features of tetraploid plants could be used to identify putative tetraploids, the leaf length, width and size of stomatal apparatus were evaluated and compared with diploid control plants. The leaves of tetraploid plants appeared normal in shape when compared with diploid plants. The differences in length and width of leaf for 30-day-old diploid and tetraploid in vitro were not so significant, but were obvious in the 6-month-old glasshouse-grown plants [Table 3]. The length and width of glasshouse-grown diploid leaves were about 15.08 cm and 1.71 cm, respectively, while in glasshouse-grown tetraploid plant leaves were about 17.89 cm and 2.12 cm, respectively.
Table 3.
Leaf characteristics of diploid and tetraploid Zingiber officinale
The sizes of stomatal apparatus of tetraploid leaves in vitro and 6-month-old glasshouse-grown are listed in Table 3 [Figure 2a and b]. In general, the stomatal in tetraploid leaves were longer and wider than that of diploid leaves. The length and width of stomatal apparatus of diploid leaves in vitro were about 24.2 μm and 18.8 μm, respectively, while the same dimensions of in vitro tetraploid leaves were about 28.4 μm and 21.5 μm, respectively. The length and width of stomatal apparatus in glasshouse-grown diploid leaves were about 31.8 μm and 22.8 μm, respectively, while in glasshouse-grown tetraploid leaves were about 34.8 μm and 26.2 μm, respectively. From these results, we also found that there was a significant difference when the same ploidy material was grown under different conditions [Table 3]. The glasshouse-grown diploids material possessed longer and wider stomatal apparatus when compared with in vitro diploids material. The same phenomena were also found in tetraploids material.
Figure 2.
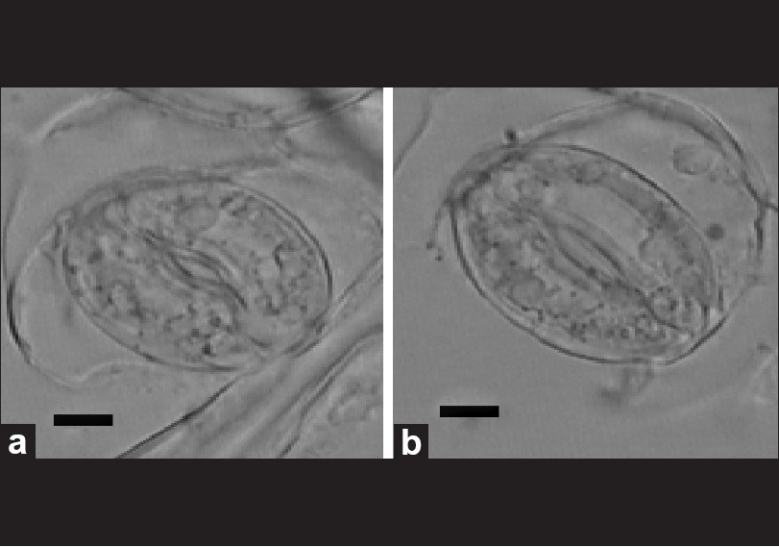
(a) Stomatal apparatus of diploid plant from glasshouse (bar = 6.50 × 10-4 cm); (b) Stomatal apparatus of tetraploid plant from glasshouse (bar = 6.50 × 10-4 cm). Each stomatal apparatus was obtained from the same part of diploid 2(a) and tetraploid 2(b) leaves in the glasshouse
Determination of rhizome yield and major chemical constituents
One of the important purposes of ginger breeding is to improve the yield of rhizomes, so the rhizome yields of all diploid and tetraploid plants were evaluated. The rhizome yield in each tetraploid line is shown in Table 4. The results indicate that all of the tetraploid lines showed higher rhizome yield than that of control. The highest rhizome yield of two-year-old tetraploid was found in line A-1, which was about 0.90 times higher than that of diploid plants [Table 4, Figure 3].
Table 4.
The rhizome yield and contents of volatile oil and gingerol in diploid and tetraploid rhizomes of Zingiber officinale
Figure 3.
Rhizome of line D7-1
The content of effective compounds is very important for medicinal plants. In order to determine the content of the effective constituent of tetraploid plants of Z. officinale Rosc., the volatile oil content and gingerol content of each tetraploid plant were analyzed.
The content of volatile oil in each tetraploid line is shown in Table 4. The results indicated that most of the tetraploid lines showed higher but not significant (≤125%) content of volatile oil than the control, although there were some differences in volatile oil content among tetraploid lines. The highest content of volatile oil in tetraploid lines was found in line D7-1, and the volatile oil content was 2.55 ml per 500 g, about 23% higher than that of the control. Even though most of two-year-old tetraploid plants had not so high volatile oil content, all of tetraploid plants showed higher overall productivity (yield of volatile oil per plant) than the control, due to their higher rhizome fresh weight. For example, the content of volatile oil in line A-1 was 2.26 ml/500 g, only 9% higher than that of the control, but the rhizome weight of plant was 2 177.4 g. The yield of volatile oil in line A-1 was therefore as high as 2.07 times per plant of that in the control [Table 4]. Therefore, the yield of volatile oil per plant of all the 18 tetraploid lines were significant higher than the control (>125%). The yield of volatile oil per plant in lines 7-1, E3, A-1, and D7-1 were more than one time higher than the control due to their high rhizome fresh yield and volatile oil content. These superior lines will be further selected to develop into new varieties in further research work [Table 4].
The gingerol were extracted and analyzed by UV-spectrophotometer (Beckman DU-600, USA) at 280 nm. The Vanillin (purchased from National Institute for the Control of Pharmaceutical and Biological Products, P.R. China) was used as the external standard. The content of gingerol in each tetraploid line is shown in Table 4. The results indicated that most of the tetraploid lines showed higher gingerol content than the control. The highest gingerol content in tetraploid lines was 1.77% in line A-1, 34% higher than that of control. All of the rest tetraploid lines showed a little higher or lower gingerol contents (%) than the control (≤125%), but all of tetraploid plants had higher (>125%) overall productivity of gingerol (yield of gingerol per plant) owing to their higher rhizome dry weight. From all the 18 tetraploid lines investigated, gingerol yield per plant in five lines were about one time higher than that of control, and these lines were therefore selected for further study. They were lines 7-1, A-1, D7-1, 7-15, and E3, and the gingerol yield per plant were 1.29, 1.26, 0.99, 0.99, and 0.98 times higher than that of the control, respectively.
DISCUSSION AND CONCLUSION
Since Blakeslee and Avery found that plant chromosome number can be doubled by colchicine in 1937,[21] colchicine began to play an important role in plant breeding. It is clear that the colchicine is a phytoalkaloid that binds to tubulin and prevents its polymerization into microtubules, so it can block the formation of mitotic spindle and arrest nuclear division at metaphase.[22] There were a few researches that had reported to obtain polyploid ginger by colchicine treatment.[18] But some differences existed between our experiment and the earlier reports. In the research of Ravindren and Nirmal,[23] they found that all of the polyploid plants returned to diploid in their subsequent study, but in our research, we have not found any chromosome number loss in the 20th generation of subculture materials in vitro and in the two-year-old glasshouse-grown materials by root-tip chromosome determination. Adaniya and Shirai found that the polyploid ginger had higher pollen fertility and germination rates,[24] but we never found any flower from the diploid and tetraploids in our research. These phenomena might be caused by plant germplasm variances, but need further study.
In our research, we found that the optimum concentration of colchicine treatment for the induction of tetraploid was 0.2%, and the optimum treated time was 30 hours. But there were some side effects of colchicine. During the tetraploid induction procedure, we found that the treated buds grew more slowly than the untreated buds when inoculated into the solid MS medium supplemented with 2.0 mg/l BAP and 0.2 mg/l NAA for 20 days. But after being subcultured for another 30 days, the growth of the treated buds recovered, and some of them were better than the untreated buds. This effect may be caused by the toxicity of the colchicine,[25] which may result in a reduced rate of cell division, even result in a death of explants.[26]
The morphological feature is an effective means for distinguishing plants with different ploidy levels in many plant types,[27,28] because polyploids possess longer and wider stomatal apparatus and leaf sizes. In our observation, we found that the size of stomatal apparatus and leaf of tetraploids were larger than that of the diploid plants. In addition, the stomatal apparatus and leaf of ex vitro material was larger than that of in vitro material, which perhaps had connection with the high humidity, weak light, and heterotrophy environment of in vitro material.[29] The larger size of stomatal apparatus and leaf of tetraploids means that the leaf surface area of tetraploid is larger than that of the diploid plants, and the larger leaf surface area may cause stronger photosynthesis, which will provide more materials and energies for the plant growing, as a result, we can obtain higher yield of rhizomes from tetraploid ginger.
Beside the stomatal apparatus and leaf, the stems, roots, and flowers in polyploid plants are usually bigger than those of the diploid plants.[30] Thus, the polyploid plants may have an increased biomass and yield. In medicinal species, the leaves, stems, flowers, and roots are often the medical parts and the source of the desired active compounds, so the increased biomass of polyploid plants is a very attractive characteristic. The higher yield or higher active compound content of these plants is important for the extraction of natural products and their clinical use in China and other Asian countries. Reports showed that the active compound content in tetraploid plants is higher when compared with diploid plants.[29,31] In our research, we found that all of the 18 tetraploid lines possessed great higher yield of rhizome than that of the diploid plants, and the volatile oil yield per plant and gingerol yield per plant were significantly higher than that of the diploid plants, which was a great improvement in ginger breeding.
In summary, 48 tetraploid lines of ginger were generated from adventitious buds by submerging into colchicine water solution. Eighteen tetraploid lines of ginger showed higher rhizome yield and volatile oil and gingerol yield per plant than the diploid mother plant lines. Five lines of tetraploid with high rhizome yield and good quality were selected to be used in further breeding programs to obtain superior new varieties for commercial production.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Hu WY, Zhang RP, Tang LP, Liu G. Research progress in the chemical constituents and pharmacology of ginger. Chin J Ethnomed Ethnopharm. 2008;9:10–4. [Google Scholar]
- 2.Dedov VN, Tran VH, Duke CC, Connor M, Christie MJ, Mandadi S, et al. Gingerols: A novel class of vanilloid receptor (VR1) agonists. Br J Pharmacol. 2002;137:793–8. doi: 10.1038/sj.bjp.0704925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang H, Xie Z, Koo HJ, McLaughlin SP, Timmermann BN, Gang DR. Metabolic profiling and phylogenetic analysis of medicinal Zingiber species: Tools for authentication of ginger (Zingiber officinale Rosc.) Phytochemistry. 2006;67:232–44. doi: 10.1016/j.phytochem.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Ali BH, Blunden G, Tanira MO, Nemmar Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem Toxicol. 2008;46:409–20. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- 5.Fuhrman B, Rosenbat M, Hayek T, Coleman R, Aviram M. Ginger extract consumption reduces plasma cholesterol, inhibits LDL oxidation and attenuates development of atherosclerosis in atherosclerotic, apolipoprotein E-deficient mice. J Nutr. 2000;130:124–31. doi: 10.1093/jn/130.5.1124. [DOI] [PubMed] [Google Scholar]
- 6.Bhandari U, Sharma JN, Zafar R. The protective action of ethanolic ginger (Zingiber officinale) extract in cholesterol fed rabbits. J Ethnopharmacol. 1998;61:167–71. doi: 10.1016/s0378-8741(98)00026-9. [DOI] [PubMed] [Google Scholar]
- 7.Nicoll R, Henein MY. Ginger (Zingiber officinale Roscoe): A hot remedy for cardiovascular disease. Int J Cardiol. 2009;131:408–9. doi: 10.1016/j.ijcard.2007.07.107. [DOI] [PubMed] [Google Scholar]
- 8.Shukla Y, Singh M. Cancer preventive properties of ginger: A brief review. Food Chem Toxicol. 2007;45:683–90. doi: 10.1016/j.fct.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Hosoki T, Sagawa Y. Clonal propagation of ginger (Zingiber officinale Rosc.) through tissue culture. HortScience. 1977;12:451–2. [Google Scholar]
- 10.Bhagyalakshmi, Singh NS. Meristem culture and micropropagation of a variety of ginger (Zingiber officinale Rosc.) with a high-yield of oleoresin. HortScience. 1988;63:321–7. [Google Scholar]
- 11.Malamug FJ, Inden H, Asahira T. Plantlet regeneration and propagation from ginger callus. HortScience. 1991;48:89–97. [Google Scholar]
- 12.Rout GR, Palai SK, Samantaray S, Das P. Effect of growth regulator and culture conditions on shoot multiplication and rhizome formation in ginger (Zingiber officinale Rosc.) in vitro. In Vitro Cell Dev Biol Plant. 2001;37:814–9. [Google Scholar]
- 13.Dekker AJ, Rao AN, Gob CJ. In vitro storage of multiple shoot cultures of ginger at ambient temperatures of 24–29°C. HortScience. 1991;47:157–67. [Google Scholar]
- 14.Tyagi RK, Agrawal A, Yusuf A. Conservation of Zingiber germplasm through in vitro rhizome formation. HortScience. 2006;108:210–9. [Google Scholar]
- 15.Kackar A, Bhat SR, Chandel KP, Malik SK. Plant regeneration via somatic embryogenesis in ginger. Plant Cell Tissue Organ Cult. 1993;32:289–92. [Google Scholar]
- 16.Guo YH, Zhang ZX. Establishment and plant regeneration of somatic embryogenic cell suspension cultures of the Zingiber officinale Rosc. HortScience. 2005;107:90–6. [Google Scholar]
- 17.Ramachandran K. Polyploidy induced in ginger by colchicine treatment. Curr Sci. 1982;51:288–9. [Google Scholar]
- 18.Liu ZW, Shi XJ, Zhao JH, Li LG. Research Progress on Ginger Breeding. Northern Hort. 2009;6:135–6. [Google Scholar]
- 19.Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissues cultures. Physiol Plant. 1962;15:473–9. [Google Scholar]
- 20.Zhang MC, Li J, Meng JC. Determination of the content of gingerol from Zingiber officinale Rosc. by UV spectrophotometry. Guizhou Med J. 2003;27:283–4. [Google Scholar]
- 21.Blakesleel A, Avery A. Methods of inducing doubling of chromosome in plants. J Hered. 1937;28:393–411. [Google Scholar]
- 22.Jordan MA, Wilson L. The use and action of drugs in analyzing mitosis. Methods Cell Biol. 1998;61:267–95. doi: 10.1016/s0091-679x(08)61986-x. [DOI] [PubMed] [Google Scholar]
- 23.Ravindren PN, Nirmal Babu K. New York, Washington: CRC press; 2005. Ginger: The Genus zingiber; pp. 68–77. [Google Scholar]
- 24.Adaniya S, Shirai D. In vitro induction of tetraploid ginger (Zingiber officinale Roscoe) and its pollen fertility and germinability. HortScience. 2001;88:277–87. [Google Scholar]
- 25.Sullivan JT, Castro L. Mitotic arrest and toxicity in Biomphalaria glabrata (Mollusca: Pulmonata) exposed to colchicine. J Invertebr Pathol. 2005;90:32–8. doi: 10.1016/j.jip.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Swanson CP. New Jersey: Prentice Hall; 1957. Cytology and cytogenetics. [Google Scholar]
- 27.Hamill SD, Smith MK, Dodd WA. In vitro induction of banana autotetraploids by colchicine treatment of micropropagated diploids. Aust J Bot. 1992;40:887–96. [Google Scholar]
- 28.Chakraborti SP, Vijayan K, Roy BN, Qadri SM. In vitro induction of tetraploidy in mulberry (Morus alba L.) Plant Cell Rep. 1998;17:799–803. doi: 10.1007/s002990050486. [DOI] [PubMed] [Google Scholar]
- 29.Huang HP, Gao SL, Chen LL, Jiao XK. In vitro induction and identification of autotetraploids of Dioscorea zingiberensis. In Vitro Cell Dev Biol Plant. 2008;44:448–55. [Google Scholar]
- 30.Zhang HF, Zheng H, Chen XH, Guo BL. Research progress of polyploid medicinal plants. (5).Hebei J Forestry Orchard Res. 2008;3:169–72. [Google Scholar]
- 31.Rowson JM. Increased alkaloid contents of induced polyploid of Datura. Nature. 1949;154:81–2. [Google Scholar]


