Abstract
Background:
Hwangryunhaedok-tang is a traditional herbal prescription that has sedative activity, hypotensive and anti-bacterial effects.
Objective:
In this study, we investigated the alteration of contents of components in Hwangryunhaedok-tang, antioxidant activity and neuroprotective activity by fermentation with Lactobacillus acidophilus KFRI 128.
Materials and Methods:
Contents of three marker compounds (geniposide, berberine and palmatine) and unknown compounds in the Hwangryunhaedok-tang (HR) and the fermented Hwangryunhaedok-tang (FHR) were measured and compared using the established high-performance liqued chromatograph coupled with a photodiode (HPLC-DAD) method. The antioxidant activity of HR and FHR were determined by DPPH free radical and hydrogen peroxide (H2O2) scavenging assay. Also, the neuroprotective activities of HR and FHR against glutamate-induced oxidative stress in a mouse hippocampal cell line (HT22) were evaluated by MTT assay.
Results:
The contents of geniposide and palmatine were decreased but the content of berberine was increased in the FHR. And the contents of unknown compounds (1), (2), (3), (4) and (5) in the HR were altered by fermentation. Electron donating activity (EDA, %) value of FHR was higher than HR for DPPH radical scavenging activity and H2O2 scavenging activity, respectively. In the MTT assay, FHR showed more potent neuroprotective activity than HR by 513.90%.
Conclusion:
The FHR using microorganism could convert compounds in HR and enhance the antioxidant and neuroprotective activity.
Keywords: Antioxidant activity, bioconversion, fermentation, Hwangryunhaedok-tang, neuroprotective activity
INTRODUCTION
Oxidative stress is caused by the accumulation of the reactive oxygen species (ROS). Oxidative stress leads to neurodegenerative disorders such as Alzheimer's disease, Parkinson's disease and Huntington's disease.[1–5] Glutamate is an excitatory neurotransmitter. Excessive glutamate causes neuronal cell death by inhibiting the cysteine uptake in neuronal cells, which induces decline of the antioxidant, glutathione (GSH).[6–9]
Hwangryunhaedok-tang (HR) was usually used as a traditional herbal medicine for sedative activity, and for its hypotensive and anti-bacterial effects. Effects of HR on neuroprotection were reported in recent research. HR is composed of four herbs, Coptis japonica, Phellodendron amurense, Scutellaria baicalensis and Schisandra chinensis.[10,11]
Bioconversion such as fermentation by microorganism could convert bioactive components of herbal prescriptions to other forms. Also, it could enhance the biological activity of medicinal herbs in a traditional herbal prescription.[12–14]
In this study, we investigated the conversion of the three components in fermented Hwangryunhaedok-tang (FHR) by Lactobacillus acidophilus KFRI 128. The marker component of composed herbs, geniposide (Schisandra chinensis), berberine (Coptis japonica) and palmatine (Phellodendron amurense) was selected to determinate conversion of components [Figure 1]. The neuroprotective and antioxidant activity of FHR was evaluated. Contents of three marker components in HR and FHR were measured using the established HPLC-DAD method.[15–17] Neuroprotective activity against glutamate-induced cytotoxicity in HT22 cells of HR and FHR was evaluated by MTT assay. The antioxidant activity of HR and FHR was investigated by DPPH radical and hydrogen peroxide (H2O2) scavenging activity assay. And we determined a relation of neuroprotective activity and antioxidant activity.
Figure 1.
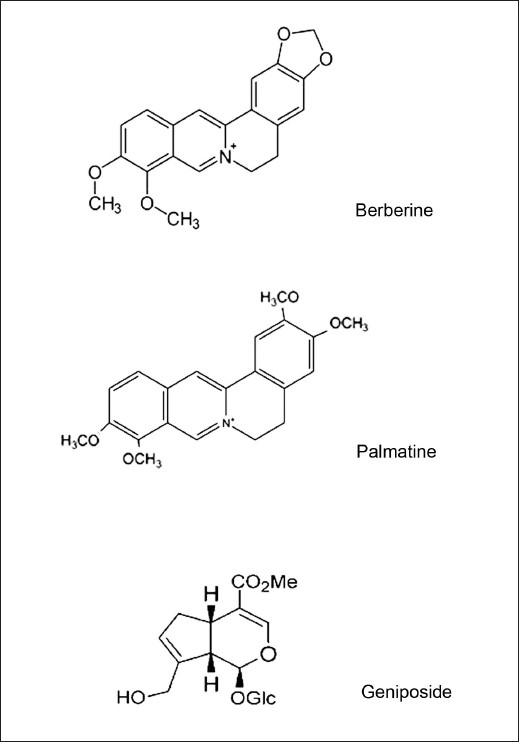
The chemical structures of three bioactive components in Hwangryunhaedok-tang. (1) genioiside, (2) berberine and (3) palmatine, respectively
MATERIALS AND METHODS
Materials and reagents
The powder of the HR sample (3.0 g) and fermentative organism, Lactobacillus acidophilus KFRI 128 were obtained from the Korea Institute of Oriental Medicine and the Korea Food Research Institute, respectively. HT22 cells were derived from the Seoul National University, Korea. Used in cell culture, Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco (Invitrogen Co., USA). L-Glutamic acid monosodium salt hydrate (Glutamate), 3-(4,5 –dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), 2,2-Di(4-tert-octylphenyl)-1-picrylhydrazyl (DPPH) 2,2-azinobis(3-ethylbenthiazolin)-6-sulfonic -acid (ABTS), 6-Hydroxy-2,5,7,8-tetramethylchroman-2-carbboxylic acid (trolox) and (+)-Sodium L-ascorbate were purchased from Sigma-Aldrich (USA). Marker components, berberine and geniposide were obtained from Korea Food and Drug Administration. Palmatine was purchased from Sigma-Aldrich (USA). The purities of three marker components were greater than 97%. Water and methanol used as solvents for HPLC grade were purchased from J.T. Baker (USA). Trifluoroacetic acid (TFA) used as solvent for analytical grade was obtained from DAE JUNG (Korea).
Fermentation condition of Hwangryunhaedok-tang
After two successive transfers of the test organisms in MRS (de Man, Rogosa and Sharp) broth for Lactobacillus acidophilus KFRI 128 at 37°C for 24 h, the activated cultures were again inoculated into each media at the same condition. It was properly diluted to obtain an initial population of 1-5 × 07 CFU/ml and served as the inoculum. The HR water extract was used as the culture media for fermentation after adjusting pH to 8.0 using 1 M NaOH and autoclaved for 15 min at 121°C. After cooling, 750 ml of HR was inoculated with 7.5 ml inoculums as described above. This was incubated at 37°C for a period of 48 h.
High-performance liquid chromatography analysis of components in the Hwangryunhaedok-tang and fermented Hwangryunhaedok-tang
The powders of HR (100 mg) and FHR (100 mg) were weighed accurately and dissolved at the concentration of 10 mg/ml in 60% MeOH. These sample solutions were stored at 4°C and filtered through a 0.45-μm membrane filter before HPLC analysis and activity assay. The HPLC system for samples’ analysis was composed of a pump (LPG 3X00), an auto sampler (ACC-3000), a column oven (TCC-3000SD) and diode array UV/VIS detector (DAD-3000(RS)). Chromatographic separation was achieved using a Phenomenex column (C18 5μm, 4.6 I.D. × 250mm) and the column temperature maintained at 30°C. The mobile phase consisted of water containing 0.1% TFA (A) and MeOH (B) with gradient elution to improve the separation capacity. The gradient elution system of Solvent A and B was optimized and performed as follows; 0-5 min, 80-70% A; 5-30 min, 70-65% A; 30-40 min, 65-60% A; 40-65 min, 60-70% A; 65 min, 70-80% A at a flow rate of 1.0 ml/min. Based on the value of UV absorption maximum of each three marker components, the detection UV wavelength was set. The UV wavelength of geniposide and berberine were set at a 230 nm and that of palmatine was set at 280 nm. The injection volume of each sample was 20 μl.
2,2-Di(4-tert-octylphenyl)-1-picrylhydrazyl free radical scavenging assay
The free radical scavenging activity of the HR and FHR was measured by DPPH assay.[18–20] Samples (150 μl) were added to 150 μl of 0.4 mM DPPH solution in 96-well plate. After incubation at room temperature for 30 min, optical density (OD) was measured at 517 nm using a microplate reader. This assay was repeated three times. The DPPH free radical activities of HR and FHR were evaluated by comparing with the value of EDA (%) (electron donating activity). The value of EDA (%) was calculated using the following equation; EDA (%) = [1- (OD of sample / OD of control)] × 100.
Hydrogen peroxide scavenging assay
The hydrogen peroxide scavenging activity of HR and FHR was evaluated by 2,2-azinobis(3-ethylbenzthiazolin)-6-sulfonicacid (ABTS) assay.[21] Samples (80 μl) were added to 20 μl of 10 mM H2O2 solution and 100 μl of 0.1 M phosphate buffer (pH 0.5) in a 96-well plate. After incubation at 35°C for 5 min, 30 μl of 1.25 mM ABTS and 30 μl of 1 U/ml peroxidase were added and incubated at 35°C for 10 min. The OD was measured at 405 nm using a microplate reader. The hydrogen peroxide scavenging activities of HR and FHR were evaluated by comparing with the value of EDA (%).
HT22 cells culture and cell viability assay
The immortalized mouse hippocampal cell line (HT22) cells are a useful model for studying the mechanism of glutamate-induced neuron cell death.[22,23] The neuroprotective activity of HT22 cells was evaluated by MTT assay to measure the degree of cell protection activity against glutamate-induced cytotoxicity. HT22 cells were incubated in DMEM supplemented with 10% (v/v) fetal bovine serum (FBS), 1% penicillin/streptomycin at 37°C in 5% CO2. HT22 cells were seeded at a density of 6.7 × 104 cells/well (300 μl) in a 48-well plate. After 24 h, HR and FHR samples (30 μl) and trolox (50 μM) were treated. Then, 30 μl of 2 mM glutamate was treated after 1 h. Trolox was used as a positive control. After 24 h, MTT solution (1 mg/ml) was added into each well and incubated for 3 h at 37°C in 5% CO2. Media was totally removed and the dark blue formazan was dissolved in 300 μl of DMSO. OD was measured at 570 nm using a microplate reader. The neuroprotective activity of samples was investigated by relative protection (%). Relative protection (%) was calculated using the following equation: (OD of glutamate-treated with sample - OD of only glutamate-treated) / (OD of control - OD of only glutamate-treated).
RESULTS AND DISCUSSION
High-performance liquid chromatography analysis of components in Hwangryunhaedok-tang and fermented Hwangryunhaedok-tang
Three marker components, geniposide, berberine and palmatine in HR and fermented by Lactobacillus acidophilus KFRI 128 were analyzed using a establish HPLC-DAD method and the results of the analysis are presented in Table 1. As a result of comparison with HR and FHR, contents of geniposide and palmatine in FHR were decreased by 8.99% and 7.24%, respectively and content of berberine was increased by 99.48%. Component content of unknown compound (1) in FHR was decreased than the HR and component contents of unknown compounds (2), (3), (4) and (5) in FHR were increased than the HR [Figure 2]. Thus, some of the compounds in the HR were converted by fermentation.
Table 1.
Contents of three marker components in the Hwangryunhaedok-tang and fermented Hwangryunhaedok-tang sample

Figure 2.
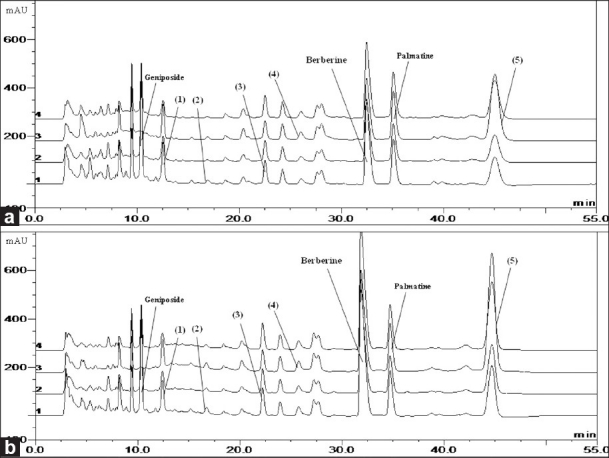
The high-performance liquid chromatography of Hwangryunhaedok-tang (a) and fermented Hwangryunhaedok-tang (b). (1 = 230 nm, 2 = 254 nm, 3 = 280 nm and 4 = 260 nm)
2,2-Di(4-tert-octylphenyl)-1-picrylhydrazyl free radical scavenging assay
DPPH free radical was reacted with the hydrogen donor, and was cleared out. The results of the assay are presented in Figure 3. Both HR and FHR had excellent antioxidant activity in a dose-dependent manner. And the value of EDA (%) of FHR was 74.07% at the concentration of 0.01 mg/ml, it was higher than that of the HR. Thus, we confirmed that the fermentation process enhanced the antioxidant activity.
Figure 3.
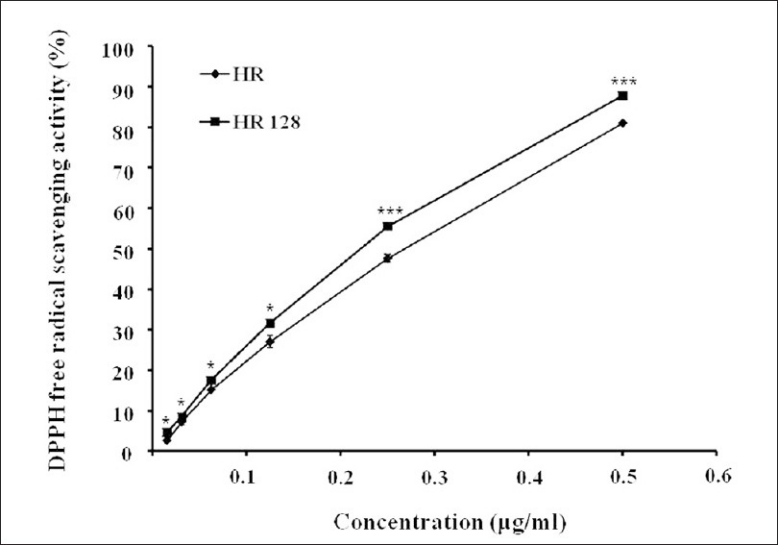
2,2-Di(4-tert-octylphenyl)-1-picrylhydrazyl free radical scavenging activity (%) of Hwangryunhaedok-tang and fermented Hwangryunhaedok-tang. Each bar represents the mean ± SD of three independent experiments. *P<0.05, **P<0.01, ***P<0.001 vs. Hwangryunhaedok-tang (ANOVA)
Hydrogen peroxide scavenging assay
The degree of hydrogen peroxide (H2O2), known to cause DNA damage in the body, promotes lipid peroxidation of the cell and aging, scavenging and antioxidant activities of HR were evaluated [Figure 4]. Both HR and FHR had a low H2O2 scavenging activity. This result suggested that both of them have no direct H2O2 scavenging activity.
Figure 4.
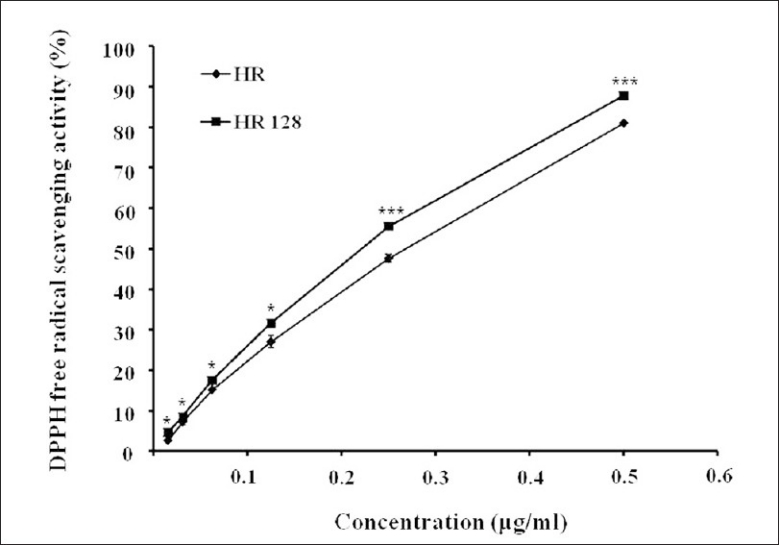
Hydrogen peroxide (H2O2) free radical scavenging activity (%) of Hwangryunhaedok-tang and fermented Hwangryunhaedok-tang. Each bar represents the mean ± SD of three independent experiments. *P<0.05, **P<0.01, ***P<0.001 vs. Hwangryunhaedok-tang (ANOVA)
Neuroprotective activity assay
We evaluated the neuroprotective activity of HR and FHR against glutamate-induced neurotoxicity in HT22 cells. The neuroprotective activity of HR was more potent than that of its single herbal medicines, such as Coptis japonica, Phellodendron amurense, Scutellaria baicalensis and Schisandra chinensis, at the concentration ranged from 1 μg/mL to 100 μg/mL, respectively [Table 2]. It could be explained by the synergistic effect of the four ingredient plant extracts. There were similar reports related to the synergistic effect of plant extracts or formulation to single compound or single herb. Ginkgo biloba extract significantly inhibited the sodium current, L-type calcium current and transient outward potassium current in a concentration-dependent manner but its main ingredients, ginkgolide A, ginkgolide B and bilobalide did not exhibit any significant effect on these cation channel currents.[24] It is interesting to note that, as with many plants, single isolated components do not provide the activity of the whole extract. In addition, FHR showed more potent neuroprotective activity by 513.90% of the activity of HR [Figure 5]. The neuroprotective effect of berberine has been reported in many studies.[25,26] We considered that increment of content of berberine induced improvement of the neuroprotective effect of HR. And the neuroprotective activity of FHR was relevant to oxidative stress-induced free radical through results of antioxidant activity test. According to these data, we suggest that fermentation converts many components in the HR and enhances the biological activity of HR.
Table 2.
The neuroprotective effects of HR, Coptis japonica, Phellodendron amurense, Scutellaria baicalensis and Schisandra chinensis on glutamate-induced cytotoxicity in HT22 cells
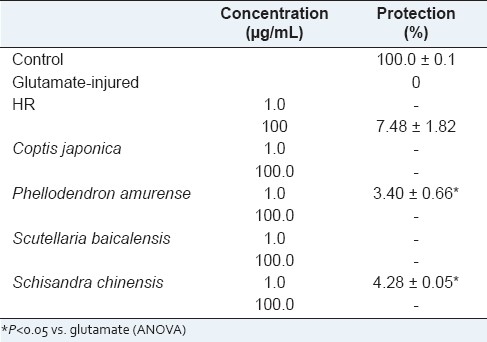
Figure 5.
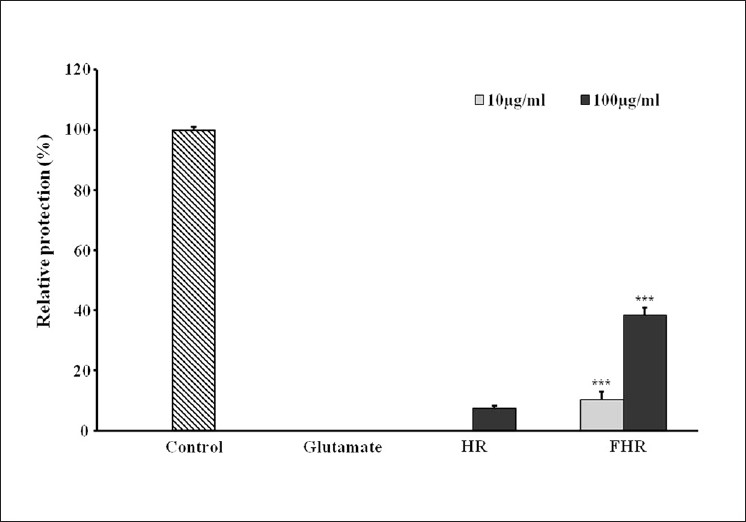
The neuroprotective effects of Hwangryunhaedok-tang and fermented Hwangryunhaedok-tang on glutamate-induced cytotoxicity in HT22 cells. HT22 cells were treated with Hwangryunhaedok-tang and fermented Hwangryunhaedok-tang at concentration of 10 and 100 μg/ml, and then incubated for 24 h with glutamate (2 mM). Trolox (50μM) that was used for positive control exhibited relative protective activity (82.89 ± 0.39%). Each bar represents the mean ± SD of three independent experiments. *P<0.05, **P<0.01, ***P<0.001 vs. Hwangryunhaedok-tang (ANOVA)
CONCLUSIONS
In this study, we analyzed the change of amount of geniposide, berberine and palmatine in HR fermented by Lactobacillus acidophilus KFRI 128. The neuroprotective and antioxidant activities of HR were compared with FHR. As a result of comparison of the contents of three marker compounds in HR and FHR, the contents of geniposide and palmatine were decreased and the content of berberine was increased. Also, amount of unknown compound (1) in FHR was decreased. Amount of unknown compounds (2), (3), (4) and (5) in FHR were increased. DPPH free radical scavenging activity and neuroprotective activity were increased by fermentation. In conclusion, we determined that the change of amount of compounds in Hwangryunhaedok-tang by fermentation might improve the neuroprotective and antioxidant activity. Finally, further study related to identification of the converted bioactive components on the fermentation process and the relationship of the conversion of components and neuroprotective activity might be necessary.
ACKNOWLEDGMENTS
This research was supported by a grant [K10050] from the Korea Institute of Oriental Medicine. We express our gratitude to Dr. Chang-Won Cho, Korea Food Research Institute, for kindly supplying the fermented Hwangryunhaedok-tang samples.
Footnotes
Source of Support: Grant [K09040] from the Korea Institute of Oriental Medicine
Conflict of Interest: None declared.
REFERENCES
- 1.Teepker M, Anthes N, Fischer S, Krieg JC, Vedder H. Effects of oxidative challenge and calcium on ATP-levels in neuronal cells. Neurotoxicol. 2007;28:19–26. doi: 10.1016/j.neuro.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Aruoma OI. Free radicals, oxidative stress and antioxidants in human health and disease. J Am Oil Chem Soc. 1998;75:199–212. doi: 10.1007/s11746-998-0032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–95. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 4.Satoh T, Enokido Y, Kubo T, Yamada M, Hatanaka H. Oxygen toxicity induces apoptosis in neuronal cells. Cell Mol Neurobiol. 1998;18:649–66. doi: 10.1023/a:1020633919115. [DOI] [PubMed] [Google Scholar]
- 5.Smith CD, Carney JM, Starke-Reed PE, Oliver CN, Stadtman ER, Floyd RA, et al. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci U S A. 1991;88:10540–3. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis JB, Maher P. Protein kinase C activation inhibits glutamate-induced cytotoxicity in a neuronal cell line. Brain Res. 1994;652:169–73. doi: 10.1016/0006-8993(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 7.Fonnum F. Glutamate: A neurotransmitter in mammalian brain. J Neurochem. 1984;42:1–11. doi: 10.1111/j.1471-4159.1984.tb09689.x. [DOI] [PubMed] [Google Scholar]
- 8.Hara J, Gerashchenko D, Wisor JP, Sakurai T, Xie XS, Kilduff TS. Thyrotropin-releasing hormone increases behavioral arousal through modulation of hypocretin/orexin neurons. J Neurosci. 2009;29:3705–14. doi: 10.1523/JNEUROSCI.0431-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim HJ, Kim JH, Son ES, Lee JM, Park HR. Neuroprotective effect of extracts from root bark of Morus alba on glutamate-induced cytotoxicity in neuronal cells. J Life Sci. 2009;19:963–7. [Google Scholar]
- 10.Kong MJ, Ha NN, Lee HY, Kim YT, Rho SJ, Kim HC. Effect of Hwangryunhaedok-tang and its modified prescription on the recovery of spatial cognitive function in the brain ischemia induced by four-vessel occlusion in rats. Kor J Herbol. 2004;19:161–8. [Google Scholar]
- 11.Hwang YS, Shin CY, Huh Y, Ryu JH. Hwangryun-Hae-Dok-tang (Huanglian-Jie-Du-Tang) extract and its constituents reduce ischemia-reperfusion brain injury and neutrophil infiltration in rats. Life Sci. 2002;71:2105–17. doi: 10.1016/s0024-3205(02)01920-3. [DOI] [PubMed] [Google Scholar]
- 12.Hyon JS, Kang SM, Han SW, Kang MC, Oh MC, Oh CK, et al. Flavonoid component changes and antioxidant activities of fermented Citrus grandis Osbeck Peel. J Kor Soc Food Sci Nutr. 2009;38:1310–6. [Google Scholar]
- 13.Park HY, Um YR, Ma JY. Quantitative analysis of marker substance in fermented Rehmanniae Radix Preparata. Korean J Pharmacogn. 2010;41:58–61. [Google Scholar]
- 14.Doh ES, Chang JP, Lee KH, Seong NS. Ginsenoside change and antioxidation activity of fermented ginseng. Korean J Med Crop Sci. 2010;18:255–65. [Google Scholar]
- 15.Hajimehdipoor H, Shekarchi M, Khanavi M, Adib N, Amri M. A validated high performance liquid chromatography method for the analysis of thymol and carvacrol in Thymus vulgaris L. volatile oil. Pharmacogn Mag. 2010;6:154–8. doi: 10.4103/0973-1296.66927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shekarchi M, Hajimehdipoor H, Khanavi M, Adib N, Bozorgi M, Akbari-Adergani B. A validated method for analysis of Swerchirin in Swertia longifolia Boiss. by high performance liquid chromatography. Pharmacogn Mag. 2010;6:13–8. doi: 10.4103/0973-1296.59961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weon JB, Yang HJ, Ma JY, Ma CJ. A HPLC-DAD method for the simultaneous determination of five marker components in the traditional herbal medicine Bangpungtongsung-san. Pharmacogn Mag. 2010;7:60–4. doi: 10.4103/0973-1296.75903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nanjo F, Goto K, Seto R, Suzuki H, Sakai M, Hara Y. Scavenging effects of tea catechins and their derivatives on 1,1-diphnyl-2-picrylhydrazyl radical. Free Radic Biol Med. 1996;21:885–902. doi: 10.1016/0891-5849(96)00237-7. [DOI] [PubMed] [Google Scholar]
- 19.Sim KS, Nurestri AM, Norhanom AW. Phenolic content and antioxidant activity of Pereskia grandifolia Haw. (Cactaceae) extracts. Pharmacogn Mag. 2010;6:248–54. doi: 10.4103/0973-1296.66945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nabavi SM, Ebrahimzadeh MA, Nabavi SF, Fazelian M, Eslami B. In vitro antioxidant and free radical scavenging activity of Diospyros lotus and Pyrus boissieriana growing in Iran. Pharmacogn Mag. 2009;5:122–6. [Google Scholar]
- 21.Müller HE. Detection of hydrogen peroxide produced by microorganism on ABTS peroxidase medium. Zentralbl Bakteriol Mikrobiol Hyg A. 1995;259:151–4. doi: 10.1016/s0176-6724(85)80045-6. [DOI] [PubMed] [Google Scholar]
- 22.Satoh T, Ishige K, Sagara Y. Protective effects on neuronal cells of mouse afforded by ebselen against oxidative stress at multiple steps. Neurosci Lett. 2004;371:1–5. doi: 10.1016/j.neulet.2004.04.055. [DOI] [PubMed] [Google Scholar]
- 23.Maher P, Davis JB. The role of monoamine metabolism in oxidative glutamate toxicity. J Neurosci. 1996;16:6394–401. doi: 10.1523/JNEUROSCI.16-20-06394.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen B, Cai J, Song LS, Wang X, Chen Z. Effects of Ginkgo biloba extract on cation currents in rat ventricular myocytes. Life Sci. 2005;76:1111–21. doi: 10.1016/j.lfs.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 25.Zhou XQ, Zeng XN, Kong H, Sun XL. Neuroprotective effects of berberine on stroke models in vitro and in vivo. Neurosci Lett. 2008;447:31–7. doi: 10.1016/j.neulet.2008.09.064. [DOI] [PubMed] [Google Scholar]
- 26.Kulkarni SK, Ashish D. Berberine: A plant alkaloid with therapeutic potential for central nervous system disorders. Phytother Res. 2010;24:317–24. doi: 10.1002/ptr.2968. [DOI] [PubMed] [Google Scholar]


