Abstract
Background:
Phyllanthus (Euphorbiaceae) species have long been used in folk medicine to treat various pathological conditions including liver diseases. Some species of Phyllanthus were found to exhibit hepatoprotective activity against drugs or toxins and this property was majorly attributed to phyllanthin and hypophyllanthin. In this study, we examined the hepatoprotective activity of five different species of Phyllanthus, namely, Phyllanthus amarus, Phyllanthus fraternus, Phyllanthus maderaspatensis, Phyllanthus urinaria, and Phyllanthus Rotundifolius. The extracts were also evaluated for the presence of key phytoconstituents, phyllanthin and hypophyllanthin.
Materials and Methods:
The extracts were evaluated for hepatoprotective activity against tert-butyl hydroxide (t-BH)-induced cytotoxicity using human hepatocarcinoma cells (HepG2 cell line).
Results:
Only P. urinaria and P. maderaspatensis exhibited significant hepatoprotective activity as evident from increased cell viability. The HPLC profile revealed that except P. amarus, the other extracts did not contain phyllanthin and hypophyllanthin.
Conclusion:
P. urinaria and P. maderaspatensis demonstrated dose-dependent hepatoprotective activity and hence, can provide promising therapeutic interventions against chemical–induced liver damage.
Keywords: Cytoprotection, hepatotoxicity, lipid peroxidation, methanolic plant extracts, silymarin
INTRODUCTION
Liver plays a pivotal role in the regulation of various physiological processes in the body such as carbohydrate metabolism and storage, fat metabolism, bile acid synthesis, etc. besides being the most important organ involved in the detoxification of various drugs as well as xenobiotics in our body. It is highly susceptible to damage by xenobiotics, drugs, etc. owing to its continuous exposure to these toxicants via the portal blood circulation.[1] Various chemicals like tert-butyl hydroperoxide (t-BH), galactosamine (GalN), paracetamol, carbon tetrachloride (CCl4), acetaminophen, and alcohol can cause potential damage to the liver cells leading to progressive dysfunction. Most of the hepatotoxic chemicals cause damage to the hepatocytes by inducing lipid peroxidation.[2,3] Thus, the disorders associated with liver are numerous and varied. However, no effective treatment that delays the disease progression and complication in liver has been found yet. Further, most of the available remedies support or promote the process of healing or regeneration of the liver. As a result, owing to the absence of proper medication, the focus has now considerably shifted toward plants as alternative therapies for the treatment of hepatic damage.
The Phyllanthus genus belongs to the Euphorbiaceae family. The genus has about 750–800 species found in tropical and subtropical regions worldwide. A number of species have been reported to have an extensive history in medicine systems.[4] A substantial number of Phyllanthus species are used widely in traditional medicine for the treatment of diabetes, jaundice, gall bladder, and liver disease.[5,6] Different species of Phyllanthus found in India are Phyllanthus fraternus, Phyllanthus urinaria, Phyllanthus virgatus, Phyllanthus maderaspatensis, and Phyllanthus debilis, among which Phyllanthus amarus is highly valued in the treatment of liver ailments and kidney stones.[5,7]
Extracts of Phyllanthus have secondary compounds like alkaloids, flavonoids, lignins, phenols, tannins, and terpene which are found in the leaf, stem, and roots of the plant. Common lipids such as sterols and flavonols also occur in the plant.[8] The major lignin's phyllanthin and hypophyllanthin have been reported to be antihepatotoxic against CCl4 -- and GalN--induced hepatotoxicity in primary cultured rat hepatocytes.[9]
The present study was designed to investigate the hepatoprotective activity of five species of Phyllanthus, namely, P. amarus, P. fraternus, P. rotundifolius, P. urinaria, and P. maderaspatensis on t-BH-induced toxicity in human hepatocellular carcinoma (HepG2) cells.
MATERIALS AND METHODS
Chemicals
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), silymarin, and t-BH were purchased from Sigma-Aldrich (St. Louis, MO, USA). Eagle's minimum essential media (EMEM) was supplied by Gibco Life Technologies (Grand Island, NY, USA) and fetal bovine serum (FBS) was purchased from Hyclone (Logan, UT, USA). Phyllanthin and hypophyllanthin were obtained from the Phytochemistry department of Natural Remedies Pvt. Ltd., Bangalore, Karnataka, India.
Plant material
Fresh plants of Phyllanthus species (P. amarus, P. fraternus, P. rotundifolius, P. urinaria, and P. maderaspatensis) were collected from the parts of Kanyakumari (Tamil Nadu, India). Voucher specimens (PYU/GJU/801, PYU/GJU/802, PYU/GJU/803, PYU/GJU/804, and PYU/GJU/805) were deposited in the herbarium of Guru Jambheswar University, Hissar (Haryana), for future reference.
Preparation of extracts
The coarsely powdered plant material (~25 g each) was extracted using methanol (~100 ml) by refluxing at 70°C for 1 h. The solution was filtered and the remaining raw material was refluxed twice with fresh methanol (75 ml) in a similar manner. The combined liquid filtrates were concentrated by distillation under vacuum to obtain a thick paste which was finally dried under vacuum at the temperature of 70°C. The final extract was weighed and %yield (w/w) was calculated [Table 1]. The samples were stored in closed containers until further analysis.
Table 1.
The % yield values of each extract

Standard and sample preparation
The standards were prepared by weighing 16 mg of phyllanthin and 15 mg of hypophyllanthin separately and then dissolved in 60 ml of methanol (HPLC grade, Qualigens). The solution obtained was then sonicated and cooled and the final volume was made to 100 ml. Similarly, the sample were prepared by weighing 500 mg of each crude herbal extract separately and dissolved in 60 ml of methanol. The resultant solution was then sonicated and cooled and the final volume was made up to 100 ml to get a concentration of 5 mg/ml.
High performance liquid chromatography methodology
The extracts were analyzed for the content of phytoconstituents, phyllanthin and hypophyllanthin, by the HPLC method. Aliquots of 5 μl of each solution were injected into the HPLC system (model LC 2010 A; Shimadzu, Japan) consisting of a quaternary pump with a UV detector, autoinjector, and column oven [RP C-18 phenomenex, Luna 3 μm (250 × 4.6 mm)] with class LC 10A software. The mobile phase consisted a mix of phosphate buffer [Solvent A, prepared by dissolving 0.140 g of anhydrous potassium dihydrogen orthophosphate (KH2PO4) in 900 ml of HPLC grade water (obtained from “Arium” Sartorious water purification system) and 0.5 ml of orthophosphoric acid (H3PO4, AR grade, Rankem) and then the final volume was made up to 1000 ml] and acetonitrile (Solvent B, HPLC grade, Qualigens). The phosphate buffer was filtered separately through a 0.45-μm membrane filter and degased by sonicating for 3 min. Both the solvents were mixed in 50:50 ratios. The flow rate of the mobile phase was maintained at 0.4 ml/min throughout the analysis and the detector wavelength was kept at 230 nm, and chromatogram was recorded. The HPLC chromatograms of each extract along with presence of phyllanthin and hypophyllanthin are shown in Figure 1.
Figure 1.
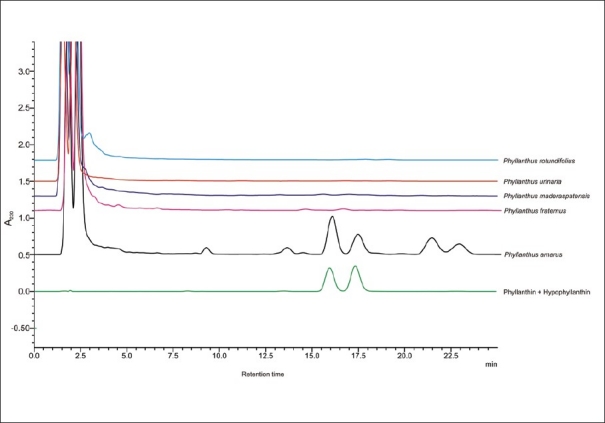
High performance liquid chromatography chromatogram of the Phyllanthus species
Cell line and culture condition
The HepG2 (hepatocellular carcinoma, #HB-8065™) cell line was procured from American Type Culture Collection (ATCC, Rockville, MD, USA). The cells were grown at 37°C in a humidified atmosphere of 5% CO2 in sterile filtered EMEM containing 10% FBS with 1 mM sodium pyruvate and 2.2 g/l sodium bicarbonate.
Cell viability assay
Cell viability was determined by a colorimetric MTT assay as described by Mosmann[10] with minor modifications. The MTT assay is based on the ability of viable cells to reduce MTT to an insoluble formazan product. In brief, HepG2 cells were cultured in 96-well plates at a seeding density of 4 × 104 cells/well. After 24 h incubation, the cells were treated with the extracts of P. amarus, P. fraternus, P. rotundifolius, P. urinaria, and P. maderaspatensis at concentrations ranging from 6.25 to 100 μg/ml in separate wells for 24 h. Thereafter, the cells were washed and incubated for 1 h with MTT. MTT was dissolved in phosphate-buffered saline (PBS) at a concentration of 5 mg/ml and added to the cells to the final concentration of 500 μg/ml. After 1 h, the medium was removed and the remaining formazan crystals were dissolved in 200 μl of DMSO. The optical density was measured using a microplate reader at a wavelength of 570 nm. Consequently, noncytotoxic concentrations were selected for hepatoprotective studies.
In vitro hepatoprotection activity study
The hepatoprotective activity of the extracts was evaluated against t-BH-induced toxicity using well-maintained HepG2 cells. Cells were seeded at a density of 5 × 104 cells/well and incubated overnight. Postincubation, the cells were treated with varying concentrations of extracts (P. amarus, P. fraternus, P. rotundifolius, P. urinaria, and P. maderaspatensis) in separate wells of a 96-well plate and incubated for 2 h. Silymarin (10 to 50 μg/ml) was used as a reference standard.[11] After incubation, the cells were treated with t-BH at a concentration of 1 mM and allowed to incubate for 2 h. Thereafter, the supernatant was discarded and the cells were washed with DPBS, and the fresh growth medium along with MTT was added and incubated for 1 h to allow the formation of formazan crystals. Finally, the medium was removed, and the formazan crystals were dissolved using DMSO; the absorbance was measured at 570 nm.[11]
Statistical analysis
Results are expressed as mean ± standard deviation (SD). A sample concentration providing 50% cytoprotection was calculated from graph plotting, percentage protection against sample concentration [Figure 2a and b]. The experiment was performed in triplicates. Statistical analysis was performed using Dunnett's multiple comparison tests and one-way analysis of variance (ANOVA) using GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA, USA). Differences between the groups were considered statistically significant at P<0.05 and P < 0.01. Results are expressed as percentage protection, i.e., the percentage increase in cell viability compared to the viability of cells treated with t-BH alone. The percent protection is calculated as below:[12]
Figure 2.
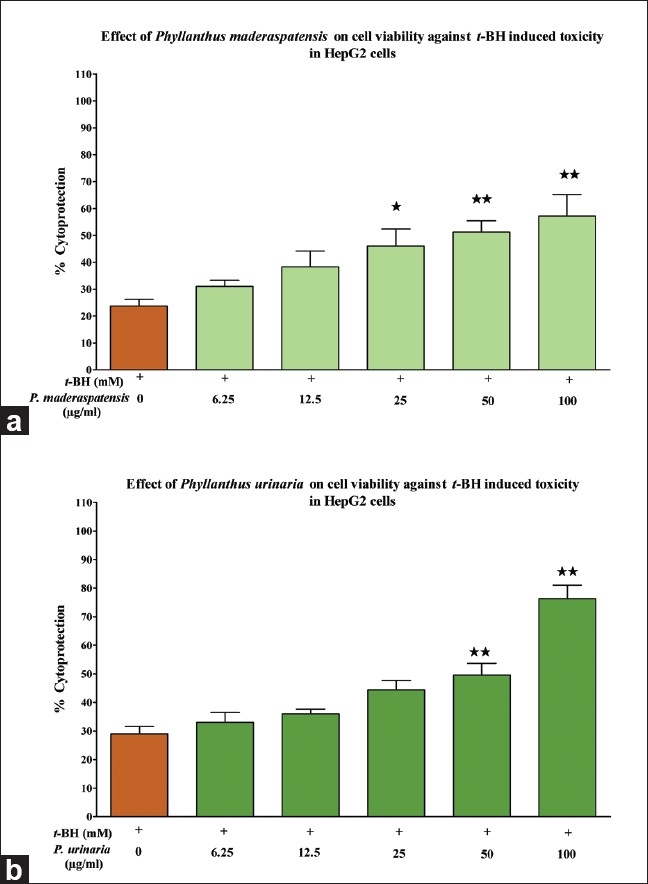
Effect of the Phyllanthus species on t-BH-induced toxicity in HepG2 cells. HepG2 cells were incubated in the presence/absence of (a) P. maderaspatensis and (b) P. urinaria for 2 h, prior to treatment with t-BH (1 mM) for 2 h. Thereafter, the cells were processed for the MTT assay. The results are expressed as mean ± SD. *P<0.05 and **P<0.001 compared to the t-BH (1 mM) control
% Protection = (Mean of sample treated – Mean of t-BH treated)/(Mean of untreated – Mean of t-BH treated) × 100.
RESULT AND DISCUSSION
Cytotoxicity of the extracts
Based on the MTT assay, the noncytotoxic concentrations of the extracts were determined. The cytotoxicity experiment showed that all tested extracts were noncytotoxic to HepG2 cells up to a concentration of 100 μg/ml.
Chemical profile of high performance liquid chromatography
The HPLC chromatograms of extracts P. amarus, P. fraternus, P. rotundifolius, P. urinaria, and P. maderaspatensis are shown in Figure 1. Only P. amarus was found to contain phyllanthin and hypophyllanthin.
Effect of the extracts on t-BH-induced hepatoxicity
We examined the hepatoprotective activity of the five species of Phyllanthus against t-BH-induced toxicity in HepG2 cells. The extracts of P. amarus, P. fraternus, P. rotundifolius, P. urinaria, and P. maderaspatensis were tested at concentrations ranging from 6.25 to 100 μg/ml in the presence of t-BH (1 mM). Among all the extracts, only P. maderaspatensis (25to100 μg/ml) and P. urinaria (50to100 μg/ml) exhibited dose-dependent cytoprotection against t-BH-induced cell toxicity at the indicated concentrations [Figure 2a, b]. P. maderaspatensis and P. urinaria showed significant cytoprotection of 44% and 66%, respectively. Based on the % cytoprotection and IC50 values, P. urinaria (IC50 = 72 μg/ml) was found to have a more potent hepatoprotective activity than P. maderaspatensis. However, the extracts showed lesser potency when compared with the reference standard, silymarin (IC50 = 49.0 μg/ml).
t-BH exerts toxicity by forming covalent bonds with cellular molecules, resulting in cell injury. It causes leakage of lactate dehydrogenase (LDH) and formation of malonydialdehyde in hepatocytes.[13] Furthermore, t-BH causes the depletion of cellular glutathione levels and can also induce DNA damage.[13,14] Since all these phenomenons resemble the conditions that occur during oxidative stress in the cells and tissues; plants with antioxidant properties can be used to treat t-BH induced cytotoxicity and the resultant liver disorders.[3,15,16]
A large number of medicines have been developed for health problems pertaining to the liver. Hepatoprotectants, therapeutic agents that prevent damage to the liver, currently include both synthetic as well as natural products. However, to search for newer and better hepatoprotective agents, natural resources such as traditional medicinal plants have always been considered as an important source for new molecules to be used as medicines.[2,17]
In this context, HepG2 cells were was selected as the model to investigate the direct effects of P. amarus, P. fraternus, P. maderaspatensis, P. urinaria, and P. rotundifolius on t-BH-induced cytotoxicity. After treatment with t-BH, cell viability was decreased with the impairment of membrane integrity as demonstrated in Figure 2a and b. Treatment with the extracts showed that the protective activity of P. urinaria (IC50 = 72 μg/ml) was higher than P. maderaspatensis. However, both the extracts showed lesser potency in comparison to the reference drug, silymarin. Interestingly, the extracts demonstrated a significant protective activity despite the absence of key active phytoconstituents, phyllanthin and hypophyllanthin, which apparently suggests the involvement of other phenolic compounds present in the extracts.
In a similar study, the ethanolic extract of P. urinaria was reported to protect against an acetaminophen overdose by down-regulating hepatic cytochrome P450 CYP2E1 protein.[18] The chemical composition analysis indicated that the protective effect of the extract was majorly due to the presence of corilagin and gallic acid.[18] The methanolic extract of P. urinaria has also been reported to protect against CCl4 -induced liver toxicity by attenuating the increase in serum glutamate-oxalate transaminase (GOT), elevating the activity of reduced glutathione peroxidase (GSH-Px)[19] and increasing intracellular free Ca2+ concentrations in liver cells.[20]
In line with our results, P. maderaspatensis has been reported to possess a significant hepatoprotective activity against acetaminophen-induced hepatotoxicity, despite the absence of phyllanthin and hypophyllanthin.[7,21]
To summarize, this study illustrates for the first time the hepatoprotective potential of P. urinaria and P. maderaspatensis against t-BH induced cytotoxicity in HepG2 cells. Besides, an interesting conclusion can also be drawn that phyllanthin and hypophyllanthin may not be exclusively responsible for the hepatoprotective activity as these are not found in P. urinaria and P. maderaspatensis. This inference opens the possibility of considering these plants an adjunctive medicine for the treatment of liver disease associated with chemical toxicities.
ACKNOWLEDGEMENT
We thank Mr. B. Murali and Dr. M. Deepak (Natural Remedies, Pvt. Ltd., Bangalore) for providing the phytochemical information on Phyllanthus species.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Pineiro-Carrero VM, Pineiro EO. Liver. Pediatrics. 2004;113:1097–106. [PubMed] [Google Scholar]
- 2.Subramoniam A, Pushpagadan P. Development of phytomedicines for liver diseases. Indian J Pharmacol. 1999;31:166–75. [Google Scholar]
- 3.Joyeux M, Rolland A, Fleurentin J, Mortier F, Dorfman P. Tert--Butyl Hydroperoxide-- induced injury in isolated Rat Hepatocytes: A model for studying antihepatotoxic crude drugs. Planta Med. 1990;56:171–4. doi: 10.1055/s-2006-960918. [DOI] [PubMed] [Google Scholar]
- 4.Unander DW, Webster GL, Blumberg BS. Record of usage or assays in Phyllanthus (Euphorbiaceae) I.Subgenera Isocladus, Kirangella, Cicca and Emblica. J Ethnopharmacol. 1990;30:233–64. doi: 10.1016/0378-8741(90)90105-3. [DOI] [PubMed] [Google Scholar]
- 5.Calixto JB, Santos AR, Cechinel Filho V, Yunes RA. A review of the plants of the genus Phyllanthus: Their chemistry, pharmacology and therapeutic potential. Med Res Rev. 1998;18:225–58. doi: 10.1002/(sici)1098-1128(199807)18:4<225::aid-med2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 6.Dhiman RK, Chawla YK. Herbal medicine for liver diseases. Dig Dis Sci. 2005;50:1807–12. doi: 10.1007/s10620-005-2942-9. [DOI] [PubMed] [Google Scholar]
- 7.Khatoon S, Rai V, Rawat AK, Mehrotra S. Comparative pharmacognostic studies of three Phyllanthus species. J Ethnopharmacol. 2006;104:79–86. doi: 10.1016/j.jep.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 8.Mdlolo CM, Shandu JS, Oyedeji OA. Phytochemical constituents and antimicrobial studies of two South African Phyllanthus species. Afr J Biotechnol. 2008;7:639–43. [Google Scholar]
- 9.Syamsunder KV, Singh B, Thakur RS, Husain A, Kiso Y, Hikino H. Antihepatotoxic principles of Phyllanthus niruri herbs. J Ethnopharmacol. 1985;14:41–4. doi: 10.1016/0378-8741(85)90026-1. [DOI] [PubMed] [Google Scholar]
- 10.Mossmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxic assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 11.Chandrasekaran CV, Sundarajan K, David K, Agarwal A. In vitro efficacy and safety of poly-herbal formulations. Toxicol Vitro. 2010;24:885–97. doi: 10.1016/j.tiv.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 12.Kinjo J, Hirakawa T, Tsuchihashi R, Okawa M, Nohara T, Okabe H. Hepatoprotective constituents in plants. Effects of Soyasapogenol B, Sophoradiol, and their Glucuronides on the cytotoxicity of tert-butyl hydroperoxide to HepG2 cells. Biol Pharm Bull. 2003;26:1357–60. doi: 10.1248/bpb.26.1357. [DOI] [PubMed] [Google Scholar]
- 13.Sohn JH, Han K, Lee S, Hwang J. Protective effects of Panduratin A against oxidative damage of tert-butyl hydroperoxide in human HepG2 cells. Biol Pharm Bull. 2005;28:1083–6. doi: 10.1248/bpb.28.1083. [DOI] [PubMed] [Google Scholar]
- 14.Hwang JM, Wang CJ, Chou FP, Tseng TH, Hsieh YS, Lin WL, et al. Inhibitory effect of berberine on tert-butyl hydroperoxide-induced oxidative damage in rat liver. Arch Toxicol. 2004;76:664–70. doi: 10.1007/s00204-002-0351-9. [DOI] [PubMed] [Google Scholar]
- 15.Park EJ, Zhao YZ, Na M, Bae K, Kim YH, Lee BH, et al. Protective effects of Honokiol and Magnolol on tertiary-butyl hydroperoxide or D-Galactosamine induced toxicity in rat primary hepatocytes”. Planta Med. 2003;69:33–7. doi: 10.1055/s-2003-37027. [DOI] [PubMed] [Google Scholar]
- 16.Thabrew MI, Gove CD, Hughes RD, McFarlane IG, Williams R. Protective effects of Osbeckia octandra against galactosamine and tert--butyl hydroperoxide-- induced hepatocyte damage. J Ethnopharmacol. 1995;49:69–76. doi: 10.1016/0378-8741(95)90033-0. [DOI] [PubMed] [Google Scholar]
- 17.Mukherjee PK, Wahile AK, Rai V, Mukherjee SK, Saha PB. Marker profiling for botanicals used for hepatoprotection in Indian system of medicines. Drug Inf J. 2006;40:131–9. [Google Scholar]
- 18.Hau DK, Gambari R, Wong RS, Yuen MC, Cheng GY, Tong CS, et al. Phyllanthus urinaria extract attenuates acetaminophen induced hepatotoxicity: Involvement of cytochrome P450 CYP2E1. Phytomedicine. 2009;16:751–60. doi: 10.1016/j.phymed.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Lee CY, Peng WH, Cheng HY, Chen FN, Lai MT, Chiu TH. Hepatoprotective effect of Phyllanthus in Taiwan on acute liver damage induced by carbon tetrachloride. Am J Chin Med. 2006;34:471–82. doi: 10.1142/S0192415X06004004. [DOI] [PubMed] [Google Scholar]
- 20.Zhou S, Xu C, Zhou N, Huang Y, Huang L, Chen X, et al. Mechanism of protective action of Phyllanthus urinaria L. against injuries of liver cells. Zhongguo Zhong Yao Za Zhi. 1997;22:109–11. [PubMed] [Google Scholar]
- 21.Asha VV, Akhila S, Wills PJ, Subramoniam A. Further studies on the antihepatotoxic activity of Phyllanthus maderaspatensis Linn. J Ethnopharmacol. 2004;92:67–70. doi: 10.1016/j.jep.2004.02.005. [DOI] [PubMed] [Google Scholar]


