Abstract
Objective:
The aim was to study the in vitro and in silico interactions of cleistanthin A and B on the adrenergic and cholinergic receptors using isolated animal tissues and bioinformatics tools.
Materials and Methods:
The alpha adrenergic receptor activities of cleistanthin A and B were studied in vitro using a guinea pig vas deferens preparation. The beta adrenergic receptor activities of cleistanthin A and B on an isolated rat heart were studied in vitro using a modified Langendorff apparatus. The effects of cleistanthin A and B on the nicotinic and muscarinic cholinergic receptors were studied in vitro using rabbit vas deferens and rabbit jejunum, respectively. All the drug responses were recorded using a data acquisition system through a variable force transducer. The receptor–ligand interactions of cleistanthin A and B with adrenergic and cholinergic receptor proteins were determined using the ArgusLab molecular modeling and drug docking program.
Results:
Cleistanthin A and B significantly inhibited the actions of the alpha adrenergic receptor and the nicotinic cholinergic receptor. Cleistanthin A and B shifted the dose–response curve to the right with an increased EC50 value of phenylephrine and acetylcholine. Both cleistanthin A and B did not have any significant effect on the beta adrenergic and muscarinic cholinergic receptors.
Conclusion:
Cleistanthin A and B block the alpha adrenergic and nicotinic cholinergic receptors, but these compounds do not interact at all with the beta adrenergic and muscarinic cholinergic receptors.
Keywords: Alpha receptor, cleistanthin A, cleistanthin B, nicotinic receptor
INTRODUCTION
Cleistanthus collinus Roxb. (Euphorbiaceae) is a poisonous plant that is widely distributed in south-eastern Asia, including India and Sri Lanka. Poisoning in humans by this plant causes cardiovascular toxicity, including hypotension, dysrhythmia, and respiratory toxicity, leading to death in 20–60% of patients.[1] Cleistanthin A and B, which are present in the leaves of C. collinus, have been reported to be responsible for the toxicity. Studies conducted with aqueous extracts of C. collinus leaves showed a direct inhibition of the alpha adrenergic receptors present in the guinea pig vas deferens.[2] No study has been carried out with cleistanthin A and B to explore their pharmacological interactions with the cholinergic and adrenergic receptors. Hence, the present study was planned to find out the possible interactions of cleistanthin A and B with both the adrenergic and cholinergic receptors.
MATERIALS AND METHODS
Plant material
Taxonomically identified C. collinus Roxb. (Euphorbiaceae) plant parts were collected in Pondicherry and in rural parts of Villupuram and Cuddalore districts of Tamil Nadu, India. They were identified and certified by the Botanical Survey of India (BSI), Coimbatore (BSI/SC/5/23/08-09/Tech.241). Leaves of C. collinus were collected in the months of February–April every year. A voucher specimen of the plant is kept in the Department of Pharmacology, JIPMER, for further reference.
Chemicals
An activated neutral alumina (aluminum oxide, Al2O3) column of chromatography grade was purchased from Spectrum Reagents and Chemicals Pvt. Ltd., Cochin, India, and Sisco Research Laboratory Ltd., Mumbai, India. Silica gel-G for thin layer chromatography (TLC) was purchased from Sisco Research Laboratory Ltd.. Acetylcholine and phenylephrine were purchased from Titan Chemicals Pvt. Ltd., India, and Sigma-Aldrich, USA, respectively. All other solvents and chemicals were of analytical grade and were purchased from Sisco Research Laboratory Ltd., and Merck Chemicals, India.
Animals
Healthy adult male guinea pigs (body weight 500–600 g), New Zealand white rabbits (1.5–2 kg), and Wistar rats (180–200 g) were used for the experiment. The animals were obtained from the Central Animal House, JIPMER, and allowed to adapt to the laboratory conditions. All the experimental animals were housed at a temperature of 25 ± 2°C and 40–50% humidity in a 12:12 ± 1 h light–dark cycle. The rats were fed with standard rat pellets (Hindustan Ltd, Bangalore, Karnataka, India) and water ad libitum. The rabbits and guinea pigs were fed with green-leafy vegetables and Bengal gram. The guinea pigs additionally received vitamin C in water once in 4 days. The study protocol was approved by the Institute Animals Ethics Committee (IAEC), and all the animal experiments were carried out in accordance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India.
Drug solutions
Cleistanthin A and B were found to be insoluble in water and soluble in benzene, acetone, and ethyl alcohol. So cleistanthin A and B were dissolved using a minimal volume of ethyl alcohol, and the required volume was made up with distilled water (q.s.). Acetylcholine and phenylephrine were dissolved in distilled water. Commercially available adrenaline injection was diluted with distilled water and used.
Isolation of cleistanthin A and B
Cleistanthin A and B were isolated from the leaves of the C. collinus plant. The leaves were dried in the shade and powered to 40 mesh size. The powered leaves were defatted with n-hexane (36 h) and extracted with acetone using cold maceration for 30 ±6 h. The acetone fraction was used for the isolation of cleistanthin A and B through column chromatography. The column was prepared using activated neutral alumina by the wet packing method. The acetone extract was dissolved in benzene and passed through the neutral alumina column and eluted with a benzene, benzene:ethyl acetate (4:1), benzene:ethyl acetate (1:1) mixture and methanol:chloroform (9.5:0.5) to isolate fractions of fatty alcohol, collinusin, cleistanthin A and cleistanthin B respectively. The fraction of cleistanthin A was purified using preparative TLC. The TLC was performed using a mobile phase chloroform:n-heptane:ethane (50:45:5) solvent system on a preactivated silica gel-G stationary phase. The fraction of cleistanthin B was purified using the crystallization method. The functional groups and facial arrangement of the cleistanthin A and B molecule were confirmed by FT-IR spectroscopy (Avatar FT-IR 330) and nuclear magnetic resonance (Bruker 300 MHz) spectroscopy.[3]
Effect of cleistanthin A and B on the alpha adrenergic receptor
A guinea pig was sacrificed by terminal exsanguination, and the abdomen was opened to expose the testes and the vas deferens.[4] The vas deferens on one side was freed from the epididymis, cut, and transferred to a Petri dish containing Kreb's solution. The tissue was mounted on an organ bath (Biopac Inc., USA) containing Kreb's solution maintained at 32 ± 1°C and aerated with oxygen. The concentration–response curves of phenylephrine and cleistanthin A and B were recorded using a data acquisition system (Biopac Inc.) through a variable transducer (50–1000 g). Since cleistanthin A and B did not have any effect on the isolated guinea pig vas deferens, the effect of phenylephrine was recorded in the presence of cleistanthin A and B.
Effects of cleistanthin A and B on the beta adrenergic receptor
An adult albino Wistar rat was anesthetised using urethane (1200 mg/kg), and the animal was fixed on the surgical table in a supine position. The animal was pre-treated with heparinized saline (5 IU/ml, i.p.) to avoid coagulation of blood in the heart chambers.[5] The chest region of the animal was shaved, cleaned and surgical area was marked. The sternum of the animal was cut carefully and the ribs were opened using a rib retractor. The heart was identified through the arch of the aorta and removed as quickly as possible and placed in a dish containing ice-cold Kreb's solution. The aortic portion of the heart was mounted in a Langendorff setup (indigenously made) and perfused with aerated Kreb's solution at a flow rate of 5 ml/min at 37±2°C. A small steel hook with a string was attached to the apex of the heart and connected to a variable transducer, and the response was recorded using a data acquisition system (Biopac Inc.). After 15–20 min of stabilization under the experimental conditions, cardiac functions such as the contractility (force of contraction) and rate of contraction were recorded. The effects of cleistanthin A and B on the force and rate of contraction were recorded at various dose levels. The effects of a known adrenergic agonist (adrenaline) and a known muscarinic agonist (acetylcholine) were evaluated after the administration of cleistanthin A and B.[6–8]
Effects of cleistanthin A and B on the nicotinic cholinergic receptor
A male rabbit was sacrificed by terminal exsanguination, and the abdomen was opened to expose the testes and the vas deferens. The prostatic half of the vas deferens was freed from the epididymis, cut, and transferred to a Petri dish containing Kreb's solution. The tissue was mounted on an organ bath (Biopac Inc.) containing Kreb's solution maintained at 32±1°C and aerated. The preparation was allowed to relax for 20–30 min with adequate washing. The concentration-response curves of acetylcholine and cleistanthin A and B were recorded using a data acquisition system through a variable transducer.[8–10]
The presence of the cholinergic receptors in the vas deferens was determined using a known cholinergic agonist (acetylcholine), and the effect of the agonist on the isolated vas deferens was evaluated in the presence of a muscarinic receptor blocker (atropine) and a nicotinic receptor blocker (d-tubocurarine). Cleistanthin A and B did not show any effect on the isolated rabbit vas deferens, and hence the effect of acetylcholine was recorded in the presence of cleistanthin A and B.
Effects of cleistanthin A and B on the muscarinic cholinergic receptor
A male rabbit was sacrificed by terminal exsanguination, and the abdomen was opened to expose the small intestine. The jejunum of the small intestine was identified, and 2-3 cm of the jejunum was separated and washed with the tyrode solution . The tissue was mounted in an organ bath (Biopac Inc.) containing the tyrode solution maintained at 32±1°C. The upright position of the tissue was not changed. The preparation was aerated, and it was allowed to relax for 20–30 min with adequate washing. The concentration–response curves of acetylcholine and cleistanthin A and B were recorded using a data acquisition system (Biopac Inc.) and a variable transducer.[11]
The presence of the cholinergic receptors in the rabbit jejunum was determined using a known cholinergic agonist (acetylcholine), and the effect of the agonist on the isolated rabbit jejunum preparation was evaluated in the presence of a muscarinic receptor blocker (atropine) and a nicotinic receptor blocker (d-tubocurarine). Cleistanthin A and B did not show any effect on the isolated rabbit jejunum, and hence the effect of acetylcholine was recorded in the presence of cleistanthin A and B.
Interactions of cleistanthin A and B with the cholinergic and the adrenergic ligands
The possible interactions between cleistanthin A and B with the adrenergic and cholinergic ligands were determined using the ArgusLab molecular modeling and drug docking program (ArgusLab 4.0.1) in December 2010. The adrenergic and cholinergic ligands were downloaded from the Internet (http://www.pdb.org/).
Statistical analysis
The mean and SEM values were calculated for each parameter. Significant differences between the groups were determined using repeated measures ANOVA followed by Bonferroni comparison of the groups. A P-value less than 0.05 was considered significant.
RESULTS
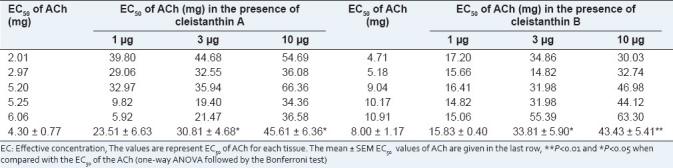
There was clear TLC separation of cleistanthin at an Rf value of 0.37–0.42. The purified cleistanthin A and B were identified using UV–visible FT-IR, and NMR spectroscopy.[3] Cleistanthin A and B when administered alone did not produce any effect on the vasa deferentia of guinea pig and rabbit. These compounds significantly inhibited the contraction induced by phenyleprine and acetylcholine in the vasa deferentia of guinea pig and rabbit, respectively [Tables 1 and 2]. Cleistanthin A and B shifted the dose–response curve to the right, as indicated by an increase in the EC50 values of phenylephrine and acetylcholine administered in the presence of increasing doses of cleistanthin A and B. In rat vas deferens, phenylephrine when administered alone caused spontaneous contractions, which disappeared in the presence of cleistanthin A and B.
Table 1.
Effects of cleistanthin A and B on the guinea pig vas deferens
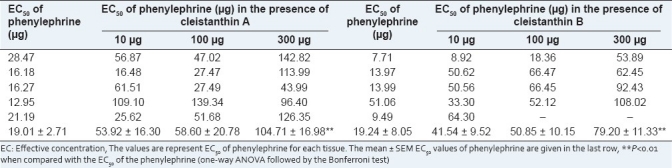
Table 2.
Effects of cleistanthin A and B on the rabbit vas deferens
Both cleistanthin A and B did not show any significant effect on the isolated rat heart or the rabbit jejunum. When administered alone, adrenaline significantly increased the force of contraction (1.44 to 1.83 g) and the number of beats (118 to 144 per minute), and acetylcholine produced the opposite effects (force of contraction 1.44 to 0.52 g, number of beats 118 to 74 per minute), and these effects of adrenaline and acetylcholine on the heart did not significantly change in the presence of cleistanthin A and B.
Cleistanthin A and B were docked with the cholinergic and the adrenergic receptors proteins. Both cleistanthin A and B did not show any significant interaction with the cholinergic and beta adrenergic receptors. While cleistanthin A showed a possible interaction with acetylcholine binding protein with a binding energy of –4.7064 kcal/mol, cleistanthin B did not show any such interaction. Both cleistanthin A and B showed a possible interaction with the alpha adrenergic receptor (data not presented).
DISCUSSION
Cleistanthin A and B blocked the effects of phenyleprine and acetylcholine on the vasa deferentia of guinea pig and rabbit, respectively, and this indicates that cleistanthin A and B act on the alpha adrenergic receptors and the nicotinic cholinergic receptors. The vas deferens of guinea pig is populated with alpha adrenergic receptors that mediate the contraction of the vas deferens. The fact that there is a shift to the right in the EC50 of phenyleprine, which is an alpha aderenergic stimulant, indicates that there is a blockade of the alpha receptors by cleistanthin A and B. Our finding is in agreement with a report that C. collinus significantly inhibited the alpha receptor action of phenylephrine in the guinea pig vas deferens.[2] The study referred to used the crude extract of the leaves and not the isolated constituents as in the present study. Hence our study confirms that it is cleistanthin A and B that are responsible for the alpha adrenergic receptor blockade. C. collinus poisoning induces hypotension in humans,[12] and it is possible that the hypotension could be due to the alpha adrenergic receptor blockade.
Different kinds of receptors including histaminic, muscarinic, adrenergic, and opioid receptors are distributed in the vas deferens of the rabbit.[13] The cholinergic receptors are distributed in both the prostatic and epididymal halves of the vas deferens. The nicotinic receptors are present in the prostatic half of the vas deferens, and both the nicotinic and muscarinic cholinergic receptors are present in the epididymal half.[9] Our study used the prostatic half of the vas deferens, and hence the inhibition of contraction by cleistanthin A and B as evidenced by a shift of the EC50 of acetylcholine to the right could be attributed to the blockade of the nicotinic cholinergic receptor. It has been reported that the crude extract of C. collinus antagonized acetylcholine on a mouse phrenic nerve-diaphragm preparation and induced a myasthenia gravis-like effect in rats.[14,15] There is a case report of C. collinus poisoning that describes symptoms similar to those of myasthenic crisis-like syndrome that responded to neostigmine.[16] These reports suggest a blockade of the neuromuscular junction, i.e., nicotinic receptor, by the crude extract of C. collinus. The results of our study with cleistanthin A and B are in conformity with these reports, and these compounds, which are present in the plant, are responsible for the neuromuscular blockade.
It is surprising that the compounds have both adrenergic and cholinergic blocking properties. While the vas deferens of guinea pig responds to lower concentrations of phenyleprine (micrograms), the vas deferens of rabbit requires higher doses (milligrams) of acetylcholine. Though there is a great difference in the sensitivity, both the agonists can be blocked by lower concentrations of cleistanthin A and B. We have not come across any compound that has been reported to exert antagonism on both alpha adrenergic and nicotinic cholinergic receptors, and cleistanthin A and B are unique in this respect. An explanation is not possible at this stage, and more studies are required to confirm the nicotinic blockade.
The absence of any effects of cleistanthin A and B on the rat heart and rabbit jejunum in the presence or absence of adrenaline or acetylcholine indicates that the test compounds have neither agonistic nor antagonistic actions on the beta adrenergic and muscarinic cholinergic receptors.
The data obtained using the bioinformatics tool also show possible interactions between cleistanthin A and B and the alpha adrenergic receptors. This adds strength to our interpretations of the data obtained from the isolated tissues. Though interactions of the study compounds with the nicotinic and muscarinic receptors were shown to be absent, cleistanthin A but not B interacted with acetylcholine binding protein. These findings contradict those of the experimental study, and we do not have any explanation for the same.
CONCLUSIONS
The present study concludes that both cleistanthin A and B have significant alpha adrenergic and nicotinic cholinergic receptor blocking activities and that they do not exert any action on the beta adrenergic and muscarinic cholinergic receptors.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Benjamin SP, Fernando ME, Jayanth JJ, Preetha B. Cleistanthus collinus poisoning. J Assoc Physicians India. 2006;54:742–4. [PubMed] [Google Scholar]
- 2.Kumar MR, Ramaswamy S, Jayanthi M, Raveendran R. Alpha-adrenergic receptor blocking effect of Cleistanthus collinus (Roxb.) Benth and Hook f. leaf extract on guinea pig isolated smooth muscle preparations. Indian J Exp Biol. 2011;49:339–42. [PubMed] [Google Scholar]
- 3.Parasuraman S, Raveendran R, Gopal V. Isolation and purification of cleistanthin A and B from the leaves of Cleistanthus collinus Roxb.(Euphorbiaceae) Arch Pharm Sci Res. 2009;1:199–202. [Google Scholar]
- 4.Kirkpatrick WR, McAtee RK, Fothergill AW, Rinaldi MG, Patterson TF. Efficacy of voriconazole in a guinea pig model of disseminated invasive aspergillosis. Antimicrob Agents Chemother. 2000;44:2865–8. doi: 10.1128/aac.44.10.2865-2868.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lucetti DL, Lucetti EC, Bandeira MA, Veras HN, Silva AH, Leal LK, Lopes AA, Alves VC, Silva GS, Brito GA, Viana GB. Anti-inflammatory effects and possible mechanism of action of lupeol acetate isolated from Himatanthus drasticus (Mart.) Plumel. J Inflamm (Lond) 2010;7:60. doi: 10.1186/1476-9255-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawrence CL, Bridgland-Taylor MH, Pollard CE, Hammond TG, Valentin JP. A rabbit Langendorff heart proarrhythmia model: Predictive value for clinical identification of torsades de pointes. Br J Pharmacol. 2006;149:845–60. doi: 10.1038/sj.bjp.0706894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inamdar N, Venkataraman BV, Aleem A. A simple and improved perfusion apparatus for isolated hearts. Indian J Pharmacol. 1994;26:262–5. [Google Scholar]
- 8.Vogel HG, editor. 2nd ed. Berlin: Springer; 2002. Drug Discovery and Evaluation. Pharmacological Assay. [Google Scholar]
- 9.Kasuya Y, Suzuki N. Regional differences in the distribution of cholinergic receptors in the rat vas deferens. Jpn J Pharmacol. 1979;29:313–4. doi: 10.1254/jjp.29.313. [DOI] [PubMed] [Google Scholar]
- 10.Fukushi Y, Wakui M. Involvement of cholinergic nerves in excitatory junction potentials through prejunctional nicotinic receptors in the guinea-pig vas deferens. J Auton Pharmacol. 1987;7:309–16. doi: 10.1111/j.1474-8673.1987.tb00159.x. [DOI] [PubMed] [Google Scholar]
- 11.Gilani AH, Mehmood MH, Janbaz KH, Khan AU, Saeed SA. Ethnopharmacological studies on antispasmodic and antiplatelet activities of Ficus carica. J Ethnopharmacol. 2008;119:1–5. doi: 10.1016/j.jep.2008.05.040. [DOI] [PubMed] [Google Scholar]
- 12.Subrahmanyam DK, Mooney T, Raveendran R, Zachariah B. A clinical and laboratory profile of Cleistanthus collinus poisoning. J Assoc Physicians India. 2003;15:1052–4. [PubMed] [Google Scholar]
- 13.Lumsden NG, Fry BG, Ventura S, Kini RM, Hodgson WC. Pharmacological characterisation of a neurotoxin from the venom of Boiga dendrophila (mangrove catsnake) Toxicon. 2005;45:329–34. doi: 10.1016/j.toxicon.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Nandakumar NV, Pagala MK, Venkatachari SA, Namba T, Grob D. Effect of Cleistanthus collinus leaf extract on neuromuscular function of the isolated mouse phrenic nerve-diaphragm. Toxicon. 1989;27:1219–28. doi: 10.1016/0041-0101(89)90030-5. [DOI] [PubMed] [Google Scholar]
- 15.Nanda Kumar NV, Vijayalakshmi KM. Experimental myasthenia gravis-like neuromuscular impairment with Cleistanthus collinus leaf extract administration in rat. Phytother Res. 1996;10:121–6. [Google Scholar]
- 16.Damodaram P, Manohar IC, Prabath Kumar D, Mohan A, Vengamma B, Rao MH. Myasthenic crisis-like syndrome due to Cleistanthus collinus poisoning. Indian J Med Sci. 2008;62:62–4. [PubMed] [Google Scholar]


