Abstract
Background:
The hypoglycemic effects of hexane, chloroform and methanol extracts of leaves of Azadirachta indica (AI) were evaluated by oral administration in streptozotocin-induced severe diabetic rats (SD).
Materials and Methods:
The effect of chronic oral administration of the extract for 28 days was evaluated in streptozotozin diabetic rats. Lipid peroxidation, glycogen content of liver and skeletal muscles, insulin, superoxide dismutase (SOD), catalase (CAT), glutathione (GSH), oxidized glutathione (GSSG) levels were determined. In addition, advanced glycation end product formation (AGEs) was evaluated.
Results:
The most active extracts were obtained with chloroform. Chloroform extract from AI shows increased levels of SOD, GSH, GSSG and CAT, hepatic glycogen content, glucose-6-phosphatase and insulin plasma levels, which also decreased the glucokinase (GK), lipid peroxidation and insulin resistance. The chloroform extract exhibited significant inhibitory activity against advanced glycation end product formation with an IC50 average range of 79.1 mg/ml.
Conclusion:
Azadirachta indica can improve hyperlipidemia and hyperinsulinema in streptozocin-induced diabetic rats and, therefore, AI can be potentially considered to be an antidiabetic-safe agent.
Keywords: Azadirachta indica, antihyperglycaemic, antihyperlipidaemic, antiglycation
INTRODUCTION
Azadirachta indica (AI), also known as neem, belongs to the family Meliaceae, which is one of the most useful medicinal plants.[1] AI has been used traditionally in the control of diabetes mellitus in many countries. The blood sugar level-lowering activity of AI oil and leaf extracts has been reported in various models of diabetic animals.[2] The aqueous extract of neem leaves significantly decreases the blood sugar level and prevents glucose-induced hyperglycemia in normal rats, streptozotocin diabetic rats and alloxan-induced diabetic rabbits.[3] The ethanol extract of neem leaves has also been shown to demonstrate antilipid peroxidative, antihyperglycemic and anti-hypercholesterolemic activities as well as to reduce the triglyceride serum levels in a diabetic rat model.[4] Treatment of AI combined with Vernonia amygdalina was assessed to determine the markers of hepatotoxicity in serum-included glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT) activities, total protein, albumin and urea, which indicated that neem provides the best protection against hepatic dysfunction.[5] More recent studies on 90 noninsulin-dependent male diabetic rats show that the capsules prepared with neem leaf are potentially helpful in the reduction of the diabetic symptoms and blood pressure of the subjects.[6] In another study, it was observed that chronic treatment with the ethanolic extract of neem in diabetic rats has an effect on blood glucose, pancreatic islet histopathology, and oxidative status of the pancreas. The result showed improved islet morphology and oxidative status in neem-treated diabetic rats.[7] The current investigation is an attempt to study the hypoglycemic effect of the chloroform leaf extract on streptozocin-induced diabetic models in rats that are known as insulin-deficiency models.
MATERIALS AND METHODS
Fresh leaves of AI were collected at Colima State Mexico and a sample specimen (no. 6532) was deposited in the Herbarium of the Escuela Nacional de Ciencias Biologicas, IPN, for further reference.
Animals
The study was conducted in the Wistar male albino rats strain, weighing about 180–225 g. Before and during the experiment, the animals were fed with the laboratory normal diet and water ad libitum. The procedures involving animals and their care conformed to the international guidelines Principles of Laboratory Animals Care.
Preparation of plant extracts
About 300 g of leaves was dried and powdered in a mechanical grinder. This powder material was extracted with 1 L of hexane, chloroform and methanol consecutively using a soxhlet apparatus. These extracts were filtered and concentrated by rotary vacuum evaporator for complete removal of solvent. An aqueous extract of the suspension was prepared using 2% (v/v) Tween-80 and used for oral administration.
Study on glucose-supplied animals (Oral glucose tolerance test)
After overnight fasting, a 0-min blood sample was taken from the diabetic control rats group and another sample was taken from the group of rats treated with the plant extract. The rats of both groups were supplied with glucose orally (2 g/kg, p.o.) 30 min after the administration of the drug or the vehicle (for control). Blood glucose levels were measured at 30, 60, 120, and 180 min after the glucose was supplied to assess the effect of different doses of chloroform extract of the leaves of AI on blood glucose levels of the animals.
Induction of diabetes mellitus
Rats were not supplied with meals for 24 h before the induction of diabetes by STZ injection. Experimentally induced SD conditions were developed in a group of normoglycemic male Wistar albino rats by a single intravenous injection of STZ at a dose of 65 mg/kg body weight/rat in 0.5 ml of physiological saline. This single dose of STZ produced SD or type-1 diabetes, having fasting blood glucose level more than 250 mg/dl after 24 h of STZ injection, and this diabetic state was maintained throughout the experimental schedule:
Severe diabetic: it was treated with AI extracts, 300 mg/kg body weight, and the animals of control group received only 2% Tween-80 solution.
All groups were treated with the extract of chloroform or vehicle for 28 days. The fasting blood glucose was monitored on day 0 at 2, 4, 6, 8, and 12 h after the administration of the extracts. The drug solutions or vehicle or standards such as tolbutamide (40 mg/kg body weight) or glibenclamide (0.5 mg/kg body weight) were administered orally by gastric intubations once daily at 9:00 am. On the 28th day, diabetic animals were sacrificed by an ether overdose and the insulin level was determined.[8,9] Plasma blood glucose levels were determined by using the glucose oxidase–peroxidase (GOD-POD) method.
Measurements of reduced superoxide dismutase, catalase, glutathione, and oxidized glutathione
Livers were homogenized with a homogenizer (Ultra Turrax T25, Rose Scientific Ltd., Edmonton, Canada) in 10 volumes of a 50 mM sodium phosphate buffer (pH 7.4) at 4°C and the supernatant obtained was used for the following antioxidant enzyme measurements using commercial kits: reduced superoxide dismutase (SOD) (Oxis International), catalase (CAT) (Cayman Chemical company, Michigan USA), glutathione peroxidase (Oxis International), and glutathione reductase (Oxis International, California USA).
Serum insulin
Diabetic rats were orally administered with 300 mg/kg extracts daily for 28 days. Afterwards, blood samples were withdrawn in order to examine the insulin levels. Serum insulin was measured using a GLAZYME INSULIN-EIA TEST. The level of insulin in serum was expressed in μIU/ml.
Insulin tolerance test
Insulin tolerance test was performed at the end of repeated administration of the plant extract. After overnight (18 h) fasting, the insulin (0.5 U/kg body weight) solution was administered subcutaneously (s.c.). Blood sample were collected before administration of insulin and at 30, 60, and 120 min after the administration of insulin.
Assay of Glycogen content, Glucose-6-Phosphatase, and glucokinase activities in the liver
Rats of each group were orally administered with 300 mg/kg of extracts for 28 days; afterwards, they were sacrificed and their livers and skeletal muscle were removed. Glycogen content of liver and skeletal muscles was determined by using anthrone reagent and the results were expressed in mg/g of tissue. The amount of phosphate liberated per unit time, determined as blue phosphor–molybdous complex, was measured using a spectrophotometer at 700 nm, which showed the Glucose-6-Phosphatase (G6Pase) activity. Protein content of the liver extract was quantified with Bradford reagent.[10] G6Pase activity (mU) was expressed as mmol of phosphate released/min/mg of protein.
GK activity was measured using a spectrophotometric method as previously described by Briefly.[11] β-nicotinamide-adenine dinucleotide phosphate (NADPH) generated by GK was measured using a spectrophotometer at 340 nm. GK activity was estimated by the standard method, i.e. by subtracting the rate of NADPH formation in the presence of 0.5 mM glucose from that obtained in the presence of 100 mM glucose. Protein concentration was quantified with Bradford reagent, and one unit of enzyme activity (mU) was defined as mmol of substrate molecules converted by 1 mg protein per minute.
Inhibition test on protein glycation in vitro
Evaluation for glycation inhibitory activity of extracts was performed as previously described.[12] Bovine serum albumin (10 mg/mL) was incubated with 250 mM D-fructose in the presence or absence of test materials for 21 days in 0.2 M potassium phosphate buffer (pH 7.4) at 37°C. After incubation, the fluorescence was measured at excitation 370 nm and emission 440 nm. Both incubations and measurements were carried out in triplicate. Aminoguanidine hydrochloride (Sigma-Aldrich Corporation, St. Louis, USA) (AG) dissolved in distilled water was used as a positive control. The concentration of each test sample showed 50% inhibition of the activities (IC50), which was estimated from the least-squares regression line of the logarithmic concentration plotted against the remaining activity.
Statistical analysis
Data are shown as mean ± SEM and statistical analyses were performed by means of the Student's t-test or by one-way analysis of variance (ANOVA), followed by Dunnett΄s multiple-comparison test (DMRT) using SPSS software when appropriate. The statistical significance was assumed at P-levels <0.05. Concentrations to produce 50% of the maximal responses (EC50) were calculated by a nonlinear curve fitting procedure using Sigma Plot version 8.0.
RESULTS
Effect on oral glucose tolerance test
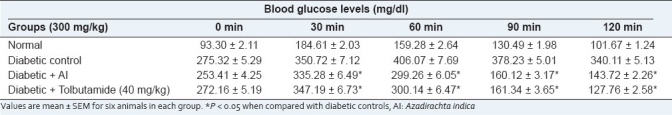
Table 1 shows the changes in the levels of blood glucose in the diabetic groups after oral administration of glucose (2 g/kg). The diabetic rats exhibit a significant increase in the blood glucose at 60 and 90 min. The extract of AI significantly (P < 0.05) decreased the blood glucose levels at 60, 90 and 120 min after glucose loading when compared with the control, which decreased after 60 min. AI-treated animals tend to give values to near-normal.
Table 1.
Effect of the chloroform extract of Azadirachta indica on oral glucose tolerance
Thiobarbituric acid assay
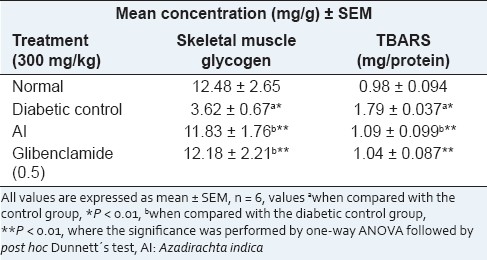
Table 2 shows thiobarbituric acid reactive substances (TBARS) as index of lipid peroxidation in plasma, which increased in diabetic rats during the 28-day duration of the experiment. Chloroform extracts are significantly lower compared with those in the diabetic control.
Table 2.
Effect of chloroform extract of leaves of Azadirachta indica on the level of TBARS and glycogen content in skeletal muscle in STZinduced diabetic rats
Glycogen level and Glucose-6-Phosphatase
As shown in Table 3, the glycogen content was increased by AI as compared with the diabetic group. The result in our studies shows that there is an increase in the glycogen content of the liver and skeletal muscle of diabetic rats treated with extracts. G6Pase activity was assessed in all groups [Table 2]. Compared with the diabetic control, the G6Pase effect was decreased by the AI chloroform extract. GK activity was increased by the AI chloroform extract. AI extracts decreased the G6Pase activity and increased the glycogen and GK activity in the liver, which indicates increased hepatic glucose uptake and decreased hepatic glucose release. Thus, the chloroform extract enables hypoglycemic activity probably by reducing HGO via decreasing G6Pase activity and increasing GK activity.
Table 3.
Effect of chloroform extract of Azadirachta indica after the 28-day treatment on plasma insulin, glycogen levels, activities of liver glucose 6-phosphatase, and glucokinase in STZ-induced SD

Effect on glutathione, superoxide dismutase, catalase, and oxidized glutathione
Table 4 shows the results of the antioxidant defense system in the liver. In STZ-diabetic rat livers, the SOD, GSH, GSSG, and CAT levels were significantly lower in the diabetic control rats compared with the nondiabetic rats. The supplementation of chloroform extract applied to rats results in an increase in these parameters, restoring them toward the control levels.
Table 4.
Effect of the chloroform extract of Azadirachta indica on antioxidant enzyme superoxide dismutase, catalase, glutathione, and oxidized glutathione in SD rats

Effect of Azadirachta indica on plasma insulin
The serum insulin level was decreased in diabetic rats with streptozotocin-induction as compared with the control group. There was also a significant difference of this parameter between the two groups [Table 3]. After 28 days of extract supplementation to SD rats, there was a significant increase in the serum insulin level with respect to the SD group.
Insulin tolerance test
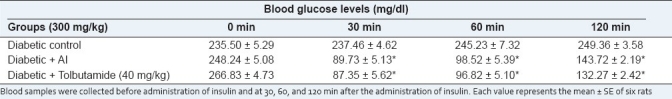
Three hundred milligrams per kilogram of AI tended to decrease the blood glucose levels after 30 min when compared with the controls [Table 5].
Table 5.
Effect of the chloroform extract of Azadirachta indica on insulin tolerance in diabetic rats
Effect of AI on advanced glycation end products formation
The BSA-fructose model adopted in this study provides a useful tool for assessing the effect of extracts on nonoenzymatic glycation process. Chloroform extracts of the leaves of AI displayed comparable activity to aminoguanidine, an advanced glycation end products (AGEs) inhibitor tested in clinical trials for the treatment of diabetic complications [Table 6].
Table 6.
Effect of hexane and chloroform extract of leaves of Azadirachta indica on the formation of advanced glycation end products

DISCUSSION
Streptozotocin, a monofunctional nitrosourea derivative, derives diabetogenic activity due to its ability to induce oxidative stress and damage in β-cells. Streptozotocin can selectively attack pancreatic β-cells by producing free radicals of oxygen, nitrogen monoxide, and reducing intracellular NAD and NADP, which are crucial for the electron delivery and energy metabolism in β-cells. These experiments focused on exploring the potential of a chloroform extract of the leaves of AI for the treatment of diabetes to substantiate a folklore claim. The differences observed between initial and final fasting blood glucose levels of different groups revealed a significant diminution in blood glucose level in diabetic controls as compared with normal animals at 6 h.
In this present investigation, the main focal points were the evaluation of the effectiveness of the chloroform extract of AI in the correction of SD, where β cell degeneration is dramatic. In an oral glucose tolerance test, a hypoglycemic effect was observed at 60 min after administration of the extract. This reflects the efficiency of the extract to control elevated blood glucose levels. Maintenance of blood glucose levels in diabetic rats with extract treatment indicates the effectiveness of the extract, which may involve increasing the peripheral utilization of glucose. The chloroform extract improved glucose tolerance, suggesting a decrease in insulin resistance.[13]
The results also demonstrated a significant control of serum lipid profiles in the extract-treated diabetic rats, with responses comparable with those of the standard drug. Diabetes is associated with hyperlipidemia.[14] It is well known that insulin activates enzyme lipoprotein lipase, which hydrolyzes triglyceride under normal conditions. Destruction of beta cells leads to the depletion of plasma insulin, which results in hyperlipidemia and hypercholesterolemia caused by derangement of metabolic abnormalities.[15] These elevated levels in diabetic rats might be due to the stimulation of hepatic triglyceride synthesis as a result of free fatty acid influx.[16] Repeated administration of AI thus had a beneficial effect on the hyperlipedimia associated with hyperglycemia.
Induction of diabetes in rats with STZ uniformly results in an increase in lipid peroxidation. TBARS is considered to be the index of endogenous lipid peroxidation, an indirect evidence of intensified free radical production.[17] Increased TBARS in diabetic rats suggests an increase in oxygen radicals, which could be due to either their increased production or decreased destruction.[18] The TBARS levels in our study were found to be elevated in the liver in the diabetic control group, and were significantly reduced upon administration of the extract.
Excessive hepatic glycogenolysis and gluconeogenesis associated with decreased utilization of glucose in the tissue is the fundamental mechanism underlying hyperglycemia in the diabetic state.[19] Aberration of liver glycogen synthesis or glycogenolysis in diabetes may be due to a lack of or resistance to insulin, which is essential to activate the glycogen synthase system.[20] The significant increase of liver glycogen level in the extract-treated diabetic groups may be due to reactivation of the glycogen synthase system. The experimental results indicate that leaves of AI possess significant antidiabetic activity and are capable of maintaining serum lipid profile and liver glycogen level.
Insulin resistance is a condition where normal or elevated insulin level produces an attenuated biological response. In diabetes, insulin resistance is the central pathophysiological event.[21]
Hyperglycemia-induced oxidative stress may also cause liver cell damage. The administration of extracts improves impairments of SOD, GSH, GSSG, and CAT activities in SD. These results suggest that the extract prevents oxidative stress, acts as a suppressor against liver cell damage, and inhibits the progression of liver dysfunction induced by chronic hyperglycemia. In other studies, polyphenols from AI were found in higher concentrations, and had a high antioxidant capacity. Although antioxidant activity and total phenolics were not evaluated in the current study, these are correlated with antiglycation activity.[22]
The possible mechanism of antihyperglycemic action of this extract appears to be both pancreatic and extrapancreatic, which have been supported here by the serum insulin assay in SD rats. The extrapancreatic effect of this extract has been focused here by the significant recovery of G6Pase activity in the liver in SD rats. The extrapancreatic effect may be by the sensitization of insulin receptor in target organ or by inhibiting insulinase activity in both the liver and the kidney.[23] From the glucose tolerance test, it has been indicated that this extract did not execute the antihyperglycemic effect by modulating the absorption of glucose in the intestine.
CONCLUSION
In conclusion, leaves of AI have beneficial effects on hyperlipidemia, hypoinsulinemia. Thus, this study provides supporting evidence for the therapeutic potential of leaves of AI that may also be helpful to prevent and/or delay the onset of diabetes. AI can be considered as a safe food supplement with potential as an antidiabetic agent; however, the investigation of major active constituents is still in progress.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kausik BL, Ranajet KB. Biological activities and medicinal plant propiertes of neem plant. Curr Sci. 2002;82:1336–45. [Google Scholar]
- 2.Mostofa M, Chouhury ME, Hossain MA, IslamIslam MH. Antidiabetic effects of Catharanthus roseus, Azadirachta indica, Allium sativum and glimepride in experimentally diabetic rat. Bangladesh J Vet Med. 2007;5:99–102. [Google Scholar]
- 3.Biswas K, Chattopadhyay I, Ranajit KB, Bandyopadhyay Biological activities and medicinal properties of neem (Azadirachta indica) Curr Sci. 2002;82:1336–44. [Google Scholar]
- 4.Ekaidem IS, Akpan HD, Usoh IF, Etim OE, Ebong PE. Effect of ethanolic extract of Azadirachta indica leaves on lipid peroxidation and serum lipids of diabetic Wistar rats. Acta Biol Szgedensis. 2007;51:17–20. [Google Scholar]
- 5.Ebong PE, Atanwho IJ, Eyon EU, Egbun GE. The antidiabetic efficacy of combined extracts from two continental plants: Azadirachta indica (A.Juss) neem and Vernonia amygdalina (Del). (African bitter leaf) Am J Biochem Biotech. 2008;4:239–44. [Google Scholar]
- 6.Kochhar A, Sharma N, Sachdeva R. Effect of supplementation of tulsi (Ocimum sanctum) and neem (Azadirachta indica) leaf powder on diabetic symptoms, anthropometric parameters and blood pressure on non-insulin dependent male diabetic. Ethno Med. 2009;3:5–9. [Google Scholar]
- 7.Akinola OB, Ezekiel AC, Dini L. Chronic treatment with ethanolic extract of the leaves of Azadirachta indica ameloriates lesion of pancreatic islets in streptozotocin diabetes. Int J Morphol. 2010;28:291–302. [Google Scholar]
- 8.Hardy KJ, McNutty SJ. Oral hypoglycemic agents. Med Dig. 1997;23:5–9. [Google Scholar]
- 9.Noawaboot J, Pannangpetch P, Kukongviriyapan V, Kongyingyoes B, Kukongviriyapan U. Antihyperglycemic, antioxidant and antiglycation activities of Mulberry leaf extract in streptozotocin-induced chronic diabetic rats. Plant Foods Hum Nutr. 2009;64:116–21. doi: 10.1007/s11130-009-0112-5. [DOI] [PubMed] [Google Scholar]
- 10.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1997;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 11.Panserat S, Capilla E, Gutierrez J. Glucokinase is highly induced and glucose-6-phosphatase poorly repressed in liver of rainbow trout (Oncorhynchus mykiss) by a single meal with glucose. Comp Biochem Physiol B. 2001;128:275–83. doi: 10.1016/s1096-4959(00)00322-5. [DOI] [PubMed] [Google Scholar]
- 12.Kim HY, Kim K. Protein gJycation inhibitory and antioxidative activities of some plant extracts in vitro. J Agric Food Chem. 2003;51:1586–91. doi: 10.1021/jf020850t. [DOI] [PubMed] [Google Scholar]
- 13.Boby RG, Leelamma S. Blackgram fiber (Phaseolus mungo): Mechanism of hypoglycemic action. Plant Foods Hum Nutr. 2003;58:7–13. doi: 10.1023/a:1024023909383. [DOI] [PubMed] [Google Scholar]
- 14.Rasineni K, Bellamkonda R, Reddy SS, Desireddy S. Antihyperglycemic activity of Catharanthus roseus leaf powder in streptozotocin-induced diabetic rats. Pharmacogn Res. 2010;3:195–200. doi: 10.4103/0974-8490.65523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maghrani M, Zeggwagh NA, Lemhadri A. Study of the hyperglycemic activity of Fraxinus excelsior and Silybum marianum in an animal model of type 2 diabetes mellitus. J Ethnopharmacol. 2004;91:309–16. doi: 10.1016/j.jep.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Kumar PM, Sasmal D, Mazumder PM. The antihyperglycemic effect of aerial parts of Salvia splendens (scarlet sage) in streptozotocin-induced diabetic-rats. Pharmacogn Res. 2010;3:190–4. doi: 10.4103/0974-8490.65520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sowmya P, Rajyalakshmi P. Hypocholesterolemic effect of germinated Fenugreek seeds in human subjects. Plant Foods Hum Nutr. 1999;53:359–65. doi: 10.1023/a:1008021618733. [DOI] [PubMed] [Google Scholar]
- 18.Shende VS, Sawant VA, Turuskar AO, Chatap VK, Vijaya C. Evaluation of hypoglycemic and antihyperglycemic effects of alcoholic extract of Chonemorpha fragrans root in normal and alloxan induced diabetic rats. Pharmacogn Mag. 2009;5:36–41. [Google Scholar]
- 19.Pongtip S, Charlotte CU, Mogens A, Wandee G, Leif SH. Antioxidant effects of leaves from Azadirachta species of different provenience. Food Chem. 2007;104:1539–49. [Google Scholar]
- 20.Chackrewarthy S, Thabrew MI, Weerasuriya MK, Jayasekera S. Evaluation of the hypoglycemic and hypolipidemic effects of an ethylacetate fraction of Artocarpus heterophyllus (jak) leaves in streptozotocin-induced diabetic rats. Pharmacogn Mag. 2010;6:186–90. doi: 10.4103/0973-1296.66933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chattopadhyay RR, Bandyopadhyay M. Possible mechanism of hepatoprotective activity of leaf extract against paracetamol-induced hepatic damage in rats. Indian J Pharmacol. 2005;37:184–5. [Google Scholar]
- 22.Wu C, Yen G. Inhibitory effect of naturally occurring flavonoids on the formation of advanced glycation end-products. J Agric Food Chem. 2005;53:3167–73. doi: 10.1021/jf048550u. [DOI] [PubMed] [Google Scholar]
- 23.Kirana H, Srinivasan BP. Aqueous extract of Garcinia indica choisy restores glutathione in type 2 diabetic rats. J Young Pharm. 2010;2:265–8. doi: 10.4103/0975-1483.66806. [DOI] [PMC free article] [PubMed] [Google Scholar]


