Abstract
Background:
Teucrium polium L. (TP) have been used in herbal medicine for different purposes such as antispasmodic, antidiabetic and lowering blood lipid. In the present study, the impact of aqueous-ethanol extract of TP on blood pressure, heart rate and intraventricular pressure was investigated in rabbit.
Materials and Methods:
Twenty-four NWZ rabbits weighed (2-3 kg) were randomly divided into four groups. In each experiment, two groups of six rabbits received jugular injection of either TP extract (20, 40 and 80 mg/kg) or normal saline for blood pressure effects and two groups for intraventricular pressure. Then, blood pressure, heart rate and intraventricular pressure were measured via carotid cannula using pressure transducer connected to a power lab system, and the data were pooled from independent, single-blinded experiments for each group.
Results:
Treatment with 80 mg/kg of TP extract significantly depressed the mean arterial blood pressure (12.5%, P< 0.05). However, there was no significant decrease in the 20 or 40 mg/kg dose or normal saline treatment group. Moreover, the extract increased (dp/dt)max (P<0.05), maximum left ventricular pressure (LVPmax) (P<0.05) and decreased (dp/dt)min significantly (P<0.05), there was no meaningful effect on left ventricular end-diastolic pressure (LVEDP).
Conclusion:
The present results demonstrated the extract had no effect on the heart rate, but showed a positive inotropic on the heart and hypotensive effects. These data suggested that hypotensive effect may counterbalance by the inotropic effect of the extract.
Keywords: Blood pressure, intraventricular pressure, heart rate, Rabbit, Teucrium polium L
INTRODUCTION
The prevalence of cardiovascular diseases is very high and increases dramatically worldwide. Hypertension is a very important risk factor for development of other cardiovascular diseases such as myocardial infarction and heart failure.[1] These conditions are the most important causes of hospital admissions which are responsible for high mortality rates, disabilities and costs. Therefore, heart failure treatment, particularly, lowering blood pressure by pharma-cological treatment is an essential step for preventing cardiovas-cular diseases. In the recent years, using herbal medicine for the prophylaxis and treatment of cardiovascular diseases has been increased and scientists have been paying more attention to investigate the cardiovascular effects of herbs.[2]
Lamiaceae or Labiatae which also known as the Mint family comprises about 210 genera and 3,500 species. One of the most popular species of this family native to the Mediterranean region and the Middle East is Teucrium polium L. (TP) which has been used for over 2000 years in traditional medicine mainly for its antidiabetic, antipyretic, anti-inflammatory and antispasmodic properties. In some parts of Iran it is traditionally used for treatment of heart failure. TP contains chemical compositions such as salvigenin, cirsiliol,[3] α- and β-pinen, sabinene, myrcene, germacrene D, limonene, β-caryophyllene and spathulenol.[4–6] Previous studies have demonstrated some of the pharmacological effects of TP such as antibacterial,[7] anti-inflammatory,[8] antioxidant,[9,10] antiulcerogenic,[11,12] antinociceptive,[13,14] antidiabetic[15,16] and antispasmodic.[17–19] In addition, lowering blood lipid,[20,21] induction of vascular relaxation,[18] positive inotropic and chronotropic[22] and decreasing of blood pressure[23] has been reported to be affected by this plant. However, there is no a comprehensive study to specify the pharmacological activities of TP extract on cardiovascular system. Therefore, the present study was conducted to investigate the possible inotropic, chronotropic and blood pressure effects of the aqueous-ethanol extract of TP in vivo.
MATERIALS AND METHODS
Plant material
Aerial part of TP was collected from hills around Ferdows city (south Khorasan province, Iran) and identified by botanists in Herbarium of Pharmacy School, Mashhad University of Medical Sciences (voucher No. 152-2016-4) and then dried at room temperature.
Extraction method
Two hundred grams of aerial parts of TP were macerated with ethanol (50%) at 30°C for 24 hours and shaken intermittently. The solution was then filtered and dried by oven at 40°C. The average w/w yield was 12.5%. The dried extract was dissolved in the distilled water to make 20, 40 and 80 mg/kg doses. The extract doses were chosen after a pilot study. Teucrium polium L. showed no cytotoxic effect[24] and on the base of previous studies[14,20] the used acute doses of TP extract in this study were not toxic.
Experimental procedure
Twenty-four NWZ rabbits weighed (2-3 kg) were randomly divided into four groups. In each experiment, two groups of six rabbits received jugular injection of either TP extract (20, 40 and 80 mg/kg) or normal saline for blood pressure effects and two groups for intraventricular pressure which received jugular injection of either TP extract (20, 40 and 80 mg/kg) or normal saline.
In control groups, normal saline (37°C) with similar volumes of extracts used to rule out the effects of blood volume changes on cardiovascular parameters. The rabbits were kept in standard conditions at 22 ± 2°C and fed with standard diet. The study was permitted by the Institutional Animal Ethics Committee of Mashhad University of Medical sciences (MUMS).
Animals were anesthetized using sodium thiopental (50 mg/kg, ip) and then jugular vein and carotid artery were cannulated. TP extracts were introduced through the jugular vein. The carotid cannula with a pressure transducer connected to a power lab machine (AD Instruments, Australia) was used for blood pressure, heart rate and LVP measurement. The data were pooled from independent, single-blinded experiments for each group.
Data analysis
The changes in blood pressure and intraventricular pressure before and after injections were analyzed, using the Wilcoxon signed rank test. The results represent mean±SEM and the differences were considered significant if P<0.05.
RESULTS
Effects on blood pressure
Treatment with TP extract at 80 mg/kg reduced the mean arterial blood pressure (79.6 vs 68.6 mmHg), significantly [Figure 1]. However, in the 20 and 40 mg/kg doses of TP extracts the mean blood pressure reduced, the changes were not reaching a significant value [Figure 1]. As expected, there was no significant differences in the normal saline treatment groups for mean arterial blood pressure, heart rate, LVPmax (maximum left ventricular pressure), LVEDP (left ventricular end diastolic pressure), (dp/dt)max (maximum rate of rise of left ventricular pressure during ventricular contraction) and (dp/dt)min (maximum rate of fall of LVP during left ventricular relaxation) [Table 1].
Figure 1.
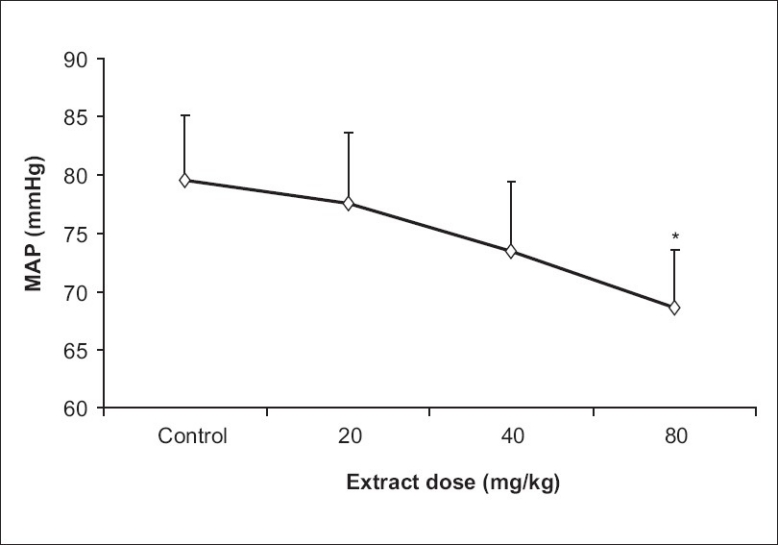
Effect of Teucrium polium L. extract on mean arterial pressure (n=6, *P<0.05).
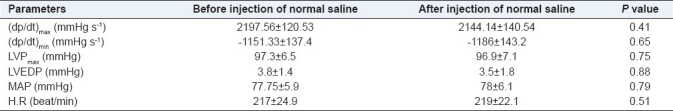
Table 1.
Effect of normal saline injection by the same volume of Teucrium polium L. extract on intraventricular pressure, mean arterial pressure and heart rate. (n=6 for intraventricular pressure control group, n=6 for mean arterial pressure control group)
Effects on inrtaventricular pressure
TP extract injection increased the mean of (dp/dt)max (1288 vs 1508 mmHg s-1 for 20 mg/kg and 1540 mmHg s-1 for 40 mg/kg and 1561 mmHg s-1 for 80 mg/kg, P<0.05) [Figure 2a]. The extract also increased the mean of LVPmax (95 vs 112 and 110 mmHg for 40 and 80 mg/kg, respectively P<0.05), significantly [Figure 3a]. Furthermore, the mean of (dp/dt)min, decreased after TP injection which was statistically meaningful (-1143 vs -1456 and -1449 mmHg s-1 for40 and 80 mg/kg respectively, P<0.05) [Figure 2b]; however, there was no significant effect of TP injection on LVEDP [Figure 3b].
Figure 2.
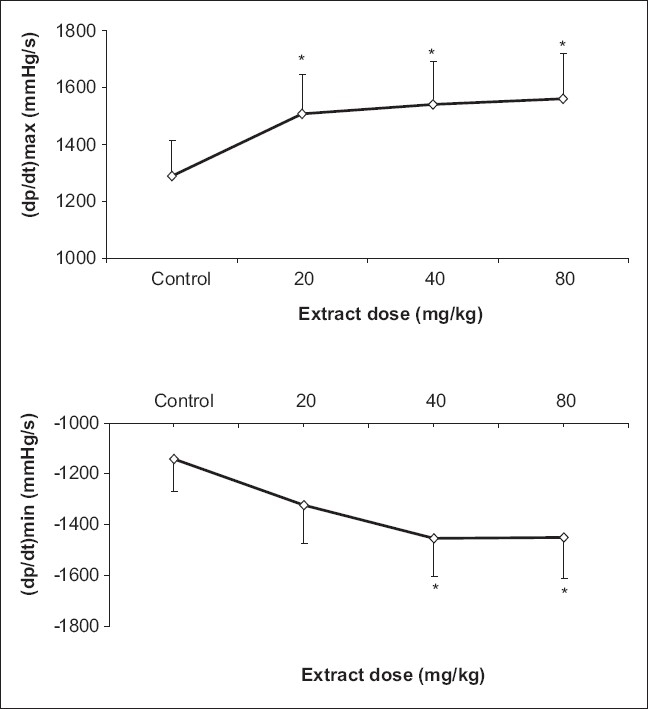
Effect of Teucrium polium L. extract on maximum rate of rise of left ventricular pressure during ventricular contraction [(dp/dt)max] (a) and maximum rate of fall of left ventricular pressure during left ventricular relaxation [(dp/dt)min] (b) (n=6, *P<0.05).
Figure 3.
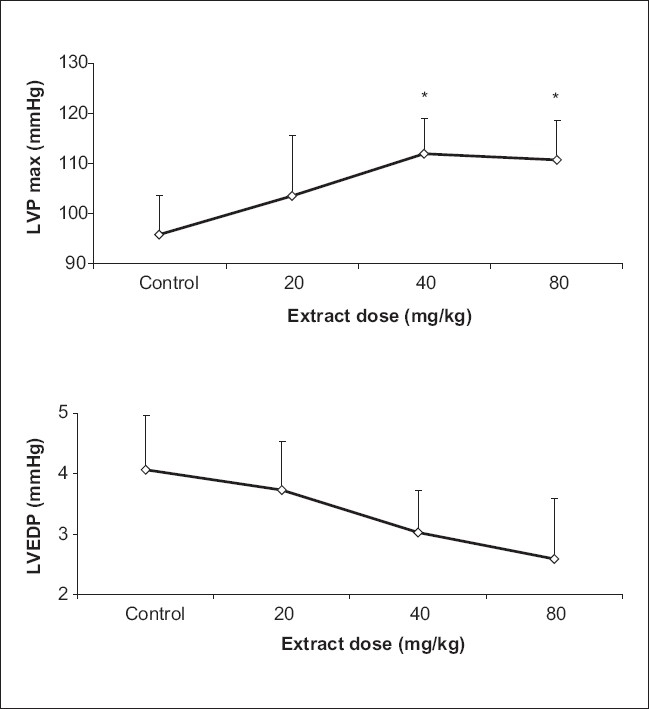
Effect of Teucrium polium L. extract on LVPmax (maximum left ventricular pressure) (a) and left ventricular end diastolic pressure (b) (n=6)
DISCUSSION
In the present study the injection of normal saline with the same volume of TP extract in blood pressure and intarventricular pressure control groups had not significant effects on heart functions, thus the results of control groups could be attributed to TP extract groups. The TP extract (80 mg/kg) significantly reduced mean arterial blood pressure (14%) in normotensive condition which indicated a relaxation effect on vascular smooth muscles. Previous studies have also demonstrated that TP extract had relaxation effects on ileum[17] and vascular smooth muscles.[18] Moreover, TP contains important ingredients such as salvigenin, cirsiliol, pinen-α and β, Sabinene, myrcene, germacrene D, limonene, β-caryophyllene, spathulenol which can influence on vascular smooth muscle tone. For instance, in many studies the antispasmodic effect of some components such as α- and β-pinen,[25,26] cirsiliol,[27,28] spathulenol,[29] limonene[30] and salvigenin[31] from other members of Lamiaceae family have been demonstrated. Taking together, it seems that the TP ingredients can induce the hypotensive effects in rabbits.
Blood pressure is not determined by vascular factors alone and cardiac interaction is also important for determining the final arterial blood pressure value. We recently demonstrated positive inotropic effects of TP extract on isolated Guinea pig heart.[22] This effect may counterbalance the relaxant effect of lower doses of TP extracts on vascular smooth muscle; consequently, in such situation, the blood pressure could not be reduced significantly. In other words, the positive inotropic effect of TP extract may suppress its relaxant properties in vivo.
However, in several studies the relaxation effect of TP extract has been observed in contracted smooth muscle in vitro,[17,18] in the present study this effect has been investigated in normotensive condition in vivo which may differ in methodology.
Although, different TP extract doses had not any significant effect on heart rate in this study. In the pervious study,[22] we demonstrated that the TP extract had a positive chronotropic effect. The logical reason for these differences between such studies could be explained by different methodological approaches. There are many factors such as cardiac, vascular, hormonal and neural that can influence the heart rate in vivo. Therefore, further studies needed to be conducted to evaluate the influences of such factors on heart rate in TP-treated animals.
Furthermore, in the present study, the effects of TP extracts on cardiac parameters such as (dp/dt)max, (dp/dt)min, LVPmax and LVEDP were investigated. The data shows that (dp/dt)max was significantly increased by TP extract treatment (17.1%, 19.5% and 21.1% for 20, 40 and 80 mg/kg dosages, respectively). This parameter is an indicator for maximum rate of rise of LVP during ventricular contraction and it is a valid criterion for inotropic effect of a substance. We have seen the inotropic effect of TP extract in vitro and in vivo. However, these findings are in contrast with other findings that demonstrated a relaxant effect on vascular smooth muscle, thus it can be concluded that the mechanism of TP activity on smooth and cardiac striated muscles are different. TP extract also significantly decreases (dp/dt)min (27.4% and 26.7% for 40 and 80 mg/kg dosages, respectively). This parameter is an indicator for maximum rate of fall of LVP during left ventricular relaxation and its reduction indicates the rapidity of ventricular relaxation, ventricular efficiency and positive inotropic effect. Therefore, the positive effect of the extract on (dp/dt)max and negative effect on (dp/dt)min after injection shows an enhancement of cardiac performance and indicates a positive inotropic effect.
LVPmax is significantly increased by TP extract (16.2% and 11.4% for 40 and 80 mg/kg dosages respectively) which also indicates the positive inotropoic effect. Taking together our findings in the present and the previous study,[22] reveal that TP extract may has a significant positive inotropic effect. More interestingly, in Iranian traditional medicine, TP is used for treatment of CHF, therefore it seems that the results of the present study can strengthen such applications. In conclusion, for better understanding of the effects of TP extract and the mechanism/s of actions on the cardiovascular system, particularly on blood pressure in hypertension status, further studies are needed to be conducted.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Androulakis ES, Tousoulis D, Papageorgiou N, Tsioufis C, Kallikazaros I, Stefanadis C. Essential hypertension: Is there a role for inflammatory mechanisms? Cardiol Rev. 2009;17:216–21. doi: 10.1097/CRD.0b013e3181b18e03. [DOI] [PubMed] [Google Scholar]
- 2.Ho JW, Jie M. Pharmacological activity of cardiovascular agents from herbal medicine. Cardiovasc Hematol Agents Med Chem. 2007;5:273–7. doi: 10.2174/187152507782109854. [DOI] [PubMed] [Google Scholar]
- 3.Rizk AM, Hammouda FM, Rimpler H, Kamel A. Iridoids and flavonoids of Teucrium polium herb. Planta Med. 1986;52:87–8. [PubMed] [Google Scholar]
- 4.Talal A, Mohammad H, Vanni C. Composition of the Essential Oil from Jordanian Germander (Teucrium polium L.) J Essent Oil Res. 2006;8:97–9. [Google Scholar]
- 5.Cakir A, Emin Dum M, Harmandar M. Volatile constituents of Teucrium polium L. from Turkey. J Essent Oil Res. 1998;10:113–5. [Google Scholar]
- 6.Vokou D, Bessiere JM. Volatile constituents of Teucrium polium. J Nat Prod. 1985;48:498–9. [Google Scholar]
- 7.Autore G, Capasso F, De Fusco R, Fasulo MP, Lembo M, Mascolo N, et al. Antipyretic and antibacterial actions of Teucrium polium. Pharmacol. Res Commun. 1984;16:21–9. doi: 10.1016/s0031-6989(84)80101-0. [DOI] [PubMed] [Google Scholar]
- 8.Tariq M, Ageel AM, al-Yahya MA, Mossa JS, al-Said MS. Anti-inflammatory activity of Teucrium polium. Int J Tissue React. 1986;11:185–8. [PubMed] [Google Scholar]
- 9.Kadifkova Panovska T, Kulevanov S, Stefov M. In vitro antioxidant activity of some Teucrium species (Lamiaceae) Acta Pharm. 2005;55:207–14. [PubMed] [Google Scholar]
- 10.Ljubuncic P, Dakwar S, Portnaya I, Cogan U, Azaizeh H, Bomzon A. Aqueous Extracts of Teucrium polium Possess Remarkable Antioxidant Activity in Vitro. Evid Based Complement Alternat Med. 2006;3:329–38. doi: 10.1093/ecam/nel028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alkofahi A, Atta AH. Pharmacological screening of the anti-ulcerogenic effects of some Jordanian medicinal plants in rats. J Ethnopharmacol. 1999;67:341–5. doi: 10.1016/s0378-8741(98)00126-3. [DOI] [PubMed] [Google Scholar]
- 12.Galati EM, Mondello MR, D’Aquino A, Miceli N, Sanogo R, Tzakou O. Effects of Teucrium divaricatum Heldr. ssp divaricatum decoction on experimental ulcer in rats. J Ethnopharmacol. 2000;72:337–42. doi: 10.1016/s0378-8741(00)00280-4. [DOI] [PubMed] [Google Scholar]
- 13.Abdollahi M, Karimpour H, Monsef-Esfehani HR. Antinociceptive effects of Teucrium polium L. total extract and essential oil in mouse writhing test. Pharmacol Res. 2003;48:31–5. [PubMed] [Google Scholar]
- 14.Baluchnejadmojarad T, Roghani M, Roghani-Dehkordi F. Antinociceptive effects of Teucrium polium leaf extract in the diabetic rat formalin test. J Ethnopharmacol. 2005;97:207–10. doi: 10.1016/j.jep.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 15.Gharaibeh NMN, Elayan HE, Salhab AS. Hypoglycemic effects of Teucrium polium. J Ethnopharmacol. 1988;24:93–9. doi: 10.1016/0378-8741(88)90139-0. [DOI] [PubMed] [Google Scholar]
- 16.Yazdanparast R, Esmaeili MA, Ashrafi J. Teucrium polium extract effects pancreatic function of streptozotocin diabetic rats: A histopathological examination. Iran Biomed J. 2005;9:81–5. [Google Scholar]
- 17.Parsaee H, Shafiee-Nick R. Anti-Spasmodic and Anti-nociceptive effects of Teucrium polium aqueous extract. Iran Biomed J. 2006;10:145–9. [Google Scholar]
- 18.Suleiman MS, Abdul-Ghani AS, Al-Khalil S, Amir R. Effect of Teucrium polium boiled leaf extract on intestinal motility and blood pressure. J Ethnopharmacol. 1988;22:111–6. doi: 10.1016/0378-8741(88)90236-x. [DOI] [PubMed] [Google Scholar]
- 19.Sadraei H, Hajhashemi V, Ghannad A, Mohseni M. Antispasmodic effect of aerial part of Teucrium polium L. essential oil on rat isolated ileum in vitro. Med J Islam Rep Iran. 2001;14:355–8. [Google Scholar]
- 20.Rasekh HR, Khoshnood-Mansourkhani MJ, Kamalinejad M. Hypolipidemic effects of Teucrium polium in rats. Fitohterapia. 2001;72:937–9. doi: 10.1016/s0367-326x(01)00348-3. [DOI] [PubMed] [Google Scholar]
- 21.Shahraki MR, Arab MR, Mirimokaddam E, Palan MJ. The effect of Teucrium polium (Calpoureh) on liver function, serum lipids and glucose in diabetic male rats. Iran Biomed J. 2007;11:65–8. [PubMed] [Google Scholar]
- 22.Niazmand S, Erfanian Ahmadpoor M, Moosavian M, Derakhshan M. The positive inotropic and chronotropic effects of Teucrium polium L. extract on Guinea pig isolated heart. Pharmacologyonline. 2008;2:588–94. [Google Scholar]
- 23.Bello R, Calatayud S, Moreno L, Beltrán B, Primo-Yúfera E, Esplugues J. Effects on arterial blood pressure of the methanol extracts from different Teucrium species. Phytother Res. 1998;11:330–1. [Google Scholar]
- 24.Ljubuncic P, Azaizeh H, Portnaya I, Cogan U, Said O, Saleh KA, et al. Antioxidant activity and cytotoxicity of eight plants used in traditional Arab medicine in Israel. J Ethnopharmacol. 2005;99:43–7. doi: 10.1016/j.jep.2005.01.060. [DOI] [PubMed] [Google Scholar]
- 25.Sadraei H, Asghari GR, Hajhashemi V, Kolagar A, Ebrahimi M. Spasmolytic activity of essential oil and various extracts of Ferula gummosa Boiss. on ileum contractions. Phytomedicine. 2001;8:370–6. doi: 10.1078/0944-7113-00052. [DOI] [PubMed] [Google Scholar]
- 26.Câmara CC, Nascimento NR, Macêdo-Filho CL, Almeida FB, Fonteles MC. Antispasmodic effect of the essential oil of Plectranthus barbatus and some major constituents on the guinea-pig ileum. Planta Med. 2003;69:1080–5. doi: 10.1055/s-2003-45186. [DOI] [PubMed] [Google Scholar]
- 27.Mustafa EH, Abu Zarga M, Abdalla S. Effects of cirsiliol, a flavone isolated from Achillea fragrantissima, on rat isolated ileum. Gen Pharmacol. 1992;23:555–60. doi: 10.1016/0306-3623(92)90127-6. [DOI] [PubMed] [Google Scholar]
- 28.Kohno S, Ohata K. Resting tonus of isolated airway smooth muscles. Nippon Yakurigaku Zasshi. 1993;102:1–10. doi: 10.1254/fpj.102.1. [DOI] [PubMed] [Google Scholar]
- 29.Perez-Hernandez N, Ponce-Monter H, Medina JA, Joseph-Nathan P. Spasmolytic effect of constituents from Lepechinia caulescens on rat uterus. J Ethnopharmacol. 2008;115:30–5. doi: 10.1016/j.jep.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 30.De Sousa DP, Júnior GA, Andrade LN, Calasans FR, Nunes XP, Barbosa-Filho JM, et al. Structure and spasmolytic activity relationships of monoterpene analogues found in many aromatic plants. Z Naturforsch. 2008;63:808–12. doi: 10.1515/znc-2008-11-1205. [DOI] [PubMed] [Google Scholar]
- 31.Uydeç-Doğan BS, Takir S, Ozdemir O, Kolak U, Topçu G, Ulubelen A. The comparison of the relaxant effects of two methoxylated flavones in rat aortic rings. Vascul Pharmacol. 2005;43:220–6. doi: 10.1016/j.vph.2005.07.002. [DOI] [PubMed] [Google Scholar]


