Abstract
Purpose
This study utilized multiple genetic analyses to detect evidence of maternal MS acquisition in children with S-ECC.
Methods
Twenty-seven mother/child pairs were selected from children with S-ECC preceding dental rehabilitation under general anesthesia. Plague samples were collected from the mother, child, and the child’s carious lesion. Arbitrarily primed-polymerase chain reaction (AP-PCR) genotyped 6–8 MS isolates from each plague sample, and unique genotypes were identified. Representative MS isolates with unique genotypes were characterized by pulsed-field gel electrophoresis (PFGE). Cluster analysis using the Dice band-based similarity coefficient was used to generate dendrograms from gel banding patterns. A Dice coefficient >70% indicated similarity or match among PFGE genotypes.
Results
In 26% (7/27) of mother/child pairs, all of the child’s isolates matched the mother. In 15% (4/27), some of the child’s genotypes matched the mother, and in 59% (16/27), no isolates matched the mother. Maternal transmission was a mode of MS acquisition in 41% (11/27) of mother/child pairs, while acquisition from non-maternal sources occurred in 74% (20/27).
Conclusions
MS genotypes that did not match maternal strains were identified in the majority of children (74%) within this S-ECC population. Evidence of maternal transmission was detected in 41% of mother /child pairs.
Keywords: CARIOLOGY, INFANT ORAL HEALTH/EARLY CHILDHOOD CARIES, MICROBIOLOGY, MUTANS STREPTOCOCCI, TRANSMISSION
The source of mutans streptococci (MS) infection in infants and toddlers has been extensively studied in recent years. Maternal transmission has been documented as a method by which children are initially inoculated with MS.
Early studies demonstrated fidelity of maternal transfer to be as high as 71% in a cohort of Birmingham, AL, children1 and as low as 43% in Toronto, Ontario children.2 Emanuelsson found 55% maternal transmission and no paternal transmission in Swedish families.3 Other studies show that MS are readily acquired from non-maternal sources in certain populations. De Soet et al. reported that cleft palate populations receiving obturators early in life demonstrate maternal transmission in only 38% of 21 mother/child pairs.4 Emanuelsson et al. reported, from a Chinese population, 36% maternal transmission, 27% paternal transmission, and, in 1 family, an identical MS strain was shared between spouses.5 Li et al. found only 45% maternal transmission of MS in a Chinese population.6 Kozai et al conducted an extensive study of 1,908 MS isolates within a Japanese population in which plaque from 76 individuals (20 married couples and 36 of their children) yielded 70 genotypes within the children. The study found that 31% had similar MS genotypes to the father, while 51% were consistent with maternal MS genotypes.7
Tedjosansongko et al. evaluated 39 Japanese children in a day care setting and found maternal transmission in 33%, paternal transmission in 8%, and evidence of horizontal transmission from playmates in 58%.8 Similarly, Mattos-Graner reported evidence of horizontal MS transfer in a Brazilian day care population.9 Ersin et al. studied Turkish families in which 8 mother/child pairs and 3 fathers all shared identical MS genotypes.10 These studies indicate that, while maternal transmission does occur, it is only one of several modes of MS acquisition.
One of the challenges inherent to studies of bacterial transmission is the implementation of laboratory techniques capable of accurate discrimination among bacteria from different individuals. Genetic analysis is now the accepted approach for strain identification due to enhanced reliability and reproducibility.11 Recent advances in molecular diagnostics for MS strain identification include: multilocus sequence typing12; chromosomal DNA fingerprinting13; pulsed-field gel electrophoresis (PFGE)14,15; arbitrarily primed-polymerase chain reaction (AP-PCR)16,17; ribotyping18,19; and sequencing of the 16S rRNA gene.11,20,21
Studies by Saarela and by Li and Caufield validated the use of AP-PCR in discriminating MS genotypes within individuals.17,22 Although AP-PCR can accurately discriminate among samples processed on the same gel, variability in environmental conditions limits its reproducibility among laboratories.23 PFGE is the preferred method of genotyping due to its reproducibility and discriminatory power.24–26 The Centers for Disease Control and Prevention (CDC), Atlanta, GA, use PFGE for bacterial DNA fingerprinting. This technique is considered the “gold standard” for epidemiological studies of pathogenic bacterial infections.27 While different restriction enzymes have been used to study the MS genome,28–34 experience in the authors’ laboratory35–40 and reports in the literature indicated that SmaI would be the best restriction endonuclease for epidemiologic studies of MS.
Previous studies have documented different MS transmission patterns in various populations. To date, however, transmission patterns within children with severe early childhood caries (S-ECC) have not been characterized. The diagnosis of S-ECC is given to children younger than 5 years old with any smooth surface caries.41 The S-ECC prevalence in the United States is between 1% and 6%. The disease, however, disproportionately effects low socioeconomic populations.42–46
Clinically, MS transmission patterns within the population of children experiencing S-ECC are of interest, since these patterns may explain characteristics shared with children at high risk of S-ECC who would benefit from early prevention. Strategies for the prevention or delay of maternal transmission of cariogenic bacteria have been recommended by the CDC and are written into oral health policies of pediatric health care organizations.47–49 This strategy, however, is predicated on maternal transmission of MS being the primary means by which S-ECC children acquire cariogenic bacteria.
The purpose of this study was to utilize multiple genetic analyses to detect evidence of maternal MS acquisition in children with S-ECC.
Methods
MS isolation
This institutionally approved, experimental cohort consisted of medically healthy children between the ages of 18 months and 6 years who were diagnosed with S-ECC (minimum def score = 6) and presented for full-mouth dental rehabilitation under general anesthesia at Children’s Hospital, Birmingham, AL. The child’s biological mother had to consent to the collection of an oral microbial (plaque swab) sample. Pretreatment plaque swab samples were collected by passing a sterile cotton swab over the teeth and gingiva of the subject and the mother. A site-specific bacterial plaque sample was collected from carious lesions of the S-ECC children with a sterile tooth-pick. Clinical samples were labeled (Table 1) and placed in a reduced transport fluid media50 for subsequent laboratory identification. MS isolates were cultivated from salivary samples grown on selective mitis salivarius with bacitracin agar, and 8 isolates were saved in Todd Hewitt (TH) broth (Becton Dickinson, Sparks, MD) with 15% glycerol and frozen at −70°C.
Table 1.
LABELING CONVENTION FOR SAMPLES
| Sample Type* | Label |
|---|---|
| Mother’s Plaque | M |
| Child’s Plaque | BP |
| Plaque from Child’s Caries | BC |
Real-time polymerase chain reaction (real-time PCR)
MS isolates in this study were identified using SYBR green-based, real-time PCR methodology.51,52 Briefly, 23 µl of a polymerase chain reaction mixture in final concentrations of 1X iQ SYBR green master mix, 0.15 µM forward and reverse Streptococcus mutans-specific primers, 5’ ATTTGATAATGCTCCTGTTTCATCAAAGAAGA 3’ and 5’ ATGGAACTCTTCAAAAGAATTATGCTTTAAACAT 3’, respectively, were pipetted into microplate wells. Subsequently, 2 µl of template DNA (~75 ng) from each MS isolate was added to the corresponding wells, and the entire plate was sealed.
These real-time PCR mixtures were subjected to precise thermal cycling on an iQ5 thermal cycler (BioRad Laboratories, Hercules, CA) using the following cycle parameters: 1 cycle of 95°C for 3 minutes followed by 35 cycles of 20 seconds at 95°C, 20 seconds at 55°C and 20 seconds at 72°C. Finally, reaction specificity was determined by performing a gradient melting curve. Analysis of amplification and melting curve data was performed using version 2.0 BioRad iQ5 standard system software (BioRad Laboratories, Hercules, CA).
AP-PCR analysis
Unique genotypes were identified by applying AP-PCR protocols reported by Li and Caufield to each MS isolate from each plaque sample.17 OPA-02 (5’-TGCCGAGCTG), a commercially available, single stranded oligonucleotide primer (Operon Technologies Inc, Alameda, CA) was used to arbitrarily prime polymerase amplification of the bacterial chromosomal DNA. All amplification reactions were conducted in a volume of 50 µl containing 5 µl of 10× reaction buffer (100 mM Tris-HCl, 500 mM KCl), 10 mM each of dATP, dCTP, dGTP, and dTTP and 0.2 µl of Taq DNA polymerase. All AP-PCR reagents were obtained from Perkin-Elmer Applied Biosystems (Branchburg, NJ).
Amplification conditions were 45 cycles consisting of 30 seconds at 94°C, 30 seconds at 36°C, and 1 minute at 72°C. Amplification was conducted in a GeneAmp PCR System 9700 (Perkin-Elmore Applied Biosystems, Branchburg, NJ). The amplification products were separated by electro-phoresis in a 1% agarose gel in 1× Tris-borate-EDTA at 45 volts for 3 hours and stained with ethidium bromide for 45 minutes. Gels were photographed with red filters under UV310 light and Polaroid 667 black and white film and recorded with an Alphalmager 1000 digital imaging system (Alpha Innotech Corp, San Leandro, CA) for visual comparison.
Three independent observers visually compared AP-PCR fingerprints for unique MS genotypes within the 8 MS isolates from the clinical sample, and MS genotype matches among samples from mother and child. MS isolates representing unique AP-PCR genotypes were selected and further characterized by PFGE.
PFGE analysis
Genotypic similarity between intrafamiliar MS samples was analyzed with PFGE. TH broth (3 ml) was inoculated with a single colony of MS from an overnight TH agar plate isolate grown anaerobically with 5% CO2 at 35°C. The turbidity of the broth culture was adjusted to an Ab610 of 0.9–1.3. A 200 µl aliquo was placed into a microfuge tube and centrifuged at 12,000 × g for 5 minutes, the supernatant was aspirated, and the pellet resuspended in 200 µl of Tris-EDTA (TE) buffer,35 washed 3 times and resuspended in 160 µl of TE buffer. Agarose plugs were prepared by equilibrating the cell suspension in a 40°C water bath for 10 minutes, adding 50 units of mutanolysin (Sigma, St. Louis, MO) and 160 µl of 1.8% (weight/volume) pulse field certified agarose (BioRad Laboratories) in TE buffer (50°C). Mixtures were dispensed into 3 wells of a disposable plug mold (BioRad Laboratories) and allowed to solidify. Cell lysis was performed in situ using 4 ml of lysis buffer35 incubated at 37°C overnight.
The buffer was replaced with a fresh lysis buffer and incubated at 37°C for 8 hours. Plugs were then protein digested in 10 ml of ESP buffer35 in a 50°C water bath overnight, washed 3 times, and stored in TE buffer (10mM Tris, 1mM Ethylenediaminetetra acetic Acid, pH 8.0, Invitrogen, Carlsbad, CA) at 4°C until used. Restriction enzyme digestion was performed on one-half of an agarose plug using 20 units of SmaI incubated at 25°C in the dark for 6 hours. An additional 20 units of SmaI was added and incubation continued overnight. Plugs were loaded into wells of 1% agarose gel (BioRad pulsed-field certified agarose, Biorad Laboratories, Hercules, CA) in 0.5× TBE buffer, and electrophoresis was performed using a CHEF Mapper XA electrophoresis system (BioRad Laboratories, Hercules, CA) at 14°C with a gradient of 6 V/cm (200 V), an included angle of 120°, an initial switch time of 1 second, and a final switch time of 40 seconds using a linear ramping factor for 22 hours. S. mutans Ingbritt C was selected as the reference strain for gel comparisons (Figure 1).
Figure 1.
S. mutans Ingbritt C was used as the reference strain for PFGE gel comparisons. DNA fragment size is expressed in kilobases (kb) and compared to lambda (λ) phage.
Bands were visualized by staining with ethidium bromide and digitized using a Gel Doc 2000 (BioRad Laboratories, Hercules, CA). Digital images were imported into Bionumerics software v4.6 (Applied Maths, Inc, Austin, TX). Patterns were normalized based upon a standard strain of S. mutans Ingbritt C (Figure 1). Cluster analysis using the Dice band-based similarity coefficient and unweighted pair group method using arithmetic averages were used to generate dendrograms to assess strain relatedness (percent similarity). A Dice coefficient greater than 70% indicated similarity or match among PFGE genotypes.
Statistical Methods
Exact 95% confidence intervals for proportions were calculated, based on the binomial distribution.
Results
Bacterial samples from 27 mother/child pairs represented 23 mothers and 27 children, of whom there were 4 sibling pairs. Among the 18 (78%) males and 5 (22%) females, the children’s mean age was 41.7 months (range = 19–82 months). Four of the children were African Americans (18%), 18 were Caucasians (78%), and 1 was of Middle Eastern descent (4%). The S-ECC children had a mean def of 9.7 teeth (range = 6–19). All MS isolates were identified as S. mutans utilizing a SYBR green-based real-time PCR assay.
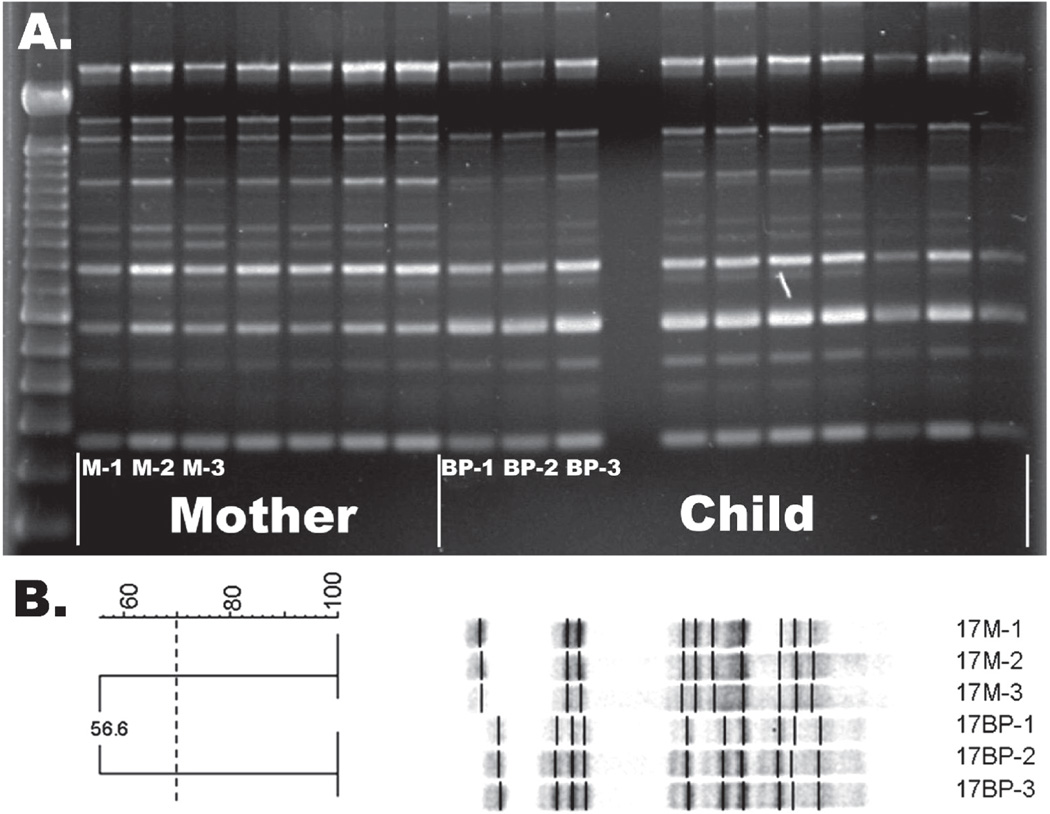
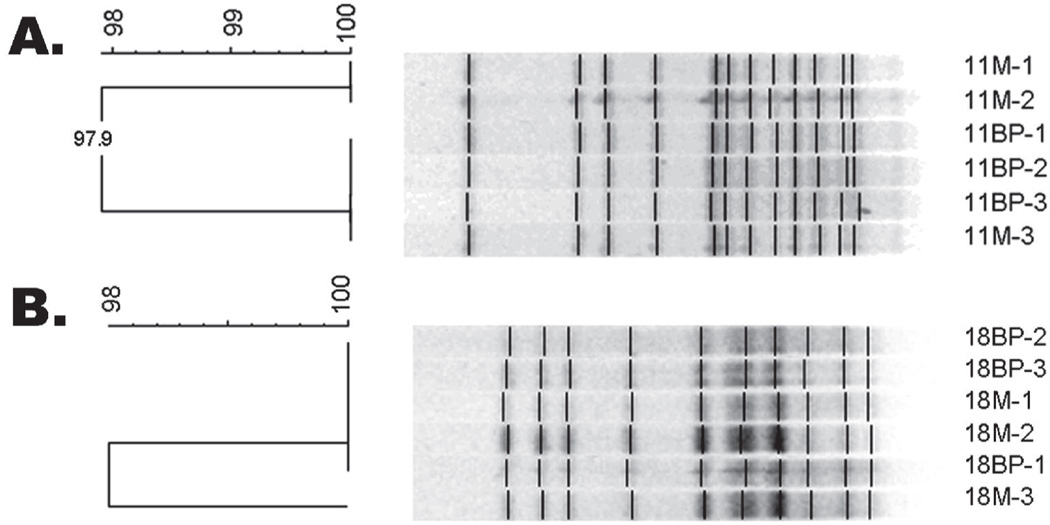
AP-PCR fingerprinting identified an average of 1.45 unique MS isolates in mothers and 1.15 unique MS isolates in children, with matching MS isolates occurring in 41% of mother-child pairs (Figure 2a). PFGE fingerprinting confirmed the AP-PCR results, as represented in Figure 2b. Table 2 summarizes the distribution of intrafamilial MS strains, as determined by PFGE. In 26% (7/27) of mother/child pairs, the isolate from the child’s mouth could be matched to isolates from their mother (Figure 3); in each of these cases, the child only had 1 isolate. In another 15% (4/27), a child possessing multiple genotypes had both matching and non-matching genotypes (Figure 4).
Figure 2.
Representative comparison of AP-PCR gel with PFGE gel for mother/child pair #17. (A) AP-PCR gel demonstrating maternal genotypes do not visually match genotypes from the child. (B) PFGE gel confirming representative genotypes from mother (M-1, M-2, M-3) and child (BP-1, BP-2, BP-3) do not match (DICE coefficient = 56.6%) -Dice coefficient greater than 70% indicates match.
Table 2.
DISTRIBUTION OF INTRAFAMILIAL GENOTYPES OF MS STRAINS ISOLATED FROM CHILD-MOTHER PAIRS
| Child/Mother pairs |
Child’s MS strains* |
Mother’s MS strains* |
|---|---|---|
| 1 | A | B, C |
| 2 | A | B |
| 5† | A | B |
| 6† | A | B |
| 8 | A | B, C |
| 9 | A, B | A |
| 10 | A | B |
| 11 | A | A |
| 12 †† | A | C |
| 13 †† | A, B | C |
| 14††† | A | B, C |
| 15 ††† | A | B, C |
| 16 | A | A, B, C |
| 17 | A | B |
| 18 | A | A |
| 19 | A, B, C | A, D |
| 20 †††† | A | A |
| 21 | A | B |
| 22 | A | A, B |
| 23 †††† | A | A |
| 24 | A | B,C |
| 25 | A | A |
| 26 | A,B | A |
| 27 | A,B | A |
| 28 | A | B |
| 29 | A | B |
| 30 | A | B,C |
Letters represent unique MS genotypes within a Child/Mother Pair. Approximately 8 MS strains were isolated per individual providing a 0.9922 probability of finding 2 or more unique genotypes, and a 0.8834 probability of finding 3 or more unique genotypes assuming that different genotypes occur at equal frequency.
Indicates children who have the same mother.
Figure 3.
Representative PFGE analysis where each genotype in child matches mother. (A) Mother/Child Pair #11 where all MS genotypes share at least 97.9% similarity. (B) Mother/Child Pair #18 where all MS genotypes share at least 96.3% similarity. -Dice coefficient greater than 70% indicates match.
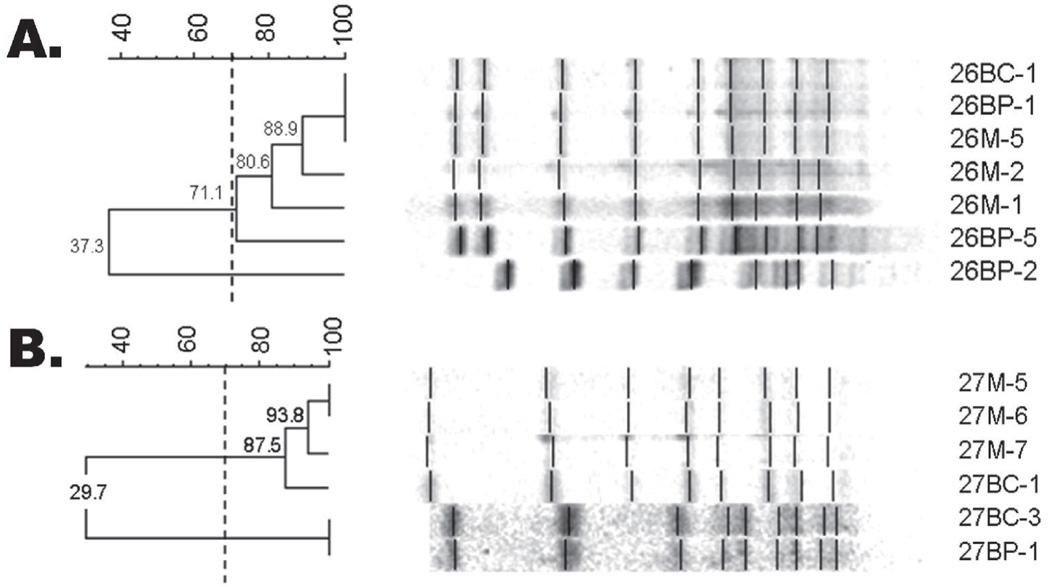
Figure 4.
Representative PFGE gels where genotypes in child partially match mother. Mother/Child Pair #26 where child’s MS genotype 26BP-2 does not match mother, while other child genotypes (26BP-1,5,BC-1) match maternal genotypes (26M-1,2,5). (B) Mother/Child Pair #27 where genotypes 27BP-1, 27BC-3 do not match maternal genotypes, while child’s 27BC-1 matches maternal genotypes (27M-5,6,7). -Dice coefficient greater than 70% indicates match.
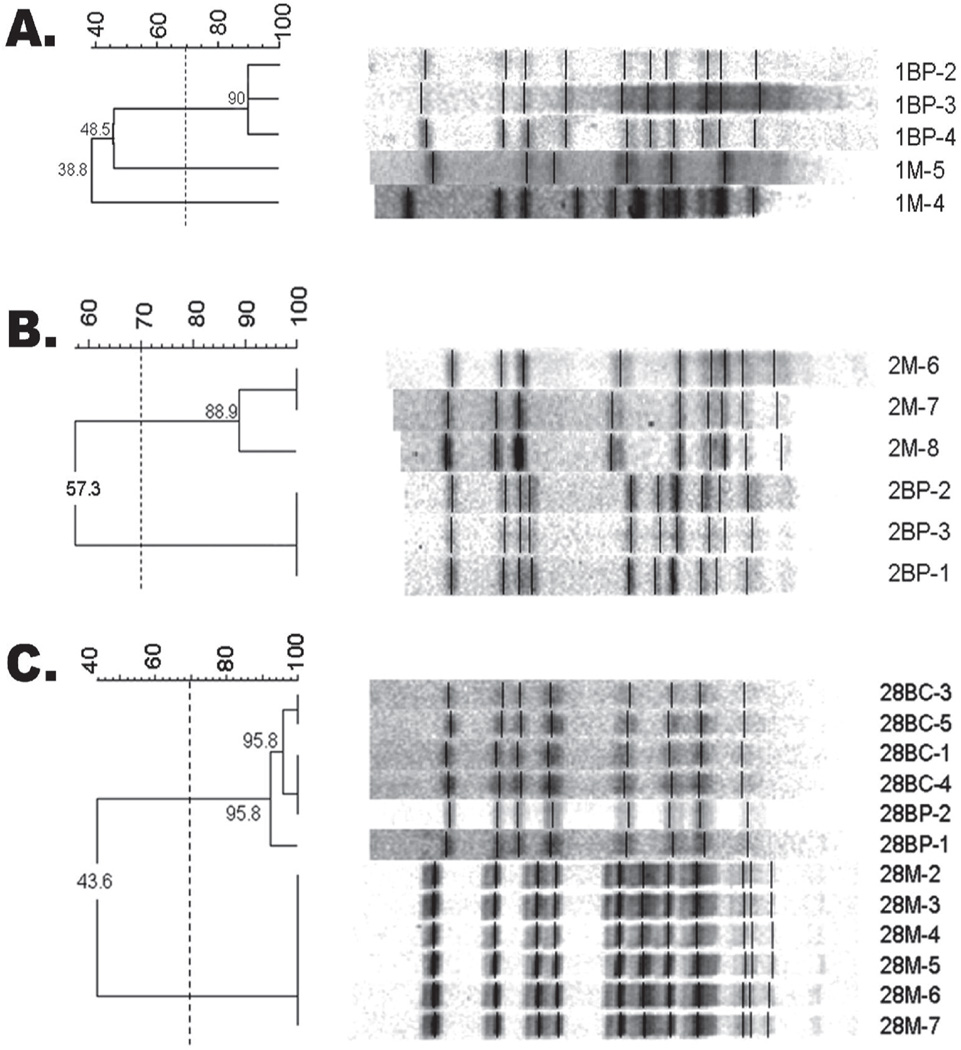
Therefore, evidence of maternal transmission as a mode of MS acquisition was found in 41% of cases. In 59% (16/27) of pairs, however, none of the child’s isolates matched the mother’s (Figure 5), and in 15% (4/27), a child with multiple genotypes possessed at least 1 genotype that did not match his or her mother’s. Interestingly, each child with multiple genotypes (5/27) possessed at least 1 genotype that did not match the mother. Overall, acquisition from non-maternal sources occurred in 74% (20/27) of mother/child pairs.
Figure 5.
Representative PFGE gels where child does not match mother. (A) Mother (1M) and child (1BP) share less than 49 percent similarity. (B) Mother (2M) and child (2BP) share less than 60 percent similarity. (C) Mother (28M) and child (28BP/28BC) share less than 44 percent similarity. -Dice coefficient greater than 70 percent indicates match.
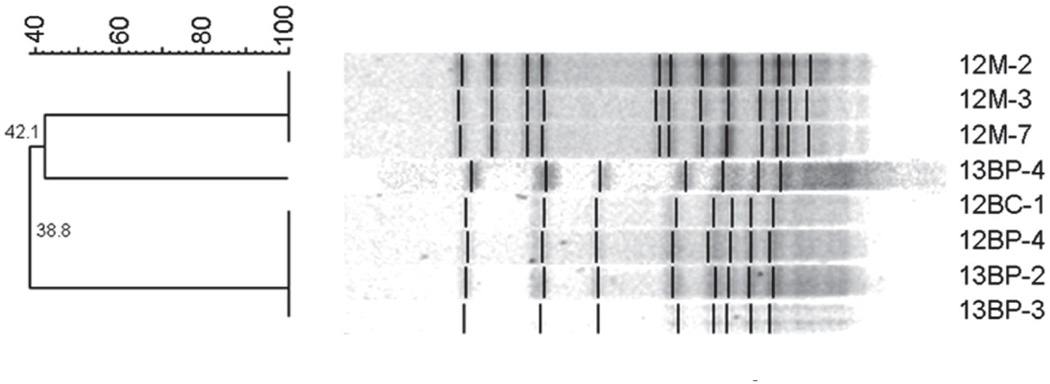
Four sibling pairs (subjects 5/6, 12/13, 14/15, and 20/23) were also evaluated to compare isolates within their oral cavities. Both AP-PCR and PFGE found all 4 pairs shared at least 1 genotype, as illustrated with sibling pair 12/13 (Figure 6). Only sibling pair 20/23 shared a genotype with the mother (Figure 7); all other sibling pairs did not match their mothers’ genotypes (Table 2).
Figure 6.
PFGE gel from siblings (12BP/BC and 13BP/BC) who possess genotypes that match one another, but do not match maternal genotypes (12M). Sibling genotypes 12BC-1, 12BP-4, 13BP-2 and 13BP-3 share 100 percent match. Neither child matches maternal genotypes (DICE coefficient <45 percent). -Dice coefficient greater than 70 percent indicates match.
Figure 7.
PFGE gel for siblings (20BP and 23BP) whose genotypes match one another and maternal genotypes (23M) (Dice coefficient >90 percent). -Dice coefficient greater than 70 percent indicates match.
Discussion
This study’s results add to an expanding body of literature demonstrating diversity in patterns of MS acquisition in human populations. While evidence for maternal transmission was observed, it was neither the exclusive nor the predominant mode of MS acquisition within this S-ECC child population. The fidelity of maternally transmitted strains observed within this high caries population (41%) is consistent with observations in a similar high caries Hispanic population reported by Tan et al.53 and a cleft palate population reported by de Soet.4,54 The evidence of acquisition of non-maternal strains of MS found in 74% (20/27) of this study’s (Table 2) population is also consistent with Lindquist and Emilson’s results, where only 9 of 26 genotypes found in their child cohort were identical to their mother’s genotypes.55 Klein et al. reported evidence of maternal transmission of S. mutans in 81% of the cohort. Strains that could not be identified as maternal, however, were also found in 75% (12/16) of children.56 Even in Kohler’s study where maternal transmission was detected in 88% of children, 50% also had non-maternal strains.57
MS transmission studies, as reported in the literature to date, have been conducted with a small sample size. This study’s sample size (27) is consistent with these other studies. A small sample size presents a statistical challenge, however, because it results in a wide confidence interval (CI) for the results. These wide CIs, however, may contribute to the broad variation in reported fidelity of MS transmission. This study has a 95% CI of positive maternal MS transmission from 22% to 61%, representing a difference of 39 percentage points between the interval’s upper and lower limit.
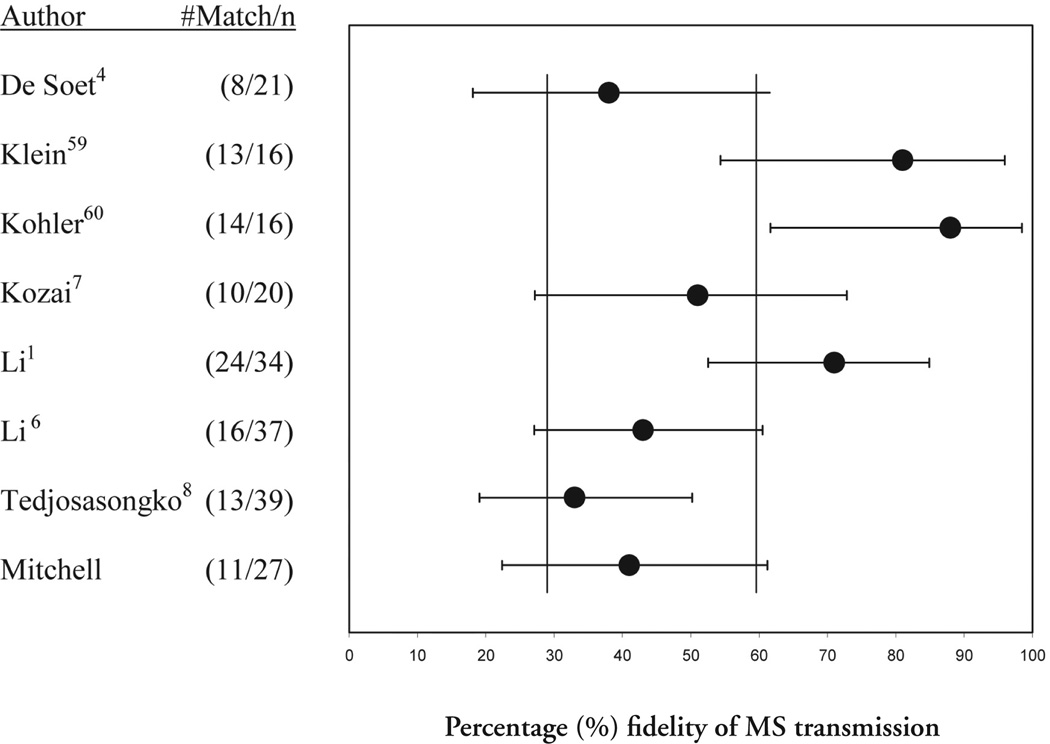
While this is the only known study that focuses on a S-ECC population and may, therefore, have unique characteristics inconsistent with non-ECC populations, a comparison with other studies illustrates some interesting trends. Figure 8 illustrates this study’s CI, and compares it with CIs calculated for 7 other studies from the literature. There is a very wide range for each study, with no study having less than 30 percentage points between the CI’s upper and lower limits, with an average range of 37 points. Six of 8 studies share 60% within their CIs, while 5 share 30%. These numbers will vary depending on the studies compared, but together illustrate the need for a study with a large sample size and much smaller CIs. This study has points within its CI that are in common with every other study in the list.
Figure 8.
95% confidence intervals for fidelity of reported maternal MS transmission. Reported transmission percentages are marked by •. Calculated confidence intervals are demarcated by error bars. Vertical bars indicate studies sharing 30% or 60% fidelity.
In this study, the children were not sampled at the time of initial acquisition. Consequently it is not known if the genotypes detected in this study may have replaced original, maternally acquired genotypes. This explanation would suggest that MS colonization is not a stable ecosystem in S-ECC patients, thus allowing new genotypes to be acquired and pre-existing genotypes to be lost.57 It is equally plausible that the genotypes detected in this study are the original MS genotypes acquired by the child, indicating that initial transmission came from a non-maternal source. The 4 sets of S-ECC siblings in the study demonstrated higher degrees of genotypic similarity with each other than they did with their mothers. This could result from children being inoculated by the same, non-maternal source or from horizontal transmission between siblings. Regardless of the explanation, MS transmitted from non-maternal sources colonized most of the patient’s mouths in this study.
In a landmark study, Caufield et al. stated: “The association between MS levels and diet high in fermentable carbohydrates cannot be ignored as a possible influence in initial colonization of MS.”58 S-ECC children are associated with aberrant, sucrose-rich feeding behaviors resulting in MS colonization at an earlier age.59–62 MS not only appear earlier, but also achieve higher bacterial levels in S-ECC.63–66 S-ECC children also have a greater number of MS genotypes than caries-free children.67,68 Taken together, these characteristics of S-ECC populations may indicate an oral ecosystem in which excessive dietary sucrose consumption favors the establishment and growth of MS, allowing indiscriminant colonization to occur from any source.
Preventive measures targeted at reducing maternal bacterial loads and delaying maternal transmission of MS have been recommended and implemented with varying degrees of success.48,49,69–73 It seems unlikely, however, that such measures would have significantly impacted the MS colonization in this population, since 20 of 27 patients (74%) acquired MS from non-maternal sources and only 7 of 27 patients (26%) exclusively manifested maternal strains. Also, given the ability of non-maternal strains to colonize 74% of the cohort, it is plausible that these maternally infected patients would have acquired MS from other sources if maternal transmission had been prevented.
Conclusions
This study used AP-PCR to identify unique MS isolates within mother-child pairs in a S-ECC population. The unique isolates were then compared for intrafamiliar similarity by using PFGE, a highly reliable and reproducible genotyping method. PFGE banding patterns after SmaI digestion indicate:
MS genotypes that did not match maternal strains were identified in the majority of children (74%) within this S-ECC population.
Maternal transmission of MS occurred in 41% of mother/child pairs.
S-ECC siblings may be infected with similar MS strains independent of maternal transmission.
Additional studies are needed to validate whether preventive practices designed to minimize maternal MS transmission to a child can effectively prevent S-ECC, since MS may be acquired from other human sources.
Acknowledgments
This project received funding from a Faculty Development Grant from the Office of the Dean and the Department of Pediatric Dentistry, School of Dentistry, University of Alabama at Birmingham, and from NIDCR Grants T32 DE07026, DE016684 and DE 11147. We wish to thank the AAPD Foundation, Healthy Smiles Healthy Children, for the opportunity to present these findings at the 2007 AAPD Foundation Research Awards at Annual Session. We wish to acknowledge Dr. David Allison, UAB School of Public Health for providing statistical probability data.
References
- 1.Li Y, Caufield PW. The fidelity of initial acquisition of mutans streptococci by infants from their mothers. J Dent Res. 1995;74:681–685. doi: 10.1177/00220345950740020901. [DOI] [PubMed] [Google Scholar]
- 2.Kulkarni GV, Chan KH, Sandham HJ. An investigation into the use of restriction endonuclease analysis for the study of transmission of mutans streptococci. J Dent Res. 1989;68:1155–1161. doi: 10.1177/00220345890680070401. [DOI] [PubMed] [Google Scholar]
- 3.Emanuelsson IR, Li Y, Bratthall D. Genotyping shows different strains of mutans streptococci between father and child and within parental pairs in Swedish families. Oral Microbiol Immunol. 1998;13:271–277. doi: 10.1111/j.1399-302x.1998.tb00707.x. [DOI] [PubMed] [Google Scholar]
- 4.de Soet JJ, Bokhout B, Buijs JF, van Loveren C, de Graaff J, Prahl-Andersen B. Transmission of mutans streptococci between mothers and children with cleft lip and/or palate. Cleft Palate Craniofac J. 1998;35:460–464. doi: 10.1597/1545-1569_1998_035_0460_tomsbm_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 5.Emanuelsson IM, Wang XM. Demonstration of identical strains of mutans streptococci within Chinese families by genotyping. Eur J Oral Sci. 1998;106:788–794. doi: 10.1046/j.0909-8836.1998.eos106305.x. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Wang W, Caufield PW. The fidelity of mutans streptococci transmission and caries status correlate with breast-feeding experience among Chinese families. Caries Res. 2000;34:123–132. doi: 10.1159/000016579. [DOI] [PubMed] [Google Scholar]
- 7.Kozai K, Nakayama R, Tedjosasongko U, et al. Intrafamilial distribution of mutans streptococci in Japanese families and possibility of father-to-child transmission. Microbiol Immunol. 1999;43:99–106. doi: 10.1111/j.1348-0421.1999.tb02380.x. [DOI] [PubMed] [Google Scholar]
- 8.Tedjosasongko U, Kozai K. Initial acquisition and transmission of mutans streptococci in children at day nursery. J Dent Child. 2002;69:284–288. [PubMed] [Google Scholar]
- 9.Mattos-Graner RO, Li Y, Caufield PW, Duncan M, Smith DJ. Genotypic diversity of mutans streptococci in Brazilian nursery children suggests horizontal transmission. J Clin Microbiol. 2001;39:2313–2316. doi: 10.1128/JCM.39.6.2313-2316.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ersin NK, Kocabas EH, Alpoz AR, Uzel A. Transmission of Streptococcus mutans in a group of Turkish families. Oral Microbiol Immunol. 2004;19:408–410. doi: 10.1111/j.1399-302x.2004.00163.x. [DOI] [PubMed] [Google Scholar]
- 11.Whiley RA, Beighton D. Current classification of the oral streptococci. Oral Microbiol Immunol. 1998;13:195–216. doi: 10.1111/j.1399-302x.1998.tb00698.x. [DOI] [PubMed] [Google Scholar]
- 12.Nakano K, Lapirattanakul J, Nomura R, et al. Streptococcus mutans clonal variation revealed by multilocus sequence typing. J Clin Microbiol. 2007;45:2616–2625. doi: 10.1128/JCM.02343-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caufield PW, Walker TM. Genetic diversity within Streptococcus mutans evident from chromosomal DNA restriction fragment polymorphisms. J Clin Microbiol. 1989;27:274–278. doi: 10.1128/jcm.27.2.274-278.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jordan C, LeBlanc DJ. Influences of orthodontic appliances on oral populations of mutans streptococci. Oral Microbiol Immunol. 2002;17:65–71. doi: 10.1046/j.0902-0055.2001.00083.x. [DOI] [PubMed] [Google Scholar]
- 15.Roberts MC, Riedy CA, Coldwell SE, et al. How xylitol-containing products affect cariogenic bacteria. J Am Dent Assoc. 2002;133:435–441. doi: 10.14219/jada.archive.2002.0201. [DOI] [PubMed] [Google Scholar]
- 16.Gronroos L, Alaluusua S. Site-specific oral colonization of mutans streptococci detected by arbitrarily primed PCR fingerprinting. Caries Res. 2000;34:474–480. doi: 10.1159/000016626. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Caufield PW. Arbitrarily primed polymerase chain reaction fingerprinting for the genotypic identification of mutans streptococci from humans. Oral Microbiol Immunol. 1998;13:17–22. doi: 10.1111/j.1399-302x.1998.tb00745.x. [DOI] [PubMed] [Google Scholar]
- 18.Alaluusua S, Alaluusua SJ, Karjalainen J, et al. The demonstration by ribotyping of the stability of oral Streptococcus mutans infection over 5 to 7 years in children. Arch Oral Biol. 1994;39:467–471. doi: 10.1016/0003-9969(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 19.Gronroos L, Saarela M, Matto J, Tanner-Salo U, Vuorela A, Alaluusua S. Mutacin production by Streptococcus mutans may promote transmission of bacteria from mother to child. Infect Immun. 1998;66:2595–2600. doi: 10.1128/iai.66.6.2595-2600.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Becker MR, Paster BJ, Leys EJ, et al. Molecular analysis of bacterial species associated with childhood caries. J Clin Microbiol. 2002;40:1001–1009. doi: 10.1128/JCM.40.3.1001-1009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawamura Y, Hou XG, Sultana F, Miura H, Ezaki T. Determination of 16S rRNA sequences of Streptococcus mitis and Streptococcus gordonii and phylogenetic relationships among members of the genus Streptococcus. Int J Syst Bacteriol. 1995;45:406–408. doi: 10.1099/00207713-45-2-406. [DOI] [PubMed] [Google Scholar]
- 22.Saarela M, Hannula J, Matto J, Asikainen S, Alaluusua S. Typing of mutans streptococci by arbitrarily primed polymerase chain reaction. Arch Oral Biol. 1996;41:821–826. doi: 10.1016/s0003-9969(96)00049-0. [DOI] [PubMed] [Google Scholar]
- 23.Riley L. Molecular Epidemiology of Infectious Diseases. Washington, DC: ASM Press; 2004. [Google Scholar]
- 24.Tenover FC, Arbeit RD, Goering RV, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: Criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bert F, Branger C, Lambert-Zechovsky N. Pulsed-field gel electrophoresis is more discriminating than multilocus enzyme electrophoresis and random amplified polymorphic DNA analysis for typing pyogenic streptococci. Curr Microbiol. 1997;34:226–229. doi: 10.1007/s002849900173. [DOI] [PubMed] [Google Scholar]
- 26.Soll D, Pujol C, Lockhart S. Manual of Clinical Microbiology. 9th ed. Washington, DC: ASM Press; 2007. Laboratory procedures for the epidemiological analysis of micro-organisms. [Google Scholar]
- 27.Goering RV. The molecular epidemiology of nosocomial infection: Past, present, and future. Rev Med Microbiol. 2000;11:145–152. [Google Scholar]
- 28.Kiska DL, Macrina FL. Genetic analysis of fructan-hyperproducing strains of Streptococcus mutans. Infect Immun. 1994;62:2679–2686. doi: 10.1128/iai.62.7.2679-2686.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mineyama R, Yoshino S, Fukushima K. Genotypic analysis of strains of mutans streptococci by pulsed-field gel electrophoresis. Microbiol Res. 2004;159:181–186. doi: 10.1016/j.micres.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 30.Mineyama R, Yoshino S, Maeda N. DNA fingerprinting of isolates of Streptococcus mutans by pulsed-field gel electrophoresis. Microbiol Res. 2007;162:244–249. doi: 10.1016/j.micres.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 31.Motegi M, Takagi Y, Yonezawa H, et al. Assessment of genes associated with Streptococcus mutans biofilm morphology. Appl Environ Microbiol. 2006;72:6277–6287. doi: 10.1128/AEM.00614-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Connor EB, O’Riordan B, Morgan SM, et al. A lacticin 3147 enriched food ingredient reduces Streptococcus mutans isolated from the human oral cavity in saliva. J Appl Microbiol. 2006;100:1251–1260. doi: 10.1111/j.1365-2672.2006.02856.x. [DOI] [PubMed] [Google Scholar]
- 33.Okahashi N, Sasakawa C, Okada N, et al. Construction of NotI restriction map of the Streptococcus mutans genome. J Gen Microbiol. 1990;136:2217–2223. doi: 10.1099/00221287-136-11-2217. [DOI] [PubMed] [Google Scholar]
- 34.Tudor JJ, Marri L, Piggot PJ, Daneo-Moore L. Size of the Streptococcus mutans GS-5 chromosome as determined by pulsed-field gel electrophoresis. Infect Immun. 1990;58:838–840. doi: 10.1128/iai.58.3.838-840.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dobbs TE, Patel M, Wakes KB, Moser SA, Stamm AM, Hoesley CJ. Nosocomial spread of Enterococcus faecium resistant to vancomycin and linezolid in a tertiary care medical center. J Clin Microbiol. 2006;44:3368–3370. doi: 10.1128/JCM.00850-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson CN, Benjamin WH, Jr, Moser SA, et al. Genetic relatedness of levofloxacin-nonsusceptible Streptococcus pneumoniae isolates from North America. J Clin Microbiol. 2003;41:2458–2464. doi: 10.1128/JCM.41.6.2458-2464.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel M, Kumar RA, Stamm AM, Hoesley CJ, Moser SA, Waites KB. USA300 genotype community-associated methicillin-resistant Staphylococcus aureus as a cause of surgical site infections. J Clin Microbiol. 2007;45:3431–3433. doi: 10.1128/JCM.00902-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel M, Waites KB, Moser SA. Characterization of community-associated methicillin-resistant Staphylococcus aureus at a tertiary medical center. Southern Medical J. 2008;101:40–45. doi: 10.1097/SMJ.0b013e31815d3fce. [DOI] [PubMed] [Google Scholar]
- 39.Patel M, Waites KB, Moser SA, Cloud GA, Hoesley CJ. Prevalence of inducible clindamycin resistance among community- and hospital-associated Staphylococcus aureus isolates. J Clin Microbiol. 2006;44:2481–2484. doi: 10.1128/JCM.02582-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waites KB, Jones KE, Kim KH, et al. Dissemination of macrolide-resistant Streptococcus pneumoniae isolates containing both erm(B) and mef(A) in South Korea. J Clin Microbiol. 2003;41:5787–5791. doi: 10.1128/JCM.41.12.5787-5791.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.American Academy of Pediatric Dentistry. Definition of Early Childhood Caries (ECC) Reference Manual 2006–07. Pediatr Dent. 2006;28 suppl:13. [Google Scholar]
- 42.US Department of Health and Human Services. Oral Health in America: A Report of the Surgeon General. Rockville, Md: US Department of Health and Human Services, National Institute of Dental and Craniofacial Research, National Institutes of Health; 2000. [Google Scholar]
- 43.Duperon DE. Early childhood caries: A continuing dilemma. J Calif Dent Assoc. 1995;23:15–16. [PubMed] [Google Scholar]
- 44.Horowitz HS. Research issues in early childhood caries. Community Dent Oral Epidemiol. 1998;26:67–81. doi: 10.1111/j.1600-0528.1998.tb02096.x. [DOI] [PubMed] [Google Scholar]
- 45.Kaste LM, Drury TF, Horowitz AM, Beltran E. An evaluation of NHANES III estimates of early childhood caries. J Public Health Dent. 1999;59:198–200. doi: 10.1111/j.1752-7325.1999.tb03269.x. [DOI] [PubMed] [Google Scholar]
- 46.Ripa LW. Nursing caries: A comprehensive review. Pediatr Dent. 1988;10:268–282. [PubMed] [Google Scholar]
- 47.Proceedings. Conference on early childhood caries. Bethesda, M, Octorber 1997; Comm Dent Oral Epidemiol; 1998. [PubMed] [Google Scholar]
- 48.American Academy of Pediatrics. Oral Health Risk Assessment Timing and Establishment of the Dental Home. Pediatrics. 2003;111:1113–1116. doi: 10.1542/peds.111.5.1113. [DOI] [PubMed]
- 49.American Academy of Pediatric Dentistry. Policy on Early Childhood Caries (ECC) Classifications, Consequences, and Preventive Strategies. Pediatr Dent. 2006;28 suppl:31. [PubMed]
- 50.Syed SA, Loesche WJ. Survival of human dental plaque flora in various transport media. Appl Microbiol. 1972;24:638–644. doi: 10.1128/am.24.4.638-644.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Z, Saxena D, Caufield PW, Ge Y, Wang M, Li Y. Development of species-specific primers for detection of Streptococcus mutans in mixed bacterial samples. FEMS Microbiol Lett. 2007;272:154–162. doi: 10.1111/j.1574-6968.2007.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoshida A, Suzuki N, Nakano Y, Kawada M, Oho T, Koga T. Development of a 5’ nuclease-based real-time PCR assay for quantitative detection of cariogenic dental pathogens Streptococcus mutans and Streptococcus sobrinus. J Clin Microbiol. 2003;41:4438–4441. doi: 10.1128/JCM.41.9.4438-4441.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tan S, Featherstone J, Hoover C, Zhan L. Virulence Factors Related to Mutans Streptococci Transmission from Mothers to Children. San Francisco, Calif: University of California San Francisco; 2007. [Google Scholar]
- 54.de Soet JJ, Kreulen CM, Veerkamp JS, Bokhout B, van Loveren C, de Graaff J. Transmission of “Streptococcus mutans” in nursing bottle caries and cleft palate patients. Adv Exp Med Biol. 1997;418:181–183. doi: 10.1007/978-1-4899-1825-3_44. [DOI] [PubMed] [Google Scholar]
- 55.Lindquist B, Emilson CG. Colonization of Streptococcus mutans and Streptococcus sobrinus genotypes and caries development in children to mothers harboring both species. Caries Res. 2004;38:95–103. doi: 10.1159/000075932. [DOI] [PubMed] [Google Scholar]
- 56.Klein MI, Florio FM, Pereira AC, Honing JF, Goncalves RB. Longitudinal study of transmission, diversity, and stability of Streptococcus mutans and Streptococcus sobrinus genotypes in Brazilian nursery children. J Clin Microbiol. 2004;42:4620–4626. doi: 10.1128/JCM.42.10.4620-4626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kohler B, Lundberg AB, Birkhed D, Papapanou PN. Longitudinal study of intrafamilial mutans streptococci ribotypes. Eur J Oral Sci. 2003;111:383–389. doi: 10.1034/j.1600-0722.2003.00068.x. [DOI] [PubMed] [Google Scholar]
- 58.Caufield PW, Cutter GR, Dasanayake AP. Initial acquisition of mutans streptococci by infants: Evidence for a discrete window of infectivity. J Dent Res. 1993;72:37–45. doi: 10.1177/00220345930720010501. [DOI] [PubMed] [Google Scholar]
- 59.Mohan A, Morse DE, O’Sullivan DM, Tinanoff N. The relationship between bottle usage/content, age, and number of teeth with mutans streptococci colonization in 6- to 24-month-old children. Community Dent Oral Epidemiol. 1998;26:12–20. doi: 10.1111/j.1600-0528.1998.tb01918.x. [DOI] [PubMed] [Google Scholar]
- 60.Berkowitz RJ. Mutans streptococci: Acquisition and transmission. Pediatr Dent. 2006;28:106–109. discussion 92-8. [PubMed] [Google Scholar]
- 61.Law V, Seow WK, Townsend G. Factors influencing oral colonization of mutans streptococci in young children. Aust Dent J. 2007;52:93–100. doi: 10.1111/j.1834-7819.2007.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 62.Tiberia MJ, Milnes AR, Feigal RJ, et al. Risk factors for early childhood caries in Canadian preschool children seeking care. Pediatr Dent. 2007;29:201–208. [PubMed] [Google Scholar]
- 63.Berkowitz RJ, Turner J, Hughes C. Microbial characteristics of the human dental caries associated with prolonged bottle-feeding. Arch Oral Biol. 1984;29:949–951. doi: 10.1016/0003-9969(84)90097-9. [DOI] [PubMed] [Google Scholar]
- 64.Milnes AR, Bowden GH. The microflora associated with developing lesions of nursing caries. Caries Res. 1985;19:289–297. doi: 10.1159/000260858. [DOI] [PubMed] [Google Scholar]
- 65.van Houte J, Gibbs G, Butera C. Oral flora of children with “nursing bottle caries.”. J Dent Res. 1982;61:382–385. doi: 10.1177/00220345820610020201. [DOI] [PubMed] [Google Scholar]
- 66.Marchant S, Brailsford SR, Twomey AC, Roberts GJ, Beighton D. The predominant microflora of nursing caries lesions. Caries Res. 2001;35:397–406. doi: 10.1159/000047482. [DOI] [PubMed] [Google Scholar]
- 67.Alaluusua S, Matto J, Gronroos L, et al. Oral colonization by more than one clonal type of mutans streptococcus in children with nursing-bottle dental caries. Arch Oral Biol. 1996;41:167–173. doi: 10.1016/0003-9969(95)00111-5. [DOI] [PubMed] [Google Scholar]
- 68.Napimoga MH, Kamiya RU, Rosa RT, et al. Genotypic diversity and virulence traits of Streptococcus mutans in caries-free and caries-active individuals. J Med Microbiol. 2004;53:697–703. doi: 10.1099/jmm.0.05512-0. [DOI] [PubMed] [Google Scholar]
- 69.Soderling E, Isokangas P, Pienihakkinen K, Tenovuo J. Influence of maternal xylitol consumption on acquisition of mutans streptococci by infants. J Dent Res. 2000;79:882–887. doi: 10.1177/00220345000790031601. [DOI] [PubMed] [Google Scholar]
- 70.Soderling E, Isokangas P, Pienihakkinen K, Tenovuo J, Alanen P. Influence of maternal xylitol consumption on mother-child transmission of mutans streptococci: 6-year follow-up. Caries Res. 2001;35:173–177. doi: 10.1159/000047452. [DOI] [PubMed] [Google Scholar]
- 71.Kohler B, Bratthall D, Krasse B. Preventive measures in mothers influence the establishment of the bacterium Streptococcus mutans in their infants. Arch Oral Biol. 1983;28:225–231. doi: 10.1016/0003-9969(83)90151-6. [DOI] [PubMed] [Google Scholar]
- 72.Dasanayake AP, Caufield PW, Cutter GR, Stiles HM. Transmission of mutans streptococci to infants following short term application of an iodine-NaF solution to mothers’ dentition. Community Dent Oral Epidemiol. 1993;21:136–142. doi: 10.1111/j.1600-0528.1993.tb00738.x. [DOI] [PubMed] [Google Scholar]
- 73.Dasanayake AP, Wiener HW, Li Y, Vermund SV, Caufield PW. Lack of effect of chlorhexidine varnish on Streptococcus mutans transmission and caries in mothers and children. Caries Res. 2002;36:288–293. doi: 10.1159/000063922. [DOI] [PubMed] [Google Scholar]