Abstract
A validated method for quantifying methadone, 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine, cocaine, benzoylecgonine, 6-acetylmorphine, morphine, and codeine in human placenta by liquid chromatography–ion trap mass spectrometry is described. Specimens (1 g) were homogenized and subjected to solid-phase extraction. Chromatographic separation was performed on a Synergi Polar RP column with a gradient of 0.1% formic acid and acetonitrile. The method was linear from 10 to 2000 ng/g for methadone and 2.5 to 500 ng/g for other analytes. Limits of detection were 0.25–2.5 ng/g, imprecisions < 9.1%CV, analytical recoveries 84.4–113.3%, extraction efficiencies > 46%, matrix effects −8.0–129.9%, and process efficiencies 24.2–201.0%. Method applicability was demonstrated by analysis of five placenta specimens from opioid-dependent women receiving methadone pharmacotherapy, with methadone doses ranging from 65 to 95 mg on the day of delivery. These are the first data on placenta concentrations of methadone and metabolites after controlled drug administration. Detection of other common drugs of abuse in placenta will also improve our knowledge of the usefulness of this matrix for detecting in utero drug exposure and studying disposition of drugs in the maternal-fetal dyad.
Introduction
The abuse of opioids and other illicit drugs during pregnancy may be associated with serious obstetrical, fetal, and neonatal complications (1-3). Methadone maintenance is the only approved pharmacotherapy for treatment of opioid-dependent pregnant women in the U.S. (4,5). Methadone maintenance treatment reduces fetal exposure to illicit drugs and other maternal risk behaviors and also improves prenatal care and enhancement of neonatal outcomes (6-9). Furthermore, methadone maintenance results in better outcomes for the mother and child than methadone-assisted heroin withdrawal (4). Despite these clear benefits, extensive in utero exposure to opioids frequently leads to neonatal abstinence syndrome (NAS) (10,11). Unfortunately, many women also relapse and consume other licit and/or illicit drugs (2,3,10). Early evidence of in utero drug exposure could improve care of the mother and newborn and promote appropriate allocation of health care and mental health resources.
Analytical methods have been developed for different biological matrices from the mother and neonate, including urine (12-14), blood (15,16), oral fluid (17), hair (18,19), sweat (20), meconium (21-24), amniotic fluid, and umbilical cord tissue (25-27). A distinct advantage of monitoring placenta for detecting in utero drug exposure is its easy and non-invasive collection at the time of delivery, whereas meconium expulsion can be delayed for up to five days. Although neonatal hair is an excellent matrix for testing, mothers are frequently reluctant to cut infants’ hair, and it is sometimes difficult to obtain sufficient specimen. Although placenta performs critical functions supplying the fetus with nutrients, producing hormones, and exchanging wastes, it is discarded at the time of birth. Thus, this tissue is immediately available at birth for identifying fetal drug exposure.
The goal of our research was to develop and fully validate an analytical method for the simultaneous quantification of methadone, cocaine, 6-acetylmorphine (6AM), and metabolites in human placenta by ion trap liquid chromatography–mass spectrometry (LC–MS). The method will be applied to the analysis of specimens following controlled administration of methadone in opioid-dependent pregnant women, but it could also be applied to the analysis of other biological tissues. To our knowledge, these are the first data on the concentrations of methadone and metabolites in placenta of methadone-maintained women.
Experimental
Chemicals and reagents
Methadone, 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP), cocaine, benzoylecgonine (BE), morphine, codeine, and 6AM standards for calibration and methadone-d9, EDDP-d3, cocaine-d3, BE-d8, morphine-d6, codeine-d6, and 6AM-d6 internal standards (IStd) were obtained from Cerilliant™ (Round Rock, TX). Methadone, cocaine, and BE for preparing quality control samples (QC) were purchased from Lipomed (Cambridge, MA); morphine, codeine, and 6AM QC solutions were different lot numbers of materials from Cerilliant, and for EDDP, a different vial from the same lot number was employed. Reagent-grade formic and perchloric acids were from Sigma-Chemical (St. Louis, MO). Dichloromethane, acetonitrile, and ammonium hydroxide were supplied by J.T. Baker (Phillipsburg, NJ), and Strata™ XC cartridges (6 mL 100 mg) were from Phenomenex (Torrance, CA).
Preparation of solutions
Mixed standard and QC working solutions containing all analytes were prepared at a concentration of 20 μg/mL for methadone and 5 μg/mL for all other compounds by dilution of stock compounds with methanol. Blank homogenized placenta samples were fortified with 50 or 100 μL of working solutions at 0.2, 0.4, 0.8, 4, 10, and 20 μg/mL for methadone and 0.05, 0.1, 0.2, 1, 2.5, and 5 μg/mL for other analytes to prepare sevenpoint calibration curves. Three QC solutions for methadone were prepared at 30, 300, and 1500 ng/g by adding 75 μL of 0.4, 4, and 20 μg/mL working solutions, and three QC solutions for other analytes were prepared at 7.5, 75, and 375 ng/g by adding 75 μL of 0.1, 1, and 5 μg/mL working solutions. A mixed IStd solution was prepared at a concentration of 2 μg/mL for methadone and 0.5 μg/mL for other analytes.
Blank placenta specimens
Anonymized blank placenta specimens were generously donated by the Department of Pathology of the Johns Hopkins Bayview Medical Center. Specimens were stored at −20°C until analysis. To ensure the absence of analytes in these specimens, each placenta was tested with the method prior to use as blank matrix for the preparation of calibration curves and QC samples.
Specimen preparation
One gramof placenta was homogenized for approximately 1–2 min in 5 mL 0.1% perchloric acid in a blender (Tissue Tearor™, BioSpec Products, Bartlesville, OK). IStd solution (100 μL) was added, followed by centrifugation at 4000 rpm for 15 min. Solid-phase extraction cartridges were sequentially preconditioned with 2mL each methanol, water, and 0.1% perchloric acid. Supernatants were loaded and cartridges washed with 2 mL 0.1% perchloric acid and 2 mL methanol. After drying under vacuum for 15 min, analytes were eluted with 2 mL dichloromethane/acetonitrile/ammonium hydroxide (45:50:5, v/v/v), followed by evaporation with nitrogen in a TurboVap LV evaporator (Zymark, Hopkinton, MA). Extracts were reconstituted with 100 μL 0.1% formic acid, and 20 μL was injected onto the LC–MS.
LC–MS
A Surveyor HPLC system coupled to an LCQ Deca XP ion trap MS (ThermoFinnigan, San Jose, CA) was employed. Data were acquired and analyzed with Xcalibur™ software, version 1.2.
A Synergi Polar-RP column (75 mm × 2 mm, 4 μm) with a guard column of identical packing material (4.0 mm × 2 mm) (Phenomenex®) was employed for chromatographic separation at 30°C. Mobile phase was 0.1% formic acid (A) and acetonitrile (B) at a flow rate of 0.2 mL/min, applying the following gradient: 0% B for 0.5 min, increase to 85% over 9.5 min and hold for 0.5 min, followed by a return to initial conditions over 1 min, and hold for 3.5 min for equilibration of the column, yielding a total run time of 15 min. A divert valve was set to direct the LC flow to the MS from 0.2 to 12 min and to waste the remaining time.
The MS was operated with electrospray ionization in positive ion mode with the following optimized parameters: spray voltage, 4 kV; sheath gas flow rate settings, 50; auxiliary gas flow rate, 10; and transfer capillary temperature, 300°C. Precursor and product ion identifications and MSn optimization were established by direct infusion of individual analytes into the MS at a concentration of 0.1 μg/mL in methanol.
Method validation
The following validation parameters were determined: linearity, limit of quantification (LOQ), limit of detection (LOD), specificity, imprecision, analytical recovery, extraction efficiency, matrix effect, process efficiency, carryover, hydrolysis, dilution integrity, and stability. Linearity was evaluated for seven-point calibration curves from 10 to 2000 ng/g for methadone and 2.5 to 500 ng/g for other compounds on four different days. Acceptable linearity was achieved when the coefficient of determination was at least 0.99, quantification was within ± 20% of target concentrations at LOQ and ± 15% for other calibrators, and CV was < 20% and 15% for LOQ and other calibrators, respectively. LOD was empirically determined by fortifying placenta at decreasing analyte concentrations; acceptance criteria included signal-to-noise ratio for all ions of at least 3, retention times within ± 0.2 min from the average of all calibrator concentrations, and appropriate chromatography. LOQ was defined as the lowest concentration that met all LOD criteria and quantified with acceptable imprecision (CV < 20%) and analytical recovery (% of target concentration ± 20%).
Specificity of the method was evaluated for endogenous and exogenous interferences. Potential endogenous interferences in placenta tissue were evaluated in 10 different blank placentas. To study exogenous interferences, 39 common drugs of abuse and pharmaceuticals at 1000 ng/g were added to placenta samples fortified at the low QC concentration. The following drugs and metabolites were tested: buprenorphine, norbuprenorphine, THC, hydrocodone, hydromorphone, oxycodone, noroxycodone, oxymorphone, noroxymorphone, clonidine, ibuprofen, pentazocine, caffeine, diphenhydramine, chlorpheniramine, brompheniramine, aspirin, acetaminophen, PCP, nicotine, diazepam, lorazepam, oxazepam, alprazolam, bromazepam, clonazepam, flurazepam, nitrazepam, flunitrazepam, temazepam, nordiazepam, imipramine, clomipramine, fluoxetine, norfluoxetine, paroxetine, 7-aminoclonazepam, 7-aminoflunitrazepam, and 7-aminonitrazepam. In addition, placenta specimens from opioid-dependent women participating in controlled buprenorphine pharmacotherapy were also analyzed. Unfortunately, some women in this group also relapsed at times, providing the opportunity to examine these potential interferences and their metabolites as well.
Imprecision and analytical recovery, extraction efficiency, matrix effect, process efficiency, and stability were evaluated at low, medium, and high QC concentrations, 30, 300, and 1500 ng/g for methadone, and 7.5, 75, and 375 ng/g for other analytes. Intra-assay imprecision and analytical recovery were calculated by analysis of five replicates at three QC concentrations assayed in the same run. Interassay imprecision and analytical recovery were assessed by analysis of 20 replicates on a total of 4 different runs. Imprecision was determined by calculation of the coefficient of variation (%CV) following Krouwer and Rabinowitz’ recommendations (28,29). Acceptable imprecision was ≤ 15%, except at the LOQ, for which ≤ 20% was acceptable. Analytical recovery was expressed as a percentage of nominal concentration and was required to be ± 15% of target, except at the LOQ, which was required to be ± 20% of target.
Extraction efficiency, matrix effect, and process efficiency were assessed following recommendations of Matuszewski et al. (30). Extraction efficiency was determined by comparing average peak areas of blank placenta specimens fortified with analytes and IStd prior to extraction (n = 5) with those obtained in specimens fortified with the same compounds after extraction (n = 5). Matrix effect was determined by comparing average peak areas in 10 different blank placenta eluates fortified after extraction to those obtained when the same amount of analytes and IStd were added to a clean tube, evaporated, and reconstituted in 100 μL 0.1% formic acid (n = 10). Process efficiency was calculated by comparing average peak areas of specimens fortified prior to extraction to those prepared in formic acid as previously described (n = 5). Carryover was evaluated by injecting a blank placenta extract fortified with only IStd after a placenta specimen extract fortified at 4000 ng/g for methadone and 1000 ng/g for other compounds.
To document potential hydrolysis of cocaine and 6AM during sample preparation, blank placenta samples (n = 3) were fortified with only cocaine and 6AM at the high QC concentration, and percentages of BE and morphine formed were determined. Dilution integrity was examined by analyzing placenta samples at concentrations of 4000 ng/g for methadone and 1000 ng/g for others. Samples were diluted 1:4 with blank homogenized placenta, and concentrations were required to be within ± 15% of target to document dilutional integrity.
Stability of analytes in placenta matrix was evaluated (n = 5) under four different conditions: room temperature for 24 h, 4°C for 72 h, three freeze/thaw cycles, and on the autosampler (10°C) for 24 and 72 h. For autosampler stability, QC samples injected after 24 and 72 h storage were quantified with a calibration curve injected at time zero. QC samples under different conditions were quantified against freshly prepared calibration curve and QC samples.
Applicability of the method
To demonstrate the feasibility of the described methodology, five placenta specimens from opioid-dependent pregnant women were analyzed. Authentic specimens were collected as part of a large Institutional Review Board-approved clinical study to assess the efficacy and safety of methadone pharmacotherapy during pregnancy. Participants were recruited at the Center for Addiction and Pregnancy of the Johns Hopkins Bayview Medical Center. During pregnancy, women received daily methadone in conjunction with individual and group behavioral counseling, prenatal obstetrical care, and other support services. Methadone dose on the day of delivery ranged from 65 to 95 mg. Placenta specimens were collected at birth and stored at −20°C until analysis.
Results
Chromatographic conditions achieved sufficient resolution of all analytes within 10 min, with a total chromatographic run time of 15 min. Retention times for all analytes over 50 injections were < 0.74%CV (Table I).
Table I.
Mass Spectrometric Transitions at Specific Collision Energies (CE), Retention Times (Rt), and Imprecision (%CV) after 50 Injections for Morphine, Codeine, 6-Acetylmorphine (6AM), Benzoylecgonine (BE), Cocaine, 2-Ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP), Methadone, and Deuterated IStd
| Segment | Compound | Quantifier Transition (CE) | Qualifier Transitions (CE) | Rt (min) | %CV Rt (n = 50) |
|---|---|---|---|---|---|
| I | Morphine | 286.2 → 201.1 (37) | 286.2 → 229.0 (37) | 4.50 | 0.74 |
| 286.2 → 268.1 (37) | |||||
| Morphine-d6 | 292.3 → 201.1 (37) | – | 4.48 | 0.71 | |
| II | Codeine | 300.2 → 215.1 (37) | 300.2 → 243.0 (37) | 5.37 | 0.3 |
| 300.2 → 282.2 (37) | |||||
| Codeine-d6 | 306.2 → 218.1 (37) | – | 5.35 | 0.31 | |
| 6AM | 328.2 → 211.1 (38) | 328.2 → 268.1 (38) | 5.66 | 0.25 | |
| 328.2 → 193.1 (38) | |||||
| 6AM-d6 | 334.1 → 211.1 (38) | – | 5.64 | 0.25 | |
| BE | 290.1 → 168.1 (29) | 290.1 → 168.1 → 150.0 (32) | 6.10 | 0.20 | |
| 290.1 → 168.1 → 82.1 (32) | |||||
| BE-d8 | 298.2 → 171.1 (29) | – | 6.07 | 0.20 | |
| III | Cocaine | 304.1 → 182.1 (30) | 304.1 → 182.1 → 150.1 (32) | 6.92 | 0.22 |
| 304.1 → 182.1 → 82.1 (32) | |||||
| Cocaine-d3 | 307.1 → 185.1 (30) | – | 6.91 | 0.24 | |
| IV | EDDP | 278.2 → 249.1 (40) | 278.2 → 249.1 → 234.2 (34) | 8.61 | 0.50 |
| EDDP-d3 | 281.3 → 249.1 (40) | – | 8.60 | 0.49 | |
| Methadone | 310.1 → 265.0 (27) | 310.1 → 265.0 → 247.1 (31) | 8.72 | 0.62 | |
| 310.1 → 265.0 → 219.1 (31) | 8.69 | 0.65 | |||
| Methadone-d9 | 319.2 → 268.1 (27) | – |
The most abundant MS2 fragment was selected for quantification of all analytes. Two additional MS2 or MS3 fragments were monitored for identification purposes, except for EDDP, for which only one MS3 fragment was available. To ensure adequate quantification, the MS method was divided into four segments. Deuterated analogues were employed as IStd for each compound, helping to compensate for observed matrix effects. Table I shows quantification and qualification transitions for each analyte and IStd, as well as retention times.
Linearity was verified by least-squares regression with a 1/x weighting factor. Coefficients of determination were > 0.99 for all analytes. Calibrator concentrations were ± 15% of target with CV < 15%, except for the LOQ, with ± 20% of target and CV < 20%. Table II includes calibration parameters, dynamic linear range, and LOD for each analyte. Figure 1B shows chromatograms of analytes at the LOQ. The method was selective with no quantifiable peaks in 10 different blank placenta specimens. Moreover, blank placenta specimens fortified with analytes of interest at the low QC and with exogenous interferences quantified within 85–115% of target and CV < 15% for all analytes. Figure 1A shows the chromatogram obtained when a blank specimen was analyzed.
Table II.
Calibration Parameters (n = 4), Linear Dynamic Ranges, and Limits of Detection (LOD) and Quantification (LOQ) for Morphine, Codeine, 6-Acetylmorphine (6AM), Benzoylecgonine (BE), Cocaine, 2-Ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP), and Methadone
| Compound | LOD (ng/g) | LOQ(ng/g) | Range (ng/g) | Intercept | Slope | r2 |
|---|---|---|---|---|---|---|
| Morphine | 2.5 | 2.5 | 2.5–500 | 0.0098 ± 0.0028 | 0.0558 ± 0.0012 | 0.9969 ± 0.0021 |
| Codeine | 2.5 | 2.5 | 2.5–500 | −0.0083 ± 0.0101 | 0.0226 ± 0.0013 | 0.9959 ± 0.0025 |
| 6AM | 1 | 2.5 | 2.5–500 | 0.0052 ± 0.0074 | 0.0221 ± 0.0016 | 0.9954 ± 0.0020 |
| BE | 1 | 2.5 | 2.5–500 | 0.0092 ± 0.0151 | 0.0249 ± 0.0022 | 0.9976 ± 0.0023 |
| Cocaine | 0.5 | 2.5 | 2.5–500 | −0.0003 ± 0.0076 | 0.0208 ± 0.0006 | 0.9983 ± 0.0011 |
| EDDP | 0.25 | 2.5 | 2.5–500 | −0.0095 ± 0.0036 | 0.0232 ± 0.0020 | 0.9979 ± 0.0013 |
| Methadone | 0.25 | 10 | 10–2000 | −0.0190 ± 0.0054 | 0.0235 ± 0.0008 | 0.9961 ± 0.0030 |
Figure 1.
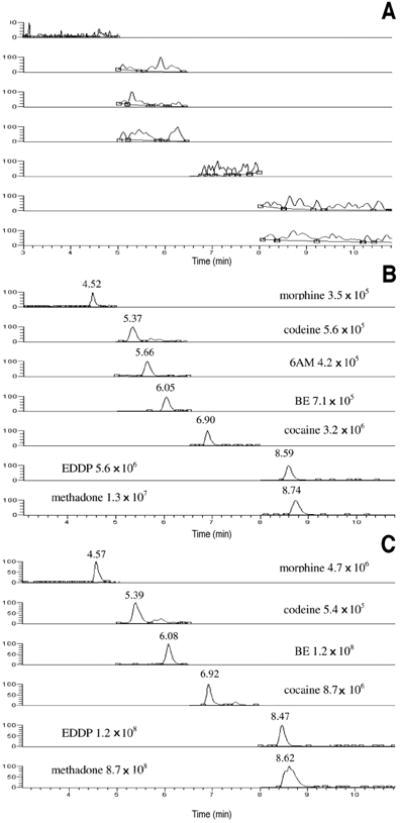
Chromatogram of the quantifier transitions for morphine, codeine, 6-acetylmorphine (6AM), benzoylecgonine (BE), cocaine, 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP), and methadone in extracted blank placenta (A), placenta fortified at the limit of quantification (B), and authentic placenta from an opioid-dependent woman maintained on daily methadone with a total cumulative dose of 11,760 mg (75 mg methadone the day of delivery) (C). The authentic placenta contained morphine (42.3 ng/g), codeine (2.6 ng/g), cocaine (7.9 ng/g), BE (496.3 ng/g), methadone (1346.3 ng/g), and EDDP (84.7 ng/g) indicating heroin or morphine, cocaine, and methadone exposure.
Intra- and interassay and total imprecision ranged from 2.7 to 8.7%, 0.0 to 9.1%, and 2.7 to 11.1%, respectively. Intraand interassay analytical recovery ranged from 84.4 to 113.3% and 92.2 to 110.2%, respectively (Table III).
Table III.
Imprecision and Analytical Recovery for Morphine, Codeine, 6-Acetylmorphine (6AM), Benzoylecgonine (BE), Cocaine, 2-Ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP), and Methadone
| Imprecision* |
Analytical Recovery† |
|||||
|---|---|---|---|---|---|---|
| Compound | QC | Intra-assay (n = 5) | Interassay (n = 20) | Total | Intra-assay (n = 5) | Interassay (n = 20) |
| Morphine | Low | 5.9 | 0.0 | 5.9 | 101.8 | 100.4 |
| Medium | 4.9 | 5.0 | 7.01 | 92.9 | 100.7 | |
| High | 7.9 | 4.1 | 8.9 | 92.8 | 97.1 | |
| Codeine | Low | 8.7 | 4.1 | 9.6 | 111.7 | 104.2 |
| Medium | 7.0 | 3.6 | 7.9 | 113.3 | 106.6 | |
| High | 5.7 | 6.8 | 8.8 | 112.1 | 103.5 | |
| 6-AM | Low | 7.8 | 7.2 | 10.6 | 89.9 | 98.1 |
| Medium | 4.5 | 0.0 | 4.5 | 111.5 | 110.2 | |
| High | 8.2 | 4.0 | 9.1 | 93.1 | 100.3 | |
| BE | Low | 8.6 | 5.1 | 10.0 | 92.4 | 97.9 |
| Medium | 6.2 | 6.7 | 9.1 | 110.7 | 101.2 | |
| High | 8.1 | 4.7 | 9.4 | 92.3 | 97.6 | |
| Cocaine | Low | 5.6 | 3.0 | 6.3 | 110.1 | 105.0 |
| Medium | 2.7 | 0.0 | 2.7 | 111.6 | 107.1 | |
| High | 4.7 | 4.1 | 6.2 | 109.7 | 102.7 | |
| EDDP | Low | 5.3 | 9.1 | 10.5 | 87.5 | 94.5 |
| Medium | 4.2 | 5.3 | 6.8 | 87.7 | 92.6 | |
| High | 5.0 | 9.8 | 11.1 | 84.4 | 92.2 | |
| Methadone | Low | 5.7 | 1.8 | 5.9 | 95.7 | 99.1 |
| Medium | 6.5 | 5.8 | 8.7 | 92.7 | 96.5 | |
| High | 4.9 | 6.3 | 8.0 | 112.1 | 100.2 | |
Imprecision is expressed as %CV.
Analytical recovery is expressed as % of target concentration.
Extraction efficiency was 65–90% for all analytes, except for BE that was ≥ 46.2%. With regard to matrix effects, suppression < 27.7% was found for 6AM, morphine, codeine, cocaine, and BE, and signal enhancement from 17.6 to 94.5% and 46.9 to 129.9% was noted for methadone and EDDP, respectively (Table IV). No quantifiable peaks were observed in a blank placenta specimen fortified with IStd, when injected after a specimen fortified at twice the upper LOQ, indicating no carryover. Hydrolysis of cocaine to BE and 6AM to morphine were 0.1% and 3.4%, respectively.
Table IV.
Extraction Efficiency (n = 5), Matrix Effect (n = 10), and Process Efficiency (n = 5) for Morphine, Codeine, 6-Acetylmorphine (6AM), Benzoylecgonine (BE), Cocaine, 2-Ethylidene-1,5-dimethyl-3,3-dip henylpyrrolidine (EDDP), and Methadone
| Compound | QC | Extraction Efficiency | Matrix Effect* |
Process Efficiency | Compound | Extraction Efficiency | Matrix Effect
|
Process Efficiency | ||
|---|---|---|---|---|---|---|---|---|---|---|
| % Effect | % CV | % Effect | % CV | |||||||
| Morphine | Low | 77.0 | −16.3 | 9.4 | 60.7 | Morphine-d6 | 75.5 | −8.3 | 3.9 | 67.2 |
| Medium | 79.9 | −8.0 | 9.5 | 71.8 | 85.1 | −13.3 | 8.0 | 71.7 | ||
| High | 80.4 | −9.5 | 6.5 | 70.9 | 76.6 | −7.4 | 7.4 | 69.1 | ||
| Codeine | Low | 90.2 | −9.3 | 9.9 | 80.9 | Codeine-d6 | 83.6 | −18.0 | 13.4 | 65.7 |
| Medium | 87.4 | −12.6 | 11.1 | 74.8 | 89.2 | −15.5 | 11.5 | 73.7 | ||
| High | 78.3 | −10.2 | 10.6 | 68.1 | 90.1 | −17.0 | 7.0 | 73.1 | ||
| 6AM | Low | 75.5 | −8.5 | 5.6 | 67.0 | 6AM-d6 | 74.7 | −9.6 | 9.2 | 63.3 |
| Medium | 88.4 | −22.3 | 9.4 | 66.0 | 81.6 | −24.6 | 9.1 | 57.0 | ||
| High | 82.0 | −14.8 | 5.9 | 67.3 | 84.4 | −16.4 | 9.1 | 58.0 | ||
| BE | Low | 51.8 | −27.7 | 6.0 | 24.2 | BE-d8 | 34.1 | −25.7 | 4.3 | 8.4 |
| Medium | 46.2 | −21.5 | 7.0 | 24.7 | 39.5 | −25.5 | 9.9 | 14.0 | ||
| High | 48.7 | −13.6 | 6.0 | 35.2 | 40.7 | −11.9 | 9.4 | 28.8 | ||
| Cocaine | Low | 86.6 | 8.7 | 11.8 | 97.3 | Cocaine-d3 | 81.1 | 11.2 | 4.9 | 92.4 |
| Medium | 80.4 | 22.5 | 8.3 | 103.0 | 84.8 | 14.6 | 10.3 | 99.4 | ||
| High | 82.0 | 5.3 | 7.0 | 87.3 | 80.3 | 2.5 | 3.4 | 82.8 | ||
| EDDP | Low | 71.0 | 129.9 | 5.4 | 201.0 | EDDP-d3 | 61.2 | 125.9 | 7.9 | 187.1 |
| Medium | 71.1 | 74.1 | 10.4 | 145.2 | 68.5 | 86.3 | 7.3 | 154.8 | ||
| High | 86.4 | 46.9 | 12.0 | 133.3 | 73.7 | 49.9 | 6.9 | 123.6 | ||
| Methadone | Low | 65.0 | 94.5 | 3.5 | 159.5 | Methadone-d9 | 61.0 | 93.3 | 6.1 | 154.3 |
| Medium | 60.6 | 81.0 | 10.3 | 141.5 | 63.4 | 64.3 | 6.8 | 126.7 | ||
| High | 67.7 | 17.6 | 5.7 | 85.3 | 69.7 | 14.7 | 6.8 | 84.4 | ||
Matrix effect is expressed as % effect, negative numbers indicating suppression and positive numbers enhancement. %CV of quality control (QC) samples in 10 different placentas are displayed for each analyte.
The method was shown to accurately quantify diluted specimens. Placenta samples fortified at 4000 ng/g for methadone and 1000 ng/g for other analytes were diluted with blank placenta tissue and quantified within 85–115% of target. Table V includes data from stability experiments. In all storage conditions, %loss was < 21%.
Table V.
Stability of Morphine, Codeine, 6-Acetylmorphine (6AM), Benzoylecgonine (BE), Cocaine, 2-Ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP), and Methadone in Placenta after Storage on the Autosampler (10°C) for 24 and 72 h, at Room Temperature for 24 h, 4°C for 72 h, and Three Freeze/Thaw Cycles, Expressed as % Loss Compared to Fresh Controls
| Compound | QC | Autosampler(10°C)
|
Room Temperature 24 h | 4°C for 72 h | Three Freeze/Thaw Cycles | |
|---|---|---|---|---|---|---|
| 24 h | 72 h | |||||
| Morphine | Low | −1.9 | −1.5 | −12.1 | −10.9 | 8.2 |
| Medium | 0.4 | 0.9 | −2.3 | −19.9 | −1.3 | |
| High | 1.4 | 5.7 | −9.3 | −14.6 | −3.7 | |
| Codeine | Low | −2.4 | 0.3 | −9.6 | −7.9 | −6.0 |
| Medium | 1.8 | 3.9 | 1.5 | −2.7 | 1.9 | |
| High | −7.2 | −2.1 | 0.3 | 4.1 | −5.6 | |
| 6AM | Low | 2.8 | −6.4 | −11.2 | −5.2 | −8.1 |
| Medium | 0.5 | 2.6 | 7.3 | −0.9 | −16.7 | |
| High | 2.4 | 1.1 | −3.3 | −11.2 | −6.5 | |
| BE | Low | 5.8 | −4.3 | 7.6 | −10.1 | −6.6 |
| Medium | 0.8 | 4.2 | −7.6 | −6.5 | −13.8 | |
| High | −2.5 | −1.2 | 14.7 | 2.0 | −14.4 | |
| Cocaine | Low | −0.6 | −0.6 | 8.4 | −1.3 | −4.7 |
| Medium | −2.6 | −1.9 | 4.6 | −0.6 | −11.4 | |
| High | 5.0 | 5.6 | 4.0 | −4.0 | −15.2 | |
| EDDP | Low | −0.4 | −1.1 | 8.5 | −21.0 | −19.3 |
| Medium | −1.6 | −0.2 | 2.4 | −5.4 | −9.0 | |
| High | 0.0 | −1.8 | −5.8 | −2.0 | −5.5 | |
| Methadone | Low | 2.8 | −3.3 | 8.9 | −11.5 | −18.0 |
| Medium | 11.2 | 0.6 | 14.4 | −2.5 | −13.9 | |
| High | 8.1 | −1.6 | 1.1 | −5.0 | −11.3 | |
Figure 1C includes the chromatogram of an authentic placenta specimen from a pregnant woman receiving daily methadone treatment with a total cumulative dose of 11,760 mg (75 mg methadone on the day of delivery). Besides methadone and EDDP, morphine, codeine, cocaine, and BE also were identified. Table VI shows the concentrations of methadone, cocaine, and opiates quantified in five authentic placenta specimens from pregnant women receiving daily methadone pharmacotherapy, demonstrating the applicability of the new assay.
Table VI.
Concentrations of Methadone, 2-Ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP), Cocaine, Benzoylecgonine (BE), Morphine, Benzoylecgonine (BE), Codeine, and 6-Acetylmorphine (6AM) in Placenta Specimens from Opioid-Dependent Women on Methadone Pharmacotherapy During Pregnancy*
| Case | Dose at Delivery (mg) | Methadone (ng/mg) | EDDP (ng/mg) | Cocaine (ng/mg) | BE (ng/mg) | Morphine (ng/mg) | Codeine (ng/mg) | 6AM (ng/mg) |
|---|---|---|---|---|---|---|---|---|
| 1 | 75 | 1346.3 | 84.7 | 7.9 | 486.3 | 41.3 | 2.6 | ND† |
| 2 | 95 | 1048.6 | 90.4 | ND | 2.32 | ND | ND | ND |
| 3 | 65 | 899.4 | 95.8 | ND | 6.8 | ND | ND | ND |
| 4 | 80 | 1909.0 | 142.2 | ND | ND | ND | ND | ND |
| 5 | 70 | 1296.8 | 85.1 | ND | ND | ND | ND | ND |
Methadone dose at delivery also is included.
None detected.
Discussion
We present the first validated methodology for simultaneous quantification of methadone, EDDP, cocaine, BE, 6-AM, morphine, and codeine in human placenta. Quantification of analytes employed the most prominent MS2 transition for all analytes. For identification purposes, two or more MS2 or MS3 fragments were monitored, except for EDDP, for which only a second MS3 fragment could be obtained. However, in all cases, the number of identification points was > 5, fulfilling the criteria of at least three identification points suggested in the Commission Decision of 12 August 2002, implementing Council Directive 96/23/EC (31).
The linear dynamic range for most analytes was from 2.5 to 500 ng/g. For methadone, an extended linear range (10 to 2000 ng/g) was needed because of daily methadone dosing. Thus, re-analysis of specimens because of high concentrations could be avoided, reducing costs and reporting times. The method was selective, as no quantifiable peaks were observed in blank placenta specimens from endogenous compounds in placenta (Figure 1A). Figure 1B shows chromatograms of a placenta specimen fortified with analytes of interest at the LOQ, demonstrating adequate sensitivity (signal-to-noise ratio > 10) and good peak shape for all analytes.
One of the difficulties of developing LC–MS methods is possible suppression or enhancement of signal, mainly caused by endogenous components in the matrix, especially when working with complex specimens (32-34). Strategies to overcome matrix effect are modifying chromatography to resolve interferences, improving specimen preparation to remove interferences, and including deuterated analogues as Istd to compensate for matrix effect (32,34). We evaluated different SPE cartridges and found the best results with Strata XC mixed-mode cartridges (reversed-phase and cation-exchange mechanisms). Placenta specimens were homogenized with 0.1% perchloric acid to assure retention of analytes by a cationexchange mechanism, thus allowing a more efficient methanolic washing step. For elution, a mixture of dichloromethane/acetonitrile in basic conditions provided a good compromise between extraction efficiency and matrix effect for most analytes (Table IV). With the selected conditions, extraction efficiency was > 46% for all analytes and matrix effect < 27% for most of them, which is similar to those reported by authors for these analytes in other matrices (35). For methadone and EDDP, an important enhancement of signal was found. However, inclusion of deuterated analogues for all analytes compensated for possible errors in imprecision and recovery, as these analytes best reproduce results for the nondeuterated analytes (Table III). The %CV in the 10 different placentas was < 12% in all cases, indicating that this effect was reproducible in the different blank authentic specimens analyzed.
A limitation of the method was partial hydrolysis of cocaine and 6AM. The % formation of BE from cocaine hydrolysis was < 0.1%, which is quite low. There would be little contribution to BE concentrations from high cocaine concentrations. However, 6AM hydrolysis was higher (3.4%), increasing morphine concentrations. As a consequence, it may not be possible to distinguish the source of morphine in placenta specimens unless 6AM is still measurable. During interpretation of opioid concentrations in placenta, the source of morphine could be from heroin, morphine or codeine.
Several analytical methods have been published for the simultaneous determination of methadone, cocaine and opioids in different matrices such as plasma or blood (36-38), urine (13,38), oral fluid (17,35,39), hair (40,41), and sweat patches (20). However, no validated analytical methods were described for the determination of these compounds in human placenta.
There are few data on expected concentrations of drugs in placenta after controlled administration of licit or illicit drugs to inform interpretation of results. Furthermore, the possible window of drug detection is unknown. Luck et al. (42) measured nicotine and cotinine concentrations in placenta and amniotic fluid from tobacco-smoking mothers and compared them to concentrations in maternal and fetal serum, showing linear correlations. Martin et al. (43) described NAS after maternal consumption of mate (traditional infusion from subtropical South America prepared by steeping yerba mate, Ilex paraguariensis, in hot water) and reported the presence of caffeine and theobromine in placenta among other maternal and neonatal matrices. Garcia-Algar et al. (44) reported detection of similar concentrations of alprazolam and α-hydroxyalprazolam in placenta and meconium after alprazolam doses two or three times those normally prescribed (1.5 mg/day). Also, similar concentrations of arecoline (main alkaloid of the areca nut) were found in placenta and meconium from six newborns exposed to this compound throughout pregnancy; arecoline was detected in five out of six placentas (9–14 ng/g) and in four meconium specimens (8–17 ng/g) (45). Ramsey et al. (46) characterized maternal and transplacental pharmacokinetics of azithromycin in term gravid women receiving 1 g of this drug at different time intervals before delivery, comparing azithromycin levels in several matrices including placenta. In only one report were methadone, EDDP, and EMDP quantified in human placenta by HPLC–UV, although no validation data were reported to evaluate the method (47). Thus, our new analytical method will permit determination of methadone, cocaine, and opiates in placenta and enable the collection of these important data in humans.
Although to date there are few clinical data, some analytes of interest were previously detected in rat placental tissue among other matrices. Peters et al. (48) measured methadone alone or in conjunction with its metabolites by scintillation and spectrometric methods, respectively. Srinivasan et al. (49) and Morishima et al. (50) developed analytical methods for the quantification of cocaine and metabolites by LC–MS–MS and GC–MS, respectively, in several rat specimens including placenta. These data inform our understanding of in utero drug exposure; however, extrapolation from animals to humans should be made with caution because of interspecies differences in placental anatomy and blood flow and maternal and fetal metabolism (51-55).
This method was applied to quantification of methadone, cocaine, and opiates and their metabolites in five authentic placenta specimens from women receiving methadone pharmacotherapy (Table VI). Determination of drug disposition in placenta from opioid-dependent women on methadone treatment will provide unique data on prenatal drug exposure. These data will help define the role of placenta in exposing the fetus to or protecting it from exogenous drug exposure and determine if placenta is a viable alternative matrix for in utero drug exposure monitoring.
Acknowledgments
The authors would like to thank Drs. Askin, March, and Ruan from the Department of Pathology of the Johns Hopkins Bayview Medical Center for providing us with human blank placenta tissue for the development of this method. Funding was from the National Institutes of Health, Intramural Research Program of the National Institute on Drug Abuse, and support for Ana de Castro, PhD, was from Direccion Xeral de Investigacion, Desenvolvemento e Innovacion (Conselleria de Innovacion e Industria), Galicia, Spain.
Footnotes
This analytical method was presented at the 2008 SOFT Annual Meeting, October 25–31, 2008, Phoenix, AZ.
References
- 1.Huestis MA, Choo RE. Drug abuse’s smallest victims: in utero drug exposure. Forensic Sci Int. 2002;128:20–30. doi: 10.1016/s0379-0738(02)00160-3. [DOI] [PubMed] [Google Scholar]
- 2.Luty J, Nikolaou V, Bearn J. Is opiate detoxification unsafe in pregnancy? J Subst Abuse Treat. 2003;24:363–367. doi: 10.1016/s0740-5472(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 3.Behnke M, Eyler FD. The consequences of prenatal substance use for the developing fetus, newborn, and young child. Int J Addict. 1993;28:1341–1391. doi: 10.3109/10826089309062191. [DOI] [PubMed] [Google Scholar]
- 4.Jones HE, O’Grady KE, Malfi D, Tuten M. Methadone maintenance vs. methadone taper during pregnancy: maternal and neonatal outcomes. Am J Addict. 2008;17:372–386. doi: 10.1080/10550490802266276. [DOI] [PubMed] [Google Scholar]
- 5.Winklbaur B, Kopf N, Ebner N, Jung E, Thau K, Fischer G. Treating pregnant women dependent on opioids is not the same as treating pregnancy and opioid dependence: a knowledge synthesis for better treatment for women and neonates. Addiction. 2008;103:1429–1440. doi: 10.1111/j.1360-0443.2008.02283.x. [DOI] [PubMed] [Google Scholar]
- 6.Finnegan LP. Treatment issues for opioid-dependent women during the perinatal period. J Psychoactive Drugs. 1991;23:191–201. doi: 10.1080/02791072.1991.10472236. [DOI] [PubMed] [Google Scholar]
- 7.Daley M, Argeriou M, McCarty D, Callahan JJ, Shepard DS, Williams CN. The impact of substance abuse treatment modality on birth weight and health care expenditures. J Psychoactive Drugs. 2001;33:57–66. doi: 10.1080/02791072.2001.10400469. [DOI] [PubMed] [Google Scholar]
- 8.McMurtrie C, Rosenberg KD, Kerker BD, Kan J, Graham EH. A unique drug treatment program for pregnant and postpartum substance-using women in New York City: results of a pilot project, 1990–1995. Am J Drug Alcohol Abuse. 1999;25:701–713. doi: 10.1081/ada-100101887. [DOI] [PubMed] [Google Scholar]
- 9.Svikis DS, Lee JH, Haug NA, Stitzer ML. Attendance incentives for outpatient treatment: effects in methadone-and non-methadone- maintained pregnant drug dependent women. Drug Alcohol Depend. 1997;48:33–41. doi: 10.1016/s0376-8716(97)00101-4. [DOI] [PubMed] [Google Scholar]
- 10.Jones HE, Velez ML, McCaul ME, Svikis DS. Special treatment issues for women. In: Strain EC, Stitzer ML, editors. Methadone Treatment for Opioid Dependence. The Johns Hopkins University Press; Baltimore, MD: 1999. pp. 251–280. [Google Scholar]
- 11.Burns L, Mattick RP. Using population data to examine the prevalence and correlates of neonatal abstinence syndrome. Drug and Alcohol Rev. 2007;26:487–492. doi: 10.1080/09595230701494416. [DOI] [PubMed] [Google Scholar]
- 12.Murphy CM, Huestis MA. LC–ESI-MS/MS analysis for the quantification of morphine, codeine, morphine-3-beta-D-glucuronide, morphine-6-beta-D-glucuronide, and codeine-6- beta-D-glucuronide in human urine. J Mass Spectrom. 2005;40:1412–1416. doi: 10.1002/jms.921. [DOI] [PubMed] [Google Scholar]
- 13.Dams R, Murphy CM, Lambert WE, Huestis MA. Urine drug testing for opioids, cocaine, and metabolites by direct injection liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2003;17:1665–1670. doi: 10.1002/rcm.1098. [DOI] [PubMed] [Google Scholar]
- 14.Wingert WE, Feldman MS, Kim MH, Noble L, Hand I, Yoon JJ. A comparison of meconium, maternal urine and neonatal urine for detection of maternal drug use during pregnancy. J Forensic Sci. 1994;39:150–158. [PubMed] [Google Scholar]
- 15.Knight EM, Hutchinson J, Edwards CH, Spurlock BG, Oyemade UJ, Johnson AA, West WL, Cole OJ, Westney LS, Westney OE, Manning M, Laryea H, Jones S. Relationships of serum illicit drug concentrations during pregnancy to maternal nutritional status. J Nutr. 1994;124(6 Suppl):973S–980S. doi: 10.1093/jn/124.suppl_6.973S. [DOI] [PubMed] [Google Scholar]
- 16.Doberczak TM, Kandall SR, Friedmann P. Relationships between maternal methadone dosage, maternal-neonatal methadone levels, and neonatal withdrawal. Obstet Gynecol. 1993;81:936–940. [PubMed] [Google Scholar]
- 17.Dams R, Murphy CM, Choo RE, Lambert WE, De Leenheer AP, Huestis MA. LC–atmospheric pressure chemical ionization-MS/MS analysis of multiple illicit drugs, methadone, and their metabolites in oral fluid following protein precipitation. Anal Chem. 2003;75:798–804. doi: 10.1021/ac026111t. [DOI] [PubMed] [Google Scholar]
- 18.Eliopoulos C, Klein J, Chitayat D, Greenwald M, Koren G. Nicotine and cotinine in maternal and neonatal hair as markers of gestational smoking. Clin Invest Med. 1996;19:231–242. [PubMed] [Google Scholar]
- 19.Garcia-Bournissen F, Rokach B, Karaskov T, Koren G. Methamphetamine detection in maternal and neonatal hair: implications for fetal safety. Arch Dis Child Fetal Neonatal Ed. 2007;92(5):F351–F355. doi: 10.1136/adc.2006.100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brunet BR, Barnes AJ, Scheidweiler KB, Mura P, Huestis MA. Development and validation of a solid-phase extraction gas chromatography–mass spectrometry method for the simultaneous quantification of methadone, heroin, cocaine and metabolites in sweat. Anal Bioanal Chem. 2008;392:115–127. doi: 10.1007/s00216-008-2228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostrea EM, Knapp DK, Romero AI, Montes M, Ostrea AR. Meconium analysis to assess fetal exposure to nicotine by active and passive maternal smoking. J Pediatr. 1994;124:471–476. doi: 10.1016/s0022-3476(94)70378-7. [DOI] [PubMed] [Google Scholar]
- 22.Coles R, Clements TT, Nelson GJ, McMillin GA, Urry FM. Simultaneous analysis of the Δ9-THC metabolites 11- nor-9-carboxy-Δ9-THC and 11-hydroxy-Δ9-THC in meconium by GC–MS. J Anal Toxicol. 2005;29:522–527. doi: 10.1093/jat/29.6.522. [DOI] [PubMed] [Google Scholar]
- 23.Gray TR, Shakleya DM, Huestis MA. Quantification of nicotine, cotinine, trans-3’-hydroxycotinine, nornicotine and norcotinine in human meconium by liquid chromatography tandem mass spectrometry. J Chromatogr B. 2008;863:107–114. doi: 10.1016/j.jchromb.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kacinko SL, Jones HE, Johnson RE, Choo RE, Huestis MA. Correlations of maternal buprenorphine dose, buprenorphine, and metabolite concentrations in meconium with neonatal outcomes. Clin Pharmacol Ther. 2008;84(5):604–612. doi: 10.1038/clpt.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore CM, Brown S, Negrusz A, Tebbett I, Meyer W, Jain L. Determination of cocaine and its major metabolite, benzoylecgonine, in amniotic fluid, umbilical cord blood, umbilical cord tissue, and neonatal urine: a case study. J Anal Toxicol. 1993;17:62. doi: 10.1093/jat/17.1.62. [DOI] [PubMed] [Google Scholar]
- 26.Winecker RE, Goldberger BA, Tebbett I, Behnke M, Eyler FD, Conlon M, Wobie K, Karlix J, Bertholf RL. Detection of cocaine and its metabolites in amniotic fluid and umbilical cord tissue. J Anal Toxicol. 1997;21:97–104. doi: 10.1093/jat/21.2.97. [DOI] [PubMed] [Google Scholar]
- 27.Montgomery D, Plate C, Alder SC, Jones M, Jones J, Christensen RD. Testing for fetal exposure to illicit drugs using umbilical cord tissue vs. meconium. J Perinatol. 2006;26:11–14. doi: 10.1038/sj.jp.7211416. [DOI] [PubMed] [Google Scholar]
- 28.Krouwer JS. Observations on comparisons of within-run and day-to-day precision. Clin Chem. 1981;27:202. [PubMed] [Google Scholar]
- 29.Krouwer JS, Rabinowitz R. How to improve estimates of imprecision. Clin Chem. 1984;30:290–292. [PubMed] [Google Scholar]
- 30.Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC–MS/MS. Anal Chem. 2003;75:3019–3030. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 31.Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off J Eur Communities. 2002;221:8–36. [Google Scholar]
- 32.Annesley TM. Ion suppression in mass spectrometry. Clin Chem. 2003;49:1041–1044. doi: 10.1373/49.7.1041. [DOI] [PubMed] [Google Scholar]
- 33.Dams R, Huestis MA, Lambert WE, Murphy CM. Matrix effect in bio-analysis of illicit drugs with LC–MS/MS: influence of ionization type, sample preparation, and biofluid. J Am Soc Mass Spectrom. 2003;14:1290–1294. doi: 10.1016/S1044-0305(03)00574-9. [DOI] [PubMed] [Google Scholar]
- 34.Taylor PJ. Matrix effects: the Achilles heel of quantitative high-performance liquid chromatography–electrospray-tandem mass spectrometry. Clin Biochem. 2005;38:328–334. doi: 10.1016/j.clinbiochem.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 35.Concheiro M, de Castro A, Quintela O, Cruz A, López- Rivadulla M. Determination of illicit and medicinal drugs and their metabolites in oral fluid and preserved oral fluid by liquid chromatography–tandem mass spectrometry. Anal Bioanal Chem. 2008;391:2329–2338. doi: 10.1007/s00216-008-2135-4. [DOI] [PubMed] [Google Scholar]
- 36.Rook EJ, Hillebrand MJ, Rosing H, van Ree JM, Beijnen JH. The quantitative analysis of heroin, methadone and their metabolites and the simultaneous detection of cocaine, acetylcodeine and their metabolites in human plasma by high-performance liquid chromatography coupled with tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;824:213–221. doi: 10.1016/j.jchromb.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 37.Gottardo R, Polettini A, Sorio D, Pascali JP, Bortolotti F, Liotta E, Tagliaro F. Capillary zone electrophoresis (CZE) coupled to time-of-flight mass spectrometry (TOF-MS) applied to the analysis of illicit and controlled drugs in blood. Electrophoresis. 2008;29:4078–4087. doi: 10.1002/elps.200800087. [DOI] [PubMed] [Google Scholar]
- 38.Bogusz MJ, Maier RD, Krüger KD, Kohls U. Determination of common drugs of abuse in body fluids using one isolation procedure and liquid chromatography–atmospheric-pressure chemical-ionization mass spectrometry. J Anal Toxicol. 1998;22:549–558. doi: 10.1093/jat/22.7.549. [DOI] [PubMed] [Google Scholar]
- 39.Allen KR, Azad R, Field HP, Blake DK. Replacement of immunoassay by LC tandem mass spectrometry for the routine measurement of drugs of abuse in oral fluid. Ann Clin Biochem. 2005;42:277–284. doi: 10.1258/0004563054255632. [DOI] [PubMed] [Google Scholar]
- 40.Moeller MR, Fey P, Wennig R. Simultaneous determination of drugs of abuse (opiates, cocaine and amphetamine) in human hair by GC/MS and its application to a methadone treatment program. Forensic Sci Int. 1993;63:185–206. doi: 10.1016/0379-0738(93)90273-d. [DOI] [PubMed] [Google Scholar]
- 41.Hegstad S, Khiabani HZ, Kristoffersen L, Kunoe N, Lobmaier PP, Christophersen AS. Drug screening of hair by liquid chromatography–tandem mass spectrometry. J Anal Toxicol. 2008;32:364–372. doi: 10.1093/jat/32.5.364. [DOI] [PubMed] [Google Scholar]
- 42.Luck W, Nau H, Hansen R, Steldinger R. Extent of nicotine and cotinine transfer to the human fetus, placenta and amniotic fluid of smoking mothers. Dev Pharmacol Ther. 1985;8:384–395. doi: 10.1159/000457063. [DOI] [PubMed] [Google Scholar]
- 43.Martin I, Lopez-Vilchez MA, Mur A, Garcia-Algar O, Rossi S, Marchei E, Pichini S. Neonatal withdrawal syndrome after chronic maternal drinking of mate. Ther Drug Monit. 2007;29:127–129. doi: 10.1097/FTD.0b013e31803257ed. [DOI] [PubMed] [Google Scholar]
- 44.Garcia-Algar O, Lopez-Vilchez MA, Martin I, Mur A, Pellegrini M, Pacifici R, Rossi S, Pichini S. Confirmation of gestational exposure to alprazolam by analysis of biological matrices in a newborn with neonatal sepsis. Clin Toxicol. 2007;45:295–298. doi: 10.1080/15563650601072191. [DOI] [PubMed] [Google Scholar]
- 45.Garcia-Algar O, Vall O, Alameda F, Puig C, Pellegrini M, Pacifici R, Pichini S. Prenatal exposure to arecoline (areca nut alkaloid) and birth outcomes. Arch Dis Child Fetal Neonatal Ed. 2005;90:F276–F277. doi: 10.1136/adc.2004.061325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramsey PS, Vaules MB, Vasdev GM, Andrews WW, Ramin KD. Maternal and transplacental pharmacokinetics of azithromycin. Am J Obstet Gynecol. 2003;188:714–718. doi: 10.1067/mob.2003.141. [DOI] [PubMed] [Google Scholar]
- 47.Nanovskaya TN, Deshmukh SV, Nekhayeva IA, Zharikova OL, Hankins GD, Ahmed MS. Methadone metabolism by human placenta. Biochem Pharmacol. 2004;68:583–591. doi: 10.1016/j.bcp.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 48.Peters MA, Turnbow M, Buchenauer D. The distribution of methadone in the nonpregnant, pregnant and fetal rat after acute methadone treatment. J Pharmacol Exp Ther. 1972;181:273–278. [PubMed] [Google Scholar]
- 49.Srinivasan K, Wang P, Eley AT, White CA, Bartlett MG. Liquid chromatography–tandem mass spectrometry analysis of cocaine and its metabolites from blood, amniotic fluid, placental and fetal tissues: study of the metabolism and distribution of cocaine in pregnant rats. J Chromatogr B. 2000;745:287–303. doi: 10.1016/s0378-4347(00)00283-8. [DOI] [PubMed] [Google Scholar]
- 50.Morishima HO, Whittington RA, Zhang Y, Cooper TB. The disposition of cocaethylene in rat maternal, placental, and fetal compartments. Am J Obstet Gynecol. 1999;180:1289–1296. doi: 10.1016/s0002-9378(99)70631-9. [DOI] [PubMed] [Google Scholar]
- 51.Syme MR, Paxton JW, Keelan JA. Drug transfer and metabolism by the human placenta. Clin Pharmacokinet. 2004;43:487–514. doi: 10.2165/00003088-200443080-00001. [DOI] [PubMed] [Google Scholar]
- 52.Enders AC, Blankenship TN. Comparative placental structure. Adv Drug Deliv Rev. 1999;38:3–15. doi: 10.1016/s0169-409x(99)00003-4. [DOI] [PubMed] [Google Scholar]
- 53.Mihaly GW, Morgan DJ. Placental drug transfer: effects of gestational age and species. Pharmacol Ther. 1983;23:253–266. doi: 10.1016/0163-7258(83)90015-3. [DOI] [PubMed] [Google Scholar]
- 54.van der Aa EM, Peereboom-Stegeman JH, Noordhoek J, Gribnau FW, Russel FG. Mechanisms of drug transfer across the human placenta. Pharm World Sci. 1998;20:139–148. doi: 10.1023/a:1008656928861. [DOI] [PubMed] [Google Scholar]
- 55.Schneider H. Placental transport function. Reprod Fertil Dev. 1991;3:345–353. doi: 10.1071/rd9910345. [DOI] [PubMed] [Google Scholar]


