Abstract
Steady state visual evoked potential (SSVEP) is the brain's natural electrical potential response for visual stimuli at specific frequencies. Using a visual stimulus flashing at some given frequency will entrain the SSVEP at the same frequency, thereby allowing determination of the subject's visual focus. The faster an SSVEP is identified, the higher information transmission rate the system achieves. Thus, an effective stimulus, defined as one with high success rate of eliciting SSVEP and high signal-noise ratio, is desired. Also, researchers observed that harmonic frequencies often appear in the SSVEP at a reduced magnitude. Are the harmonics in the SSVEP elicited by the fundamental stimulating frequency or by the artifacts of the stimuli? In this paper, we compare the SSVEP responses of three periodic stimuli: square wave (with different duty cycles), triangle wave, and sine wave to find an effective stimulus. We also demonstrate the connection between the strength of the harmonics in SSVEP and the type of stimulus.
1. Introduction
A brain-computer interface (BCI) translates brain activities into commands that control external devices. BCI research was initially motivated by the need of a new type of communication tools for paralyzed or elderly people [1, 2]. In recent years, many researchers have investigated BCI for computer gaming and entertainment applications [3–6], which makes noninvasive electroencephalography (EEG) a popular choice [7]. Three types of neuronal signals are most commonly used in EEG-based BCI systems: event-related potentials (ERP) [6, 8, 9], motor-imagery-related brain activity [10, 11], and steady state visual evoked potentials (SSVEP) [12–20].
Among these choices, SSVEP is viewed, by many researchers, as a promising electrophysiological source for BCI systems [21]. When looking at a light stimulus flickering at a given frequency, a user's SSVEP is entrained at the same frequency. Hence, by examining the EEG signal, a simple algorithm can identify the corresponding stimulus at which the subject is looking [22, 23]. It has been reported that SSVEPs can be elicited in the range of 4–100 Hz [24–26]. Although the strongest responses were observed in the range of 5–20 Hz, high-frequency stimuli (greater than 30 Hz) present minimal safety hazards due to photo-induced epileptic seizures [27].
Because EEG is always mixed with background noises, the efficacy of an SSVEP-based BCI system relies heavily on the signal-noise ratio. Intuitively, SSVEP will be detected much faster and with greater easy if the signal-noise ratio is high. The faster an SSVEP is identified, the faster a BCI system can correctly respond, hence a higher information throughput [28]. As it is currently unknown whether the choice of a square wave, triangle wave, or sine wave light signal affects the strength of the elicited SSVEP, these three waveforms (square wave with different duty cycles) were compared in Section 3 for their success rate in eliciting an SSVEP response. In addition, researchers have observed that a stimulus at frequency f can elicit SSVEP not only at f, but also at harmonics 2f, 3f, or sometimes even higher orders [29, 30]. This seems to suggest that harmonics may be used to detect the stimulating frequency. In order to take advantage of the harmonics in the design of a BCI system, the following question needs to be addressed: are SSVEP harmonics elicited by the fundamental frequency, that is, f, or by the artifacts of the stimulus?
From a signal perspective, the commonly used flickering stimulus is a periodic square wave with 50% duty cycle. Its spectrum contains nonzero Fourier components at ±(2k − 1)f, k = 1,2,…. Therefore, under a square wave stimulus, the cause of a 3f harmonic in SSVEP is unclear. In this paper, we explore the SSVEP responses of three periodic stimuli: square waves with different duty cycles, triangle waves, and sine waves. This group of waveforms provides us with the flexibility to adjust the strength of harmonics in a stimulus, hence allowing us to investigate the effects of artifact on SSVEPs.
The remainder of the paper is organized as follows. In Section 2, we describe the stimuli used in the experiments and the experimental setup. The results are given in Section 3. We conclude in Section 4.
2. Methodology
Three types of periodic stimuli were used in the experiments: square waves (with duty cycle τ ∈ (0,1)), triangle waves, and sine waves. If we define the relative strength of the kth harmonic frequency with respect to the fundamental frequency as r(k) = |G k/G 1|, where G 1 and G k are the Fourier coefficients for the fundamental frequency and the kth harmonic frequency, respectively, it is straightforward to show that r sine(k) = 1 for k = ±1 and 0 otherwise; r triangle(k) = [(π/2)sine(kπ/2)]2; r square(k) = |sine(kτ)/sine(τ)|. Clearly, in theory, there are no harmonic frequencies in a sine wave. In a triangle wave, the harmonic frequencies only exist for odd k. Its magnitude is proportional to 1/k 2. For a square wave with duty cycle τ = 0.5, there are also no harmonics for even k. The magnitude of odd harmonics is, however, proportional to 1/k, that is, stronger than that of a triangle wave. Note that the magnitude of harmonics of a square wave depends on its duty cycle, for example, r sine(2) > 0 for τ ≠ 0.5.
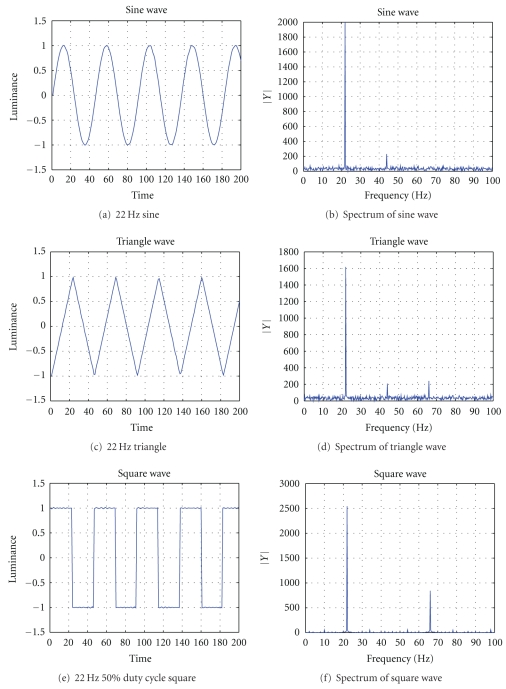
The above wave forms were rendered using an LED. In order to generate sine and triangle luminance signal, the LED needs to work in a linear (or close to linear) operating region. For the LED used in our experiments, a 3.25 V DC bias was applied. The resulting linear operating region is [3 V, 3.5 V]. The luminance of the LED was converted to an electrical signal using a Lutron LX-102 light meter. The output of the light meter was visualized using a Agilent 54621D oscilloscope and recorded using an integrated sound card. Figure 1 shows the luminance signal and its spectrum of the three waves. Note that the light signals were not perfectly sine, triangle, or square waves due to the nonlinearity of the LED. The artifacts in the sine and triangle waves were more significant than in the square wave. For example, 2f, which should not exist theoretically in sine or triangle waves, appeared in the measured luminance signal. Nevertheless, the amplitude of 2f in the measured sine or triangle luminance is roughly one order of magnitude smaller.
Figure 1.
(a), (c), and (e) are the luminance figures of an LED measured by a Lutron LX-102 light meter. Their corresponding frequency representations are given in (b), (d), and (f), respectively. The spectrum of the square wave strictly adheres to theory, that is, a peak demonstrated at fundamental frequency f as well as a peak at the 3f harmonic. The sine wave and the triangle wave do not. They have weak harmonics that should not exist at 2f. However, these harmonics should not affect the result, since their strength are one tenth that of the fundamental frequency.
Five subjects participated in this experiment. EEG was recorded with one channel over the occipital cortex at a sampling rate of 1 kHz, filtered by a 0.15 Hz high-pass filter and a 150 Hz low-pass filter. The distance between the LED and a subject was 50 cm. We examined stimuli of 11 Hz, 13 Hz, 15 Hz, 18 Hz, and 22 Hz and recorded the SSVEPs of square, triangle, and sine waves. Square waves were generated with 10%, 25%, and 50% duty cycles. In each recording session, the subject was told to look at the stimulus for 8 seconds and close their eyes for a rest period of a random duration from 10 to 20 seconds. The recorded data were discarded when muscle movements artifacts were significant.
3. Results
The primary research goals of these experiments are to find out what kind of waveforms is preferred for future SSVEP based systems. Table 1 reports the SSVEP results from all subjects. f is the fundamental frequency of the stimulus. “Valid trials” is the number of trials where the magnitude of FFT coefficients of SSVEP at f, 2f, or 3f are 50% greater than the baseline. “Total trials" is the number experiments in which a stimulus is presented to a user regardless of whether the SSVEP peaks were detected. “1f occurs, 2f occurs, and 3f occurs” are the number of observed SSVEP peaks at 1f, 2f and 3f, respectively.
Table 1.
Statistic of harmonics in SSVEP.
| 1f occurs | 2f occurs | 3f occurs | Valid trials | Total trials | |
|---|---|---|---|---|---|
| 11 Hz sine | 20 | 10 | 7 | 22 | 29 |
| 13 Hz sine | 22 | 9 | 2 | 22 | 30 |
| 15 Hz sine | 23 | 8 | 5 | 25 | 33 |
| 18 Hz sine | 23 | 9 | 6 | 25 | 34 |
| 22 Hz sine | 19 | 12 | 1 | 20 | 26 |
| 11 Hz triangle | 14 | 10 | 4 | 16 | 22 |
| 13 Hz triangle | 19 | 10 | 0 | 19 | 21 |
| 15 Hz triangle | 16 | 5 | 5 | 16 | 17 |
| 18 Hz triangle | 17 | 6 | 2 | 17 | 21 |
| 22 Hz triangle | 15 | 9 | 3 | 15 | 19 |
| 11 Hz 50% square | 20 | 11 | 15 | 20 | 21 |
| 13 Hz 50% square | 17 | 5 | 5 | 17 | 19 |
| 15 Hz 50% square | 17 | 9 | 8 | 16 | 17 |
| 18 Hz 50% square | 18 | 9 | 8 | 19 | 19 |
| 22 Hz 50% square | 18 | 9 | 8 | 18 | 19 |
| 11 Hz 25% square | 11 | 9 | 5 | 11 | 15 |
| 13 Hz 25% square | 17 | 8 | 6 | 18 | 18 |
| 15 Hz 25% square | 7 | 7 | 7 | 10 | 15 |
| 18 Hz 25% square | 17 | 14 | 10 | 18 | 18 |
| 22 Hz 25% square | 15 | 15 | 10 | 18 | 18 |
| 11 Hz 10% square | 8 | 9 | 4 | 12 | 17 |
| 13 Hz 10% square | 13 | 13 | 6 | 17 | 17 |
| 15 Hz 10% square | 15 | 12 | 11 | 19 | 20 |
| 18 Hz 10% square | 16 | 9 | 10 | 20 | 21 |
| 22 Hz 10% square | 13 | 6 | 6 | 15 | 19 |
Theoretically, SSVEP peaks appear at the stimulus frequency 1f and its harmonics 2f, 3f, and so forth. An SSVEP system has to use an recognizable 1f component to identify which frequency the subject is looking at, while it sometimes uses its harmonics to improve the accuracy. Thus, a valid trial without a 1f peak may not be acceptable in a real SSVEP system. So, we define a trial in which 1f occurs as an accurate trial, and the accuracy of a certain type of waveform of a certain frequency is Accuracywave,frequency = 1f occurs/Total trials. Figure 2 shows the accuracies of SSVEP trials driven by the three waves above.
Figure 2.
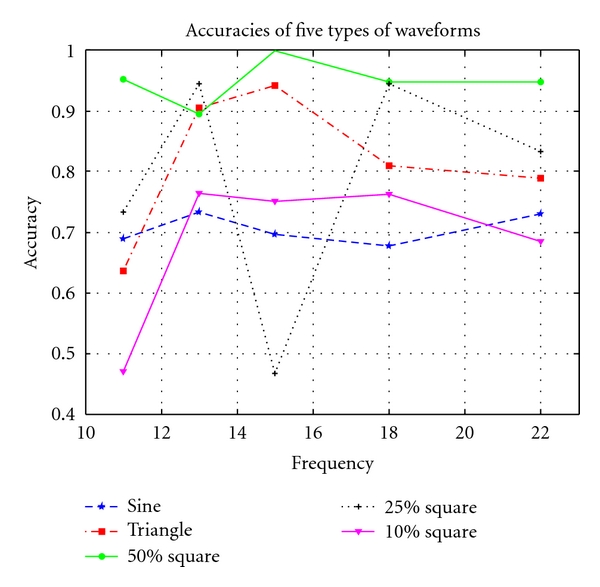
11, 13, 15, 18, and 22 Hz were used as the stimulus frequencies. The accuracies of the SSVEP experiments are computed with equation Accuracy = 1f occurs/Total trials.
We have the following observations.
A square waves with 50% duty cycle have a significantly higher accuracy than other stimuli in our experiment. As shown in Figure 2, the average accuracies (∑all frequenciesnumber of accurate trials/∑all frequenciestotal number of trials) of sine, triangle, and square waves with duty cycle 50%, 25%, and 10% were 70.4%, 81.0%, 94.7%, 79.8%, and 69.1%, respectively. Using statistic analysis techniques, we check if the performance of 50% square wave is better than that of triangle wave, which is intuitively the second best waveform as seen in Figure 2, with a significant level less than 0.05. (90/95) 50% square waves and (81/100) triangle waves evoked 1f SSVEP, thus . Since , we conclude that square waves with 50% duty cycle have a significantly higher accuracy than other stimuli in our experiment.
-
(ii)
A square wave has a higher success rate than sine or triangle waves in eliciting SSVEPs. In our experiments, the success rates (number of valid trials divided by the total number of trials) for sine, triangle, and square waves were 75.0%, 83.0%, and 90.8%, respectively.
-
(iii)
All three wave forms elicited 2f component in SSVEPs. In our experiments, the success rates for 2f component in SSVEP were 42.9% for sine waves, 48.2% for triangle waves, and 56.2% for square waves (averaged over all three duty cycles). Among the three duty cycles, 10%, 25%, and 50%, of the square wave, the 2f success rates were 43.0%, 70.7%, and 59.0%, respectively.
-
(iv)
A square wave has a significantly higher success rate than sine or triangle wave in eliciting 3f component in SSVEPs. In our experiments, the success rates for 3f component in SSVEP were 18.4% for sine waves, 14.0% for triangle waves, and 48.0% for square waves (averaged over all three duty cycles). Among the three duty cycles, 10%, 25%, and 50%, of the square wave, the 3f success rates were 44.6%, 50.7%, and 55.0%, respectively.
Although sine, triangle, and square waves with 50% duty cycle do not contain 2f component, they all elicited 2f in SSVEP with similar success rates. Square wave with 25% duty cycle contains a strong 2f component. Its 2f success rate is significantly higher (70.7%). This suggests that (1) the 2f component is primarily elicited by the fundamental frequency and (2) the artifacts in the stimuli increase the success rate of 2f in SSVEP. A similar observation is obtained for 3f. This seems to suggest that although the fundamental frequency can elicit harmonics (2f and 3f in our experiments) in SSVEP, the success rate of getting harmonics in SSVEPs is positively correlated with the strength of the artifacts in a stimulus.
4. Conclusion
Our results showed that the harmonics associated with SSVEP are elicited both by the fundamental frequency and the artifacts of the stimuli, with the 2f component mainly produced by the fundamental frequency and the 3f mainly by the artifacts of square waves. At the same time, SSVEP elicited with square waves do not always contain all the artifactual frequency components, for example, 3f, and SSVEP with sine waves may have 3f harmonics, which is not a part of the stimuli artifacts.
We also observed that square waves with 50% duty cycle have a significantly higher accuracy than other stimuli in our experiment. As a result, the use of square waves with 50% duty cycle is preferred if high 1f SSVEP eliciting rate is the goal, while sine waves for SSVEP simulation should be chosen if few harmonic artifacts are wanted.
Acknowledgments
This work was supported by the Department of Computer and Information Science and the School of Engineering, University of Mississippi. We would like to thank James Church, Joseph Smith, Xiaofei Nan, and Jing Ma for the help in data collection.
References
- 1.Hinterberger T, Kübler A, Kaiser J, Neumann N, Birbaumer N. A brain-computer interface (BCI) for the locked-in: comparison of different EEG classifications for the thought translation device. Clinical Neurophysiology. 2003;114(3):416–425. doi: 10.1016/s1388-2457(02)00411-x. [DOI] [PubMed] [Google Scholar]
- 2.Birbaumer N, Kübler A, Ghanayim N, et al. The thought translation device (TTD) for completely paralyzed patients. IEEE Transactions on Rehabilitation Engineering. 2000;8(2):190–193. doi: 10.1109/86.847812. [DOI] [PubMed] [Google Scholar]
- 3.Piccini L, Parini S, Maggi L, Andreoni G. A wearable home BCI system: preliminary results with SSVEP protocol. In: Proceedings of the 27th Annual International Conference of the Engineering in Medicine and Biology Society, (IEEE-EMBS 2005); September 2005; pp. 5384–5387. [DOI] [PubMed] [Google Scholar]
- 4.Lalor E, Kelly S, Finucane C, et al. Steady-state VEP-based brain-computer interface control in an immersive 3D gaming environment. Eurasip Journal on Applied Signal Processing. 2005;2005(19):3156–3164. [Google Scholar]
- 5.Jaganathan V, Srihari Mukesh TM, Reddy M. Design and implementation of high performance visual stimulator for brain computer interfaces. In: Proceedings of the 27th Annual International Conference of the Engineering in Medicine and Biology Society, (IEEE-EMBS 2005); September 2005; pp. 5381–5383. [DOI] [PubMed] [Google Scholar]
- 6.Pfurtscheller G, Neuper C, Guger C, et al. Current trends in Graz brain-computer interface (BCI) research. IEEE Transactions on Rehabilitation Engineering. 2000;8(2):216–219. doi: 10.1109/86.847821. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen K, Cabrera A, do Nascimento O. EEG based BCI—towards a better control. Brain-computer interface research at Aalborg University. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2006;14(2):202–204. doi: 10.1109/TNSRE.2006.875529. Article ID 1642769. [DOI] [PubMed] [Google Scholar]
- 8.Birbaumer N, Ghanayim N, Hinterberger T, et al. A spelling device for the paralysed. Nature. 1999;398(6725):297–298. doi: 10.1038/18581. [DOI] [PubMed] [Google Scholar]
- 9.Wolpaw J, Birbaumer N, McFarland D, Pfurtscheller G, Vaughan T. Brain-computer interfaces for communication and control. Clinical Neurophysiology. 2002;113(6):767–791. doi: 10.1016/s1388-2457(02)00057-3. [DOI] [PubMed] [Google Scholar]
- 10.Lee H, Cichocki A, Choi S. Nonnegative matrix factorization for motor imagery EEG classification. In: Proceedings of the 16th International Conference on Artificial Neural Networks, (ICANN 2006), vol. 4132; 2006; pp. 250–259. Lecture Notes in Computer Science. [Google Scholar]
- 11.Lou B, Hong B, Gao X, Gao S. Bipolar electrode selection for a motor imagery based brain-computer interface. Journal of Neural Engineering. 2008;5(3):342–349. doi: 10.1088/1741-2560/5/3/007. [DOI] [PubMed] [Google Scholar]
- 12.Middendorf M, McMillan G, Calhoun G, Jones KS. Brain-computer interfaces based on the steady-state visual-evoked response. IEEE Transactions on Rehabilitation Engineering. 2000;8(2):211–214. doi: 10.1109/86.847819. [DOI] [PubMed] [Google Scholar]
- 13.Kelly SP, Lalor EC, Finucane C, McDarby G, Reilly RB. Visual spatial attention control in an independent brain-computer interface. IEEE Transactions on Biomedical Engineering. 2005;52(9):1588–1596. doi: 10.1109/TBME.2005.851510. [DOI] [PubMed] [Google Scholar]
- 14.Kelly SP, Lalor EC, Reilly RB, Foxe JJ. Visual spatial attention tracking using high-density SSVEP data for independent brain-computer communication. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2005;13(2):172–178. doi: 10.1109/TNSRE.2005.847369. [DOI] [PubMed] [Google Scholar]
- 15.Muller-Putz GR, Scherer R, Brauneis C, Pfurtscheller G. Steady-state visual evoked potential (SSVEP)-based communication: impact of harmonic frequency components. Journal of Neural Engineering. 2005;2(4):123–130. doi: 10.1088/1741-2560/2/4/008. [DOI] [PubMed] [Google Scholar]
- 16.Cheng M, Gao X, Gao S, Xu D. Design and implementation of a brain-computer interface with high transfer rates. IEEE Transactions on Biomedical Engineering. 2002;49(10):1181–1186. doi: 10.1109/tbme.2002.803536. [DOI] [PubMed] [Google Scholar]
- 17.Friman O, Volosyak I, Gräser A. Multiple channel detection of steady-state visual evoked potentials for brain-computer interfaces. IEEE Transactions on Biomedical Engineering. 2007;54(4, article 20):742–750. doi: 10.1109/TBME.2006.889160. [DOI] [PubMed] [Google Scholar]
- 18.Sutter E. The brain response interface: communication through visually-induced electrical brain responses. Journal of Microcomputer Applications. 1992;15(1):31–45. [Google Scholar]
- 19.Mukesh T, Jaganathan V, Reddy M. A novel multiple frequency stimulation method for steady state VEP based brain computer interfaces. Physiological Measurement. 2006;27(1):61–71. doi: 10.1088/0967-3334/27/1/006. [DOI] [PubMed] [Google Scholar]
- 20.Teng F, Choong A, Gustafson S, Waddell D, Lawhead P, Chen Y. Steady state visual evoked potentials by dual sine waves. In: Proceedings of the Association for Computing Machinery Southeast Conference, (ACMSE); 2010. [Google Scholar]
- 21.Beverina F, Palmas G, Silvoni S, Piccione F, Giove S. User adaptive BCIs: SSVEP and P300 based interfaces. PsychNology Journal. 2003;1:331–354. [Google Scholar]
- 22.Morgan ST, Hansen JC, Hillyard SA. Selective attention to stimulus location modulates the steady-state visual evoked potential. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(10):4770–4774. doi: 10.1073/pnas.93.10.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller M, Hillyard S. Effects of spatial selective attention on the steady-state visual evoked potential in the 20–28 Hz range. Cognitive Brain Research. 1998;6(4):249–261. doi: 10.1016/s0926-6410(97)00036-0. [DOI] [PubMed] [Google Scholar]
- 24.Regan D. Human Brain Electrophysiology: Evoked Potentials and Evoked Magnetic Fields in Science and Medicine. New York, NY, USA: Elsevier; 1989. [Google Scholar]
- 25.Herrmann CS. Human EEG responses to 1–100 Hz flicker: resonance phenomena in visual cortex and their potential correlation to cognitive phenomena. Experimental Brain Research. 2001;137(3-4):346–353. doi: 10.1007/s002210100682. [DOI] [PubMed] [Google Scholar]
- 26.Garcia G, Ibanez D, Mihajlovic V, Chestakov D. Detection of high frequency steady state visual evoked potentials for brain-computer interfaces. In: Proceedings of the 17th European Signal Processing Conference (EUSIPCO 2009); 2009. [Google Scholar]
- 27.Pastor MA, Artieda J, Arbizu J, Valencia M, Masdeu JC. Human cerebral activation during steady-state visual-evoked responses. Journal of Neuroscience. 2003;23(37):11621–11627. doi: 10.1523/JNEUROSCI.23-37-11621.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allison B, Sugiarto I, Graimann B, Graser A. A display optimization in SSVEP BCIs. In: Proceedings of the Computer-Human Interaction, (CHI 2008); April 2008; Florence, Italy. [Google Scholar]
- 29.Bin G, Gao X, Yan Z, Hong B, Gao S. An online multi-channel SSVEP-based brain-computer interface using a canonical correlation analysis method. Journal of Neural Engineering. 2009;6(4):6 pages. doi: 10.1088/1741-2560/6/4/046002. Article ID 046002. [DOI] [PubMed] [Google Scholar]
- 30.Krusienski DJ, Allison BZ. Harmonic coupling of steady-state visual evoked potentials. In: Proceedings of the 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, (EMBS’08); August 2008; pp. 5037–5040. [DOI] [PubMed] [Google Scholar]