Abstract
Transforming acidic coiled coil 3 (TACC3) is a non-motor microtubule-associated protein (MAP) that is important for mitotic spindle stability and organization. The exact mechanism by which TACC3 acts at microtubules to stabilize the spindle has been unclear. However, several recent studies identified that the TACC3 complex at microtubules contains clathrin in addition to its previously identified binding partner, colonic and hepatic tumor overexpressed gene (ch-TOG). In this complex, phosphorylated TACC3 interacts directly with both ch-TOG and clathrin heavy chain, promoting accumulation of all complex members at the mitotic spindle. This complex stabilizes kinetochore fibers within the spindle by forming cross-bridges that link adjacent microtubules in these bundles. So, TACC3 is an adaptor that recruits ch-TOG and clathrin to mitotic microtubules, in an Aurora A kinase-regulated manner. In this mini-review we will describe the recent advances in the understanding of TACC 3 function and present a model that pulls together these new data with previous observations.
Key words: TACC3, clathrin, ch-TOG, kinetochore fiber, cross-linking
Introduction
At the onset of mitosis the microtubule network becomes highly dynamic, and undergoes a large-scale reorganization from the interphase array to form the mitotic spindle.1 This bipolar array of microtubules has the important job of segregating pairs of chromatids to ensure that each daughter cell receives one of each type of chromosome.1 In animal cells the centrosomes nucleate microtubule assembly early in mitosis.1 The less dynamic ‘minus’ ends of the microtubules are held at the centrosome while the highly dynamic ‘plus’ ends grow into the cytoplasm.1 Microtubules growing out from centrosomes capture chromosomes, which then become aligned at the center of the spindle as all kinetochores become attached. Once all sisters are aligned they separate and are pulled towards the spindle poles. These complicated processes are coordinated by a vast number of proteins located at centrosomes, on the microtubules, and at the kinetochores of the chromosomes.1 These include motor proteins which interact with microtubules and move along them in an ATP-dependent manner, such as kinesin 5 which contributes to spindle bipolarity and size by sliding apart overlapping microtubules originating from opposite spindle poles.2 Other microtubule-associated proteins lack motor activity but play roles in spindle stability. Over recent years many such MAPs have been identified, and their individual contributions to spindle assembly and function elucidated.2 One such protein is TACC3, a protein that is conserved across metazoans and down to yeast.3
Early work showed that TACC3 contributes to spindle stability and organization during mitosis, by acting both at centrosomes and microtubules.4,5 The precise mechanism by which TACC3 exerts its spindle-stabilizing function at microtubules was unclear, but now recent evidence has shown that TACC3 exists in a complex with ch-TOG and clathrin, that cross-links microtubules in kinetochore fibers. These fibers are bundles of microtubules that link the kinetochores to the spindle poles, and are more stable than the other types of microtubule in the spindle. These bundles have the strength to withstand the forces involved in maintaining chromosome alignment and separation, and when they are disrupted, cells have difficulty accomplishing both of these events.6
TACC3: A Conserved Mitotic Protein
The first hint that TACC domain-containing proteins had a role in mitosis came with the discovery of the Drosophila melanogaster protein D-TACC.7 This was found to be a centrosomal protein with some localization to spindle microtubules, with an important role in spindle assembly, organization and function.7 Like Drosophila, many species including Xenopus laevis and Caenhorabditis elegans contain only one TACC protein. However humans contain a family of three TACC proteins that all contain a C-terminal coiled coil domain, but vary extensively in the sequence of the N terminal part of the protein.3 Of these, TACC3 most closely resembles the TACC homologs in lower species because it functions during mitosis. In humans, TACC3 is expressed in certain proliferative tissues including testis, lung, spleen, bone marrow, thymus and peripheral blood leukocytes.3 This is similar in adult mice, but TACC3 is expressed in most tissue types during mouse embryo-genesis, and loss of TACC3 is lethal at the embryonic stage.3 These observations are consistent with a critical role for TACC3 in cell division. This review will focus on human proteins unless specified otherwise.
TACC3 expression is altered in some human tumor types.3,8 However, since it is upregulated in some cancers, while being downregulated in others, it is unclear whether this event contributes to the progression of the disease. On the other hand, there is good evidence to suggest that TACC3 promotes the resistance of tumors to challenges that could otherwise cause cell death. For example, tumor cells frequently have extra centrosomes, which could potentially cause multipolar spindles and multiplanar division, leading to cell death. TACC3 and ch-TOG are involved in clustering these centrosomes into two poles, allowing a somewhat ‘normal’ division along a single axis.9 Resistance of cancer cells to the spindle toxin paclitaxel also depends on TACC3, highlighting the potential clinical relevance of dysregulation of these proteins in tumors.8
Unraveling the Function of TACC3
In cells, TACC3 is localized to both centrosomes and spindle microtubules, but is not found at astral microtubules once the spindle has formed.5 It has a conserved function in spindle stability indicated by defects seen on depletion of the protein which include: disorganized spindles with lower microtubule density, chromosome misalignment and a longer duration of mitosis.10
Homologs of TACC3 and ch-TOG have a conserved interaction,3 and this had long been considered to be the functionally relevant complex. Although both proteins are found at centrosomes in human cells, their recruitment to these is independent of each other.5,10 Ch-TOG clearly has a critical TACC3-independent function at centrosomes where it promotes microtubule assembly and outgrowth,4,11,12 and it also contributes to spindle bipolarity.4,10,12 Now several recent studies have identified that TACC3 and ch-TOG on spindle microtubules are in complex with clathrin.13–16 These new data indicate that, at spindle fibers, TACC3 and ch-TOG function together with clathrin as part of a kinetochore fiber-stabilizing complex, rather than as a pair as previously thought.
During mitosis, clathrin has the same distribution on microtubules as TACC3 and ch-TOG.13 Like these proteins, clathrin was also found to stabilize kinetochore fibers.17 The observation that clathrin is only seen at microtubules during mitosis and not interphase suggests that spindle localization depends either upon a mitotic binding partner that is not available during interphase, or upon mitosis-specific modification of clathrin.17,18
The effect of clathrin depletion on mitosis is strikingly similar to that of TACC3 depletion.10,17 Indeed, the mitotic defects observed on depletion of either TACC3 or clathrin heavy chain (CHC) were no less severe than those seen on combined depletion of both proteins, confirming that they are part of the same functional complex at microtubules, which acts to stabilize kinetochore fibers.16
It was already known that TACC3 is required for localization of ch-TOG to the microtubules,10 but depletion of each complex member using RNA interference suggested that TACC3, CHC and ch-TOG each contribute somewhat to the localization of the other complex members to the spindle.10,13 Depletion of TACC3, and to a lesser extent depletion of ch-TOG, caused a reduction in the amount of clathrin at the spindle.13 Interestingly, CHC depletion using RNA interference resulted in a reduction in the amount of TACC3 at the spindle, even when the loss in microtubule density that occurs was accounted for.13–16 Depletion of CHC caused a loss of ch-TOG from microtubules,13,16 much like TACC3 depletion.10,13,16 From these data it seems that all three proteins help the others to become enriched at the spindle.
When GFP-TACC3 was overexpressed in cells, it recruited additional clathrin and ch-TOG to the spindle, while over-expression of CHC and ch-TOG had no effect on the other two.11,13 This suggests that TACC3 is the limiting factor that recruits the complex, but that the complex as a whole interacts more stably with the spindle than any individual components.13 This marks a distinction between recruitment and enrichment of complex members at spindles.
Co-immunoprecipitation of CHC and TACC3 was dependent on Aurora A kinase activity,14,16 and a direct interaction was found between TACC3 and CHC that is dependent upon phosphorylation of TACC3 by Aurora A kinase, at serine residue 558 and possibly serine 552.14,16 This phosphorylation event appears to be regulated by the Ran GTPase via Importin β.14 Treatment of cells with an Aurora A kinase inhibitor resulted in loss of TACC3 and clathrin from microtubules,13,19 suggesting that phosphorylation by Aurora A both facilitates initial recruitment of TACC3 to microtubules, and creates a binding site to facilitate recruitment of CHC.
Thus, there are two aspects to localization of this complex to microtubules; firstly TACC3-dependent recruitment to microtubules, and secondly the subsequent accumulation of the whole complex here.13 In this model the phosphorylation of TACC3 could cause a conformational change to permit microtubule binding, similar to the change reported for the Xenopus TACC3 homolog Maskin.20 In this way phosphorylation would contribute to initial recruitment. Accumulation would occur as the proteins interact with each other in a large complex that forms multiple contacts with microtubules. Phosphorylation would also contribute to this accumulation since the interaction of CHC with TACC3 is also phosphodependent.14,16 Further work is necessary to understand the fine detail of how the components are recruited and accumulated at spindle fibers. It is clear that the TACC3/ch-TOG/clathrin complex is required for stabilization of kinetochore fibers, so how does the complex do it?
Pulling It Together: The TACC3/ch-TOG/Clathrin Complex is an Inter-Microtubule Bridge in Kinetochore Fibers
We are beginning to understand how kinetochore fibers can be stabilized. It had been noted for many years that in electron micrographs, electron-dense bridges appear to link adjacent microtubules within these fibers, raising the possibility that these bridges act to stabilize the fibers.21 It is already appreciated that some proteins form bridges between antiparallel microtubules at the midzone of the spindle where the microtubules nucleated from the opposite poles overlap.2 But little was known about the proteins which bridge between parallel microtubules in the spindle.
Clathrin exists as a triskelion formed by association of three CHCs via their C termini, with each CHC forming one of the legs, each of which is tightly associated with a clathrin light chain (CLC).22 The globular N-terminal domain or ‘foot’ is located at the end of each CHC leg. This foot domain is needed for localization of clathrin to the spindle.17,23 This together with the kinetochore fiber stabilizing function of clathrin raised the intriguing possibility that clathrin may be an important component of one such inter-microtubule bridge, in which it could link adjacent microtubules via its connected ‘feet’.13,17 Interestingly, a CHC gene fusion that forms dimers rather than trimers that was identified in human tumors can rescue the mitotic function of clathrin in cells depleted of CHC,24 while mutants that remain monomeric cannot.23 Thus, multimerization of CHCs is essential, but it does not have to be a trimer to accomplish kinetochore fiber stabilization.
There is now ultrastructural evidence to support this idea of clathrin as an inter-microtubule bridge. When CHC was depleted there were fewer bridges within kinetochore fibers, and these microtubule bundles contained fewer total microtubules, and a lower density of microtubules.13 Immunogold labelling with an anti-clathrin antibody revealed that gold particles were frequently found in between pairs of closely located microtubules, and were often seen at inter-microtubule bridges, confirming clathrin as a bridge component.13 Furthermore, the bridges that were lost on CHC depletion were a specific subtype of short-length bridges.13
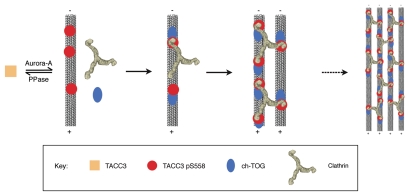
TACC3 depletion also caused loss of the same class of short bridges.13 Together this suggests that, regardless of the order of recruitment, the mode of action of this TACC3/ch-TOG/clathrin complex is to stabilize kinetochore fibers by physically bridging between adjacent microtubules. This model is represented in Figure 1. In addition to physical cross-bracing of microtubules that make up the spindle, the TACC3/ch-TOG/clathrin bridges also appear to protect the fiber from microtubule loss.13 This could occur as a consequence of the cross-bracing activity and/or via the activity of ch-TOG.13,16 It is known that in vitro the Xenopus ch-TOG homolog, XMAP215, acts as a processive tubulin polymerase, employing both a microtubule binding site and a variable number of tubulin dimer binding sites to incorporate new dimers into the end of a microtubule.25 In cells, ch-TOG promotes growth of microtubule plus ends by antagonizing mitotic centromere-associated kinesin (MCAK).12 MCAK promotes phases of microtubule depolymerization, known as ‘catastrophes’. This anti-catastrophe activity of ch-TOG may be mediated by its polymerase activity balancing MCAK activity, or possibly by physically protecting plus ends against MCAK.12 As some groups suggest, there could therefore be an additional aspect to the stabilizing function of the TACC3/ch-TOG/clathrin complex, perhaps by co-ordinating or increasing the ch-TOG anti-catastrophe activity down the microtubules.13,16 Thus, the role of TACC3 in mitosis is as a regulated adaptor that can integrate several mitotic proteins into a complex on micro-tubules which holds kinetochore fibers together.
Figure 1.
Model of recruitment and inter-microtubule cross-linking by the TACC3/ch-TOG/clathrin complex. Phosphorylation of TACC3 by Aurora A kinase enables it to bind to microtubules, where it can recruit clathrin and ch-TOG. Clathrin triskelia could interact with multiple TACC3 molecules on microtubules, including those on adjacent microtubules, in which case a bridge would be formed. Ultimately many bridges would form between closely located microtubules, helping to stabilize microtubule bundles in kinetochore fibers. As the multiple contacts form in these complexes, all the individual components would interact more stably with the spindle, causing the accumulation we observe by immunofluorescence.
Remaining Questions
Work published over the past year has accelerated our understanding of TACC3, ch-TOG and clathrin function at the mitotic spindle.13–16 However there are, as always, some unresolved questions. Clearly this complex is found at kinetochore microtubules, but whether it is also found at interpolar microtubules, and if not, how specificity for kinetochore micro-tubules would be achieved is not known.
It is also not clear how TACC3 interacts with microtubules. Maskin is able to interact directly with microtubules,26 but the human protein does not appear to do so.16 In cells, recruitment of both TACC3 and Maskin to centrosomes and spindles requires phosphorylation by Aurora A kinase.3 Together this led some groups to propose that clathrin is actually the recruitment factor for TACC3, and by extension ch-TOG, to the spindle.14–16 In this scheme it is not clear how clathrin would be recruited to microtubules. Indeed since CHC does not interact directly with microtubules and in cells only a small proportion of clathrin is found at the spindle, while the rest is found in the cytoplasm and at clathrin coated structures on membranes, clathrin is a very unlikely candidate as a spindle recruitment factor.13 Interestingly, the part of CHC that binds to phospho-TACC3 includes part of CHC repeat 0 that was identified previously as critical for recruitment of CHC to the spindle.16,18 This is consistent with the unified model presented here (Fig. 1), since if clathrin itself were the spindle recruitment factor, it would not need to be able to bind TACC3 in order to become localized to the spindle.
There are also some outstanding questions about the bridging function of this complex and the function of TACC3 at the spindle. While the trimeric structure of clathrin makes it an attractive candidate as the central bridging portion of the complex, TACC3 is also known to dimerize,27 raising the possibility of an alternative conformation in which dimerized TACC3 could bridge between adjacent microtubules by interacting with a clathrin molecule on each microtubule. Interestingly it has been shown in fission yeast that the TACC3 homolog, Alp7, cross-links both antiparallel and parallel microtubules.28
We are lacking complete structural information for all complex components. A structure has been determined for the single TOG domain from the C. elegans ch-TOG homolog, Zyg9, however the structure of the whole human ch-TOG, particularly when bound to microtubules, is unknown.29 An atomic model for clathrin has been described,22 but there is currently no structural information for TACC3. The configuration of bridges shown in Figure 1 takes into account the known spacing of clathrin triskelia and microtubules.13 Future structural studies may well refine this model. Furthermore, if clathrin is indeed the central bridging portion of the complex, as has been suggested,13 then does it exist as a single triskelion? CHC mutants that are predicted to be unable to form lattices can function in mitosis,23,24 but whether bridges are interconnected needs to be explored further.
One study suggested that G2 and S-phase expressed 1 (GTSE1) may also be part of the TACC3/ch-TOG/clathrin complex.15 Whether GTSE1 is an important part of this bridging complex is unclear at this stage. It will be interesting to see whether loss of GTSE1 affects inter-microtubule bridges.
It also remains unclear to what extent TACC3 functions at centrosomes. TACC3 has been reported to localize to centrosomes, but it is not required for ch-TOG recruitment here, suggesting that these proteins may not interact at centrosomes in human cells.5,10 Clathrin has no prominent localization at centrosomes.13,17–19,23,24 Certainly, ch-TOG acts independently of TACC3 at centrosomes to promote microtubule assembly.4 Taken together this suggests that ch-TOG functions alone at centrosomes, although the possibility that the other TACC proteins could be involved cannot be excluded.10 Once short microtubules are generated from the centrosomes by ch-TOG, TACC3 seems to aid their stability,4,11,12 which we predict is as part of the TACC3/ch-TOG/clathrin complex described in this review.
While the TACC3/ch-TOG interaction is conserved across many species, currently it has only been shown in humans and Xenopus that clathrin is also contained within this complex.13–16 Further work is needed to determine whether this is conserved, or if the function of these proteins may be clathrin-independent in some species. In particular, in some species it seems that TACC3 homologs are more involved in centrosome-based function, such as in Drosophila, where D-TACC is required for localization of the ch-TOG homolog, Msps, to centrosomes.30
Studying this complex is not without complications. Clathrin has a fundamentally important role during interphase, when clathrin mediated membrane traffic is critical to many cellular functions, making knockout mice or cell lines an unrealistic prospect.22 TACC3 knockout is also lethal before birth.3 With assembly and organization of the mitotic spindle being so tightly regulated, it is difficult to tease out the functions of any critical spindle protein by RNA interference, since any defects occurring early on in the process may mask defects at later stages. This is a challenge in cell cycle research generally, but newer methods of acute inhibition or inactivation of a protein will enable us to analyse effects on a shorter time scale.
Summary
In this short review, we have drawn together recent work on the function of TACC3 in mitosis. We have seen that TACC3 forms a complex that pulls together microtubules. These cross-linking bridges help to stabilize the kinetochore fibers that control chromosome dynamics. In this role, TACC3 seems to act as a regulated adaptor, recruiting other proteins to the microtubules once it is phosphorylated by Aurora A kinase. Once this initial recruitment is achieved, formation of multiple bridges may facilitate accumulation of all complex members at microtubules. The TACC3/ch-TOG/clathrin bridges that we have discussed represent a subset of inter-microtubule bridges. The identification of the other inter-microtubule bridges is an exciting prospect indeed for those interested in the function of the mitotic spindle.
Acknowledgments
We would like to thank members of the Royle lab for useful discussion and we apologize to those whose work we could not discuss due to space constraints. Our work is supported by a Career establishment Award from Cancer Research UK (C25425/A8722).
Abbreviations
- CHC
clathrin heavy chain
- CLC
clathrin light chain
- TACC3
transforming acidic coiled coil 3
- ch-TOG
colonic and hepatic tumor overexpressed gene
- XMAP215
Xenopus microtubule associated protein 215 kDa
- Msps
minispindles
- Zyg9
ZYGote defective: embryonic lethal family member 9
- MAP
microtubule associated protein
- MCAK
mitotic centromere-associated kinesin
- GTSE1
G2 and S-phase expressed 1
References
- 1.O'Connell CB, Khodjakov AL. Cooperative mechanisms of mitotic spindle formation. J Cell Sci. 2007;120:1717–1722. doi: 10.1242/jcs.03442. [DOI] [PubMed] [Google Scholar]
- 2.Peterman EJ, Scholey JM. Mitotic microtubule cross-linkers: insights from mechanistic studies. Curr Biol. 2009;19:1089–1094. doi: 10.1016/j.cub.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 3.Peset I, Vernos I. The TACC proteins: TACC-ling microtubule dynamics and centrosome function. Trends Cell Biol. 2008;18:379–388. doi: 10.1016/j.tcb.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Barr AR, Gergely F. MCAK-independent functions of ch-Tog/XMAP215 in microtubule plus-end dynamics. Mol Cell Biol. 2008;28:7199–7211. doi: 10.1128/MCB.01040-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gergely F, Karlsson C, Still I, Cowell J, Kilmartin J, Raff JW. The TACC domain identifies a family of centrosomal proteins that can interact with microtubules. Proc Natl Acad Sci USA. 2000;97:14352–14357. doi: 10.1073/pnas.97.26.14352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rieder CL. Kinetochore fiber formation in animal somatic cells: dueling mechanisms come to a draw. Chromosoma. 2005;114:310–318. doi: 10.1007/s00412-005-0028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gergely F, Kidd D, Jeffers K, Wakefield JG, Raff JW. D-TACC: a novel centrosomal protein required for normal spindle function in the early Drosophila embryo. EMBO J. 2000;19:241–252. doi: 10.1093/emboj/19.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt S, Schneider L, Essmann F, Cirstea IC, Kuck F, Kletke A, et al. The centrosomal protein TACC3 controls paclitaxel sensitivity by modulating a premature senescence program. Oncogene. 2010;29:6184–6192. doi: 10.1038/onc.2010.354. [DOI] [PubMed] [Google Scholar]
- 9.Fielding AB, Lim S, Montgomery K, Dobreva I, Dedhar S. A critical role of integrin-linked kinase, ch-TOG and TACC3 in centrosome clustering in cancer cells. Oncogene. 2011;30:521–534. doi: 10.1038/onc.2010.431. [DOI] [PubMed] [Google Scholar]
- 10.Gergely F, Draviam VM, Raff JW. The ch-TOG/ XMAP215 protein is essential for spindle pole organization in human somatic cells. Genes Dev. 2003;17:336–341. doi: 10.1101/gad.245603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cassimeris L, Becker B, Carney B. TOGp regulates microtubule assembly and density during mitosis and contributes to chromosome directional instability. Cell Motil Cytoskeleton. 2009;66:535–545. doi: 10.1002/cm.20359. [DOI] [PubMed] [Google Scholar]
- 12.Holmfeldt P, Stenmark S, Gullberg M. Differential functional interplay of TOGp/XMAP215 and the KinI kinesin MCAK during interphase and mitosis. EMBO J. 2004;23:627–637. doi: 10.1038/sj.emboj.7600076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Booth DG, Hood FE, Prior IA, Royle SJ. A TACC3/ch-TOG/clathrin complex stabilises kinetochore fibres by inter-microtubule bridging. EMBO J. 2011;30:906–919. doi: 10.1038/emboj.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu W, Tao W, Zheng P, Fu J, Bian M, Jiang Q, et al. Clathrin recruits phosphorylated TACC3 to spindle poles for bipolar spindle assembly and chromosome alignment. J Cell Sci. 2010;123:3645–3651. doi: 10.1242/jcs.075911. [DOI] [PubMed] [Google Scholar]
- 15.Hubner NC, Bird AW, Cox J, Splettstoesser B, Bandilla P, Poser I, et al. Quantitative proteomics combined with BAC TransgeneOmics reveals in vivo protein interactions. J Cell Biol. 2010;189:739–754. doi: 10.1083/jcb.200911091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin CH, Hu CK, Shih HM. Clathrin heavy chain mediates TACC3 targeting to mitotic spindles to ensure spindle stability. J Cell Biol. 2010;189:1097–1105. doi: 10.1083/jcb.200911120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Royle SJ, Bright NA, Lagnado L. Clathrin is required for the function of the mitotic spindle. Nature. 2005;434:1152–1157. doi: 10.1038/nature03502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hood FE, Royle SJ. Functional equivalence of the clathrin heavy chains CHC17 and CHC22 in endocytosis and mitosis. J Cell Sci. 2009;122:2185–2190. doi: 10.1242/jcs.046177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheeseman LP, Booth DG, Hood FE, Prior IA, Royle SJ. Aurora A kinase activity is required for localisation of TACC3/ch-TOG/clathrin inter-microtubule bridges. Commun Integr Biol. 2011;4:409–412. doi: 10.4161/cib.4.4.15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albee AJ, Wiese C. Xenopus TACC3/maskin is not required for microtubule stability but is required for anchoring microtubules at the centrosome. Mol Biol Cell. 2008;19:3347–3356. doi: 10.1091/mbc.E07-11-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hepler PK, McIntosh JR, Cleland S. Intermicrotubule bridges in mitotic spindle apparatus. J Cell Biol. 1970;45:438–444. doi: 10.1083/jcb.45.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Royle SJ. The cellular functions of clathrin. Cell Mol Life Sci. 2006;63:1823–1832. doi: 10.1007/s00018-005-5587-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Royle SJ, Lagnado L. Trimerisation is important for the function of clathrin at the mitotic spindle. J Cell Sci. 2006;119:4071–4078. doi: 10.1242/jcs.03192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blixt MK, Royle SJ. Clathrin heavy chain gene fusions expressed in human cancers: analysis of cellular functions. Traffic. 2011;12:754–761. doi: 10.1111/j.1600-0854.2011.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Widlund PO, Stear JH, Pozniakovsky A, Zanic M, Reber S, Brouhard GJ, et al. XMAP215 polymerase activity is built by combining multiple tubulin-binding TOG domains and a basic lattice-binding region. Proc Natl Acad Sci USA. 2011;108:2741–2746. doi: 10.1073/pnas.1016498108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Brien LL, Albee AJ, Liu L, Tao W, Dobrzyn P, Lizarraga SB, et al. The Xenopus TACC homologue, maskin, functions in mitotic spindle assembly. Mol Biol Cell. 2005;16:2836–2847. doi: 10.1091/mbc.E04-10-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simpson RJ, Yi Lee SH, Bartle N, Sum EY, Visvader JE, Matthews JM, et al. A classic zinc finger from friend of GATA mediates an interaction with the coiled-coil of transforming acidic coiled-coil 3. J Biol Chem. 2004;279:39789–39797. doi: 10.1074/jbc.M404130200. [DOI] [PubMed] [Google Scholar]
- 28.Thadani R, Ling YC, Oliferenko S. The fission yeast TACC protein Mia1p stabilizes microtubule arrays by length-independent crosslinking. Curr Biol. 2009;19:1861–1868. doi: 10.1016/j.cub.2009.09.063. [DOI] [PubMed] [Google Scholar]
- 29.Al-Bassam J, Larsen NA, Hyman AA, Harrison SC. Crystal structure of a TOG domain: conserved features of XMAP215/Dis1-family TOG domains and implications for tubulin binding. Structure. 2007;15:355–362. doi: 10.1016/j.str.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 30.Lee MJ, Gergely F, Jeffers K, Peak-Chew SY, Raff JW. Msps/XMAP215 interacts with the centrosomal protein D-TACC to regulate microtubule behaviour. Nat Cell Biol. 2001;3:643–649. doi: 10.1038/35083033. [DOI] [PubMed] [Google Scholar]