Abstract
In many animals, germ-cell fate is specified by inheritance of the germ plasm, which is enriched in maternal RNAs and proteins. Assembly of the Drosophila germ (pole) plasm begins with the localization and translation of oskar (osk) RNA at the oocyte posterior pole. osk RNA produces two isoforms, long and short Osk. Short Osk recruits other pole plasm components, and long Osk restricts them to the oocyte cortex. Although molecular functions of long Osk remain mysterious, it is known to be involved in endocytic activation and actin cytoskeletal remodeling. We identified several vesicular trafficking machinery components that act downstream of long Osk in pole plasm assembly. These included the Rab5 effector protein Rabenosyn-5 (Rbsn-5) and the Golgi/endosomal protein Mon2, both of which were crucial for Osk-induced actin remodeling and the anchoring of pole plasm components. We propose that, in response to long Osk, the Rab5/Rbsn-5-dependent endocytic pathway promotes the formation of specialized vesicles, and Mon2 acts on these vesicles as a scaffold to instruct actin nucleators like Cappuccino and Spire to remodel the actin cytoskeleton, which anchors pole plasm components to the cortex. This mechanism may be applicable to the asymmetric localization of macromolecular structures such as protein-RNA complexes in other systems.
Key words: germ plasm, oskar, cell polarization, vesicle trafficking, golgi, endosome, actin cytoskeleton
Drosophila Germ Plasm Assembly
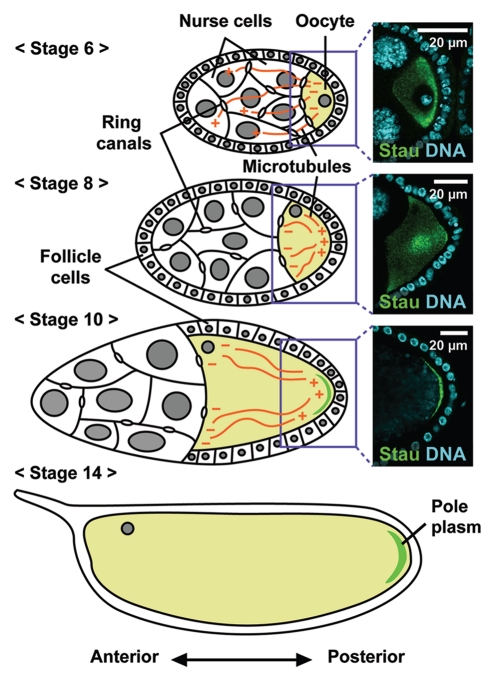
The assembly of the germ (pole) plasm in Drosophila oogenesis is a useful model system for studying cell polarization and the asymmetric localization of macromolecular complexes. Drosophila oogenesis is divided into 14 stages based on morphological criteria, beginning with the formation of a germline cyst of 16 cells that are interconnected through cytoplasmic bridges called ring canals (Fig. 1).1 One of these 16 cells develops into the oocyte; the remaining 15 cells become polyploid nurse cells. Pole plasm components are synthesized in the nurse cells and transported into the oocyte along the microtubule cytoskeleton emanating from the posterior end of the oocyte.2 During stages 6–7, intercellular signaling between the oocyte and surrounding somatic follicle cells promotes the reorganization of oocyte polarity, such that the microtubule minus ends are lost from the oocyte posterior, and instead nucleate along the lateral and anterior cortex. The plus ends transiently accumulate in the middle of the oocyte at stage 7, and subsequently point to the posterior pole. Consistent with these events, a microtubule plus-end marker, kinesinβ-galactosidase (Kin-βgal) fusion protein, and the endogenous Kinesin heavy chain protein accumulate at the oocyte posterior during stages 8–10.3,4 The assembly of pole plasm begins with the Kinesin I-dependent localization of oskar (osk) RNA to the posterior pole of the oocyte at stage 8.5,6 Functional pole plasm is assembled by stage 13, stably anchored at the posterior cortex of the oocyte, and later inherited by the germline progenitors (pole cells) during embryogenesis.
Figure 1.
Developmental stages of Drosophila oogenesis. Schematic representations of Drosophila egg chambers. Each egg chamber consists of a single oocyte and 15 nurse cells, which are surrounded by a monolayer of somatic follicle cells. Pole plasm components are synthesized in the nurse cells and transported to the oocyte through the ring canal along the microtubule cytoskeleton, which extends its plus ends into the nurse cells until stage 6. The reorganization of oocyte polarity during stage 6–7 induces a microtubule rearrangement, resulting in polarized microtubule arrays with minus ends along the lateral and anterior cortex and plus ends at the posterior. The polarized microtubule arrays direct the posterior localization of osk RNA, which is subsequently translated into proteins that recruit and anchor pole plasm components to the posterior cortex. Images of osk RNP and nuclei stained with Staufen (Stau) and DAPI, respectively, are shown at right.
The localization and translation of osk RNA are sufficient to induce pole plasm assembly, as evidenced by an Osk anterior misexpression experiment. In the osk-bcd 3′ UTR transgene, the osk coding sequence is fused with the bicoid (bcd) 3′ untranslated region (3′ UTR), which contains cis-acting signals that target the oocyte anterior. The anterior targeting of Osk by this construct results in the ectopic assembly of the pole plasm.7
osk RNA is translated only after it is localized to the oocyte posterior. Intriguingly, although no alternatively spliced forms of osk RNA has been identified, translation from a single osk RNA species produces two isoforms, long and short Osk, which have distinct functions in pole plasm assembly.8–10 Short Osk recruits downstream components of the pole plasm, such as Vasa (Vas) protein, and long Osk anchors these pole plasm components, including short Osk itself, to the posterior cortex of the oocyte. Furthermore, immunoelectron microscopy revealed that short and long Osk have different subcellular distributions in the cytoplasm at the oocyte posterior.11 Short Osk is integrated into the polar granules, which are specialized ribonucleoprotein aggregates in the pole plasm, whereas long Osk is detected on vesicular structures such as endosomes, and is undetectable on the polar granules.
Endocytic activity in the Drosophila oocyte is polarized toward the posterior A fluorescent lipophilic dye, FM4-64, is preferentially internalized from the oocyte posterior, and markers of the early, late and recycling endosomes (Rab5, Rab7 and Rab11, respectively) are all present throughout the oocyte cortex, with enrichment at the posterior pole.12,13 Interestingly, the polarized endocytosis in the oocyte depends on Osk function: osk mutant oocytes fail to maintain either the localized endocytosis or the polarized distribution of endosomal markers at the posterior. Furthermore, the anterior mis-expression of long Osk results in an ectopic accumulation of endosomal markers and increased endocytosis, implying that the vesicular trafficking is intimately associated with the pole plasm assembly.
Two Distinct Roles of the Endocytic Pathway in Pole Plasm Assembly
We performed a genetic screen to isolate mutants defective in pole plasm assembly by using GFP-Vas as a visual pole plasm marker.13 The screen recovered many genes that are known to be involved in pole plasm assembly. In addition, we identified several factors involved in vesicle trafficking. Rabenosyn-5 (Rbsn-5) is an evolutionally conserved effector of the small GTPase Rab5, which regulates the early endocytic pathway.14 Drosophila oocytes lacking Rbsn-5 were defective in endocytosis, as is also observed in yeasts and mammalian cells.14,15 We found that the osk RNA, as well as the Osk and Vas proteins, failed to accumulate at the posterior pole of the rbsn-5 oocyte, and instead diffused into the cytoplasm. The posterior localization of osk RNA depends on the proper alignment of the microtubule arrays, the plus-ends of which are targeted to the oocyte posterior.3,4 The rbsn-5 mutant oocytes failed to maintain the posterior accumulation of the microtubule plus-end marker Kin-βgal, which instead diffused into the cytoplasm along with pole plasm components. These findings indicated that the endocytic pathway is required for the polarization of microtubule arrays (Fig. 2).
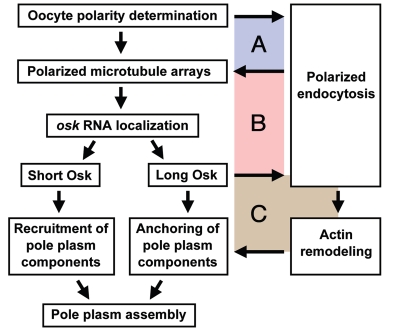
Figure 2.
Multiple interdependent relationships between pole plasm assembly and endocytosis. The polarized microtubule arrays that are induced by oocyte polarization are required for the initial activation of endocytosis at the posterior, while the increased endocytosis at the oocyte posterior maintains the plus-end targeting of microtubules to the posterior. Thus, the microtubule array polarization and local endocytic activation are interdependent (A). Polarized arrays of microtubules direct the posterior localization of osk RNA, resulting in local synthesis of the two Osk isoforms. Short Osk recruits downstream pole plasm components to the posterior, while long Osk stimulates endocytosis, thereby generating a second positive feedback loop (B). Increased endocytosis promotes the rearrangement of cortical F-actin, resulting in the formation of long F-actin projections, which anchor pole plasm components at the cortex. Because pole plasm anchoring is likely to increase the posterior localization of osk RNA, long Osk-induced endocytosis and actin remodeling form a third positive feedback loop (C).
Nevertheless, the endocytic activation by Osk implied that the endocytic pathway also acts downstream of Osk in pole plasm assembly. Unfortunately, the mislocalization of osk RNA in the rbsn-5 mutant oocytes made it impossible to assess further roles of the endocytic pathway in pole plasm assembly, which depends on the proper localization of osk RNA to the oocyte posterior. To overcome this problem, we expressed the osk RNA ectopically at the anterior pole of the oocyte by using osk-bcd 3′ UTR. In wild-type oocytes expressing osk-bcd 3′ UTR, the anterior-targeted Osk was stably anchored at the anterior cortex. However, in rbsn-5 oocytes, the anterior Osk and other pole plasm components failed to be anchored, and diffused into the cytoplasm.13 This defect was also observed in rab5 mutant oocytes.16 These data clearly demonstrated that the endocytic pathway is required for the anchorage of pole plasm components to the oocyte cortex (Fig. 2).
How does the endocytic pathway contribute to anchoring the pole plasm to the cortex? The anchoring of pole plasm components is known to be dependent on the actin cytoskeleton. When oocytes are treated with an actin polymerization inhibitor, cytochalasin D, or are deficient in any of several actin-binding proteins, such as Moesin, Bifocal and Homer, pole plasm components are not properly anchored to the posterior cortex.6,17–19 Interestingly, Osk is reported to remodel cortical F-actin as long F-actin projections from the posterior cortex of the oocyte.11 In addition, expression of the osk-bcd 3′ UTR transgene induces ectopic F-actin projections from the anterior cortex in the oocyte.13,16,20 Furthermore, when only long or short Osk is misexpressed at the anterior pole of the oocyte, only long Osk induces the ectopic F-actin projections, indicating that it is long Osk but not short Osk that remodels the actin cytoskeleton.20
Since long Osk resides on the endosomal membrane,11 we next examined whether the Osk-induced rearrangement of F-actin is dependent on the activity of endocytic components. We found that, in rbsn-5 or rab5 oocytes, ectopic anteriorly localized Osk did not induce the long F-actin projections, and instead caused aberrant F-actin aggregates, which diffused into the cytoplasm, often engulfing pole plasm components.13,16 These results revealed a novel role of the endocytic pathway in the Osk-induced rearrangement of F-actin for the pole plasm anchorage at the oocyte posterior cortex.
Osk-Induced Rearrangementof F-Actin
The above results showed that the endocytic pathway is involved in Osk-dependent actin remodeling. However, the factors that contribute to the F-actin remodeling in response to Osk and how they act remained unknown. From the same genetic screen, however, we identified the Golgi/endosomal protein Mon2 as a factor involved in this process.16 In oocytes lacking Mon2, the initial accumulation of GFP-Vas and other pole plasm components at the posterior pole was intact, but their localization was not maintained, resulting in their complete loss from the posterior pole in late-stage oocytes. Yeast Mon2p (Ysl2p) forms a large protein complex on the surface of the trans-Golgi network and early endosomes, where it is proposed to act as a scaffold to regulate antero- and retrograde trafficking among the Golgi, endosomes and vacuoles.21–24 The distribution pattern of GFP-Mon2 in the Drosophila oocyte was similar to the distributions of Golgi and endosomal markers. Detailed analyses of GFP-Mon2 localization in Drosophila S2 cells showed that it was co-localized with Golgi markers, and less frequently, with early and recycling endosomal markers. Thus, Mon2 is likely to act on vesicular structures for the anchorage of pole plasm components to the oocyte posterior cortex.
The distribution of endosomal markers and the preferential incorporation of FM4-64 dye from the posterior were intact in oocytes lacking Mon2. In contrast, no F-actin projections emanated from the posterior cortex of the mon2 oocytes. Therefore, in the genetic hierarchy for Osk-mediated pole plasm anchoring, Mon2 is placed between endocytic activation and actin remodeling. The anterior misexpression of Osk in mon2 oocytes induced faint F-actin granules instead of long F-actin projections. These results further suggest that, although Mon2 plays a pivotal role in the formation of proper F-actin projections in response to Osk, it is dispensable for the actin remodeling per se.16
Actin Remodelers that Act Downstream of Osk
To elucidate the molecular mechanism by which Mon2 coordinates proper actin remodeling in response to Osk, we sought to identify Mon2-interacting partners through yeast two-hybrid analyses.16 Using full-length Mon2 as the bait to screen a Drosophila ovarian cDNA library, one of the candidates identified was the actin nucleator, Spire (Spir). Furthermore, coimmunoprecipitation assays using S2 cell lysates showed specific interactions between Mon2 and the Drosophila Formin-family actin nucleator Cappuccino (Capu) as well as Spir, suggesting that these actin nucleators, together with Mon2, function in the Osk-dependent actin remodeling. Capu and Spir are known to form a complex and act together in the anterior-posterior axis formation in oocytes.25,26 However, because osk RNA localization in the oocyte is defective in the absence of Capu or Spir, the possible functions of these actin nucleators in the Osk-dependent formation of long F-actin projections had never been investigated.
To examine the roles of Capu and Spir in Osk-dependent actin remodeling, we again expressed Osk ectopically at the anterior pole of the oocyte.16 Anterior Osk in the capu or spir oocytes induced disorganized, fuzzy ball-like F-actin structures instead of the normal F-actin projections. The ectopic Osk was not tightly anchored at the anterior pole of the oocyte, but was detached from the cortical F-actin layer. These findings support the idea that Capu and Spir are required for pole plasm anchoring through proper remodeling of the cortical F-actin cytoskeleton in response to Osk.
What is the functional relationship between Mon2 and Capu or Spir? In both capu mon2 and mon2 spir double-mutant oocytes, Osk expressed at the anterior induced neither fuzzy ball-like F-actin structures nor F-actin projections. Instead, the ectopic Osk induced faint F-actin granules, as observed in the mon2 single mutant. Given that Capu and Spir are actin nucleators that can directly regulate actin remodeling, they are likely to act downstream of Mon2 in the genetic hierarchy for pole plasm anchoring to the oocyte cortex.16
We also found that the small GTPase Rho1, which forms a complex with Capu and Spir to regulate their actin nucleation activity, is highly concentrated at the oocyte posterior.16 Furthermore, this posterior concentration of Rho1 requires Mon2, indicating that Mon2 might regulate the Capu/Spir activity to remodel cortical F-actin. Based on these data, we propose that, in response to Osk, vesicle-localized Mon2 acts as a scaffold that instructs the Capu-Spir-Rho1 complex to form long F-actin projections, which anchor the pole plasm components to the oocyte cortex (Fig. 3).
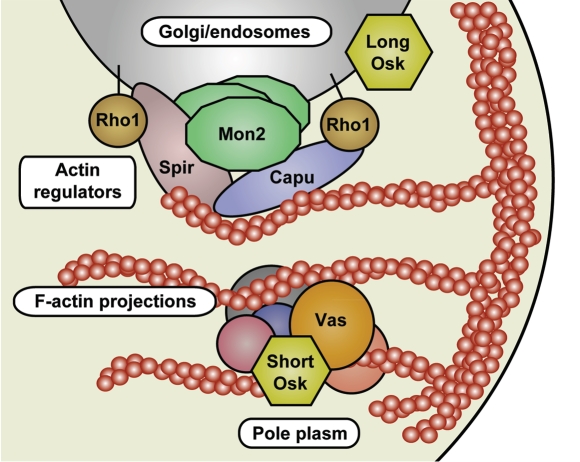
Figure 3.
A model for pole plasm anchoring to the posterior cortex of the Drosophila oocyte. At the oocyte posterior, long Osk stimulates endocytosis, producing specialized vesicles that orchestrate actin remodeling. On these vesicles, Mon2 acts as a platform for actin regulators including Capu, Spir and Rho1, to promote the formation of the long F-actin projections that are crucial for anchoring the pole plasm.
Future Directions
Our genetic analyses reveal that three positive feedback loops or interdependent relationships exist between the endocytic pathway and pole plasm assembly (Fig. 2).13 First, oocyte polarization leads to local activation of the endocytic pathway at the oocyte posterior, while the endocytic pathway is required for the formation of polarized microtubule arrays. Therefore, an interdependent relationship exists between the microtubule array polarization and local endocytic activation (Fig. 2A). Next, osk RNA localizes to the oocyte posterior via the polarized microtubule arrays. Locally synthesized long Osk subsequently stimulates endocytosis, forming a second positive feedback loop (Fig. 2B). Consistent with this scenario, Osk has been reported to contribute to the maintenance of the oocyte polarity.27 Finally, Osk-mediated activation of the endocytic pathway promotes the long F-actin projections that anchor the pole plasm, thereby recruiting additional pole plasm components, including osk RNA. Therefore, Osk and the endocytic pathway form another positive feedback loop that ensures robust pole plasm assembly at the oocyte posterior pole (Fig. 2C). The loss of Rbsn-5 function causes both the mistargeting of microtubule plus ends and the disorganization of F-actin, indicating that Rbsn-5 is associated with all three loops. In contrast, mon2-deficient oocytes show milder defects in pole plasm anchoring compared to rbsn-5 or rab5-deficient oocytes, and treatment with actin inhibitors such as cytochalasin D or latrunculin A causes similar mild defects in pole plasm anchoring.6,19 Mon2 is therefore required only for F-actin rearrangement during pole plasm assembly. In other words, Mon2 is specifically involved in the third feedback loop. The endocytic pathway consists of multiple steps, including endocytosis, endosomal recycling, late endosomal sorting, and endosome-to-Golgi trafficking. Therefore, an important remaining issue is to determine which steps of the endocytic pathway and what endosomal properties, such as lipid composition or acidification, contribute to each feedback loop during pole plasm assembly.
Furthermore, many questions regarding the long Osk-dependent pole plasm anchoring remain. An obvious unanswered question is how the long Osk stimulates endocytosis. Ultrastructural analyses have indicated that long Osk resides on endosomes.11 Therefore, long Osk might directly upregulate endocytosis by interacting with the vesicular membrane. However, no known membrane-binding motif has been identified in long Osk. Furthermore, long Osk expressed in S2 cells fails to localize to endosomes (unpublished data). Therefore, an additional factor(s), which presumably resides on endosomes in the oocyte but not in S2 cells, promotes the endosomal localization of long Osk. Identifying the binding partners of long Osk by immunoprecipitation-mass spectrometry analyses and/or proteomic approaches using purified endosomes from oocytes might reveal factors that regulate long Osk localization and activity. Long Osk on endosomes may also instruct the complex containing Mon2, Capu, Spir and Rho1 to remodel cortical F-actin appropriately. Although short Osk shares its entire sequence with long Osk, which has only a 138-amino-acid extension at the N terminal of the short isoform, the two Osk isoforms have distinct functions and cellular distributions. Therefore, the N-terminal extension on long Osk somehow promotes its endosomal localization. Furthermore, the N-terminal extension also suppresses the innate functions of Osk elicited by the short Osk domains, such as its ability to interact with several pole plasm proteins.8 Determining the three-dimensional structure of long and short Osk will be invaluable for elucidating the mechanisms by which the two Osk isoforms orchestrate pole plasm assembly and anchoring.
Acknowledgments
We thank Yasuko Kato, Kazuki Matsuda and Kazuko Hanyu-Nakamura for their help with the studies. Research in the Nakamura lab was supported in part by a Grant-in-Aid from the MEXT and JSPS.
References
- 1.Spradling AC. Development genetics of oogenesis. In: Bate M, Martinez-Arias A, editors. The Development of Drosophila melanogaster. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 1–70. [Google Scholar]
- 2.Steinhauer J, Kalderon D. Microtubule polarity and axis formation in the Drosophila oocyte. Dev Dyn. 2006;235:1455–1468. doi: 10.1002/dvdy.20770. [DOI] [PubMed] [Google Scholar]
- 3.Clark I, Giniger E, Ruohola-Baker H, Jan LY, Jan YN. Transient posterior localization of a kinesin fusion protein reflects anteroposterior polarity of the Drosophila oocyte. Curr Biol. 1994;4:289–300. doi: 10.1016/s0960-9822(00)00068-3. [DOI] [PubMed] [Google Scholar]
- 4.Palacios IM, St. Johnston D. Kinesin light chain-independent function of the Kinesin heavy chain in cytoplasmic streaming and posterior localisation in the Drosophila oocyte. Development. 2002;129:5473–5485. doi: 10.1242/dev.00119. [DOI] [PubMed] [Google Scholar]
- 5.Brendza RP, Serbus LR, Duffy JB, Saxton WM. A function for kinesin I in the posterior transport of oskar mRNA and Staufen protein. Science. 2000;289:2120–2122. doi: 10.1126/science.289.5487.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cha BJ, Serbus LR, Koppetsch BS, Theurkauf WE. Kinesin I-dependent cortical exclusion restricts pole plasm to the oocyte posterior. Nat Cell Biol. 2002;4:592–598. doi: 10.1038/ncb832. [DOI] [PubMed] [Google Scholar]
- 7.Ephrussi A, Lehmann R. Induction of germ cell formation by oskar. Nature. 1992;358:387–392. doi: 10.1038/358387a0. [DOI] [PubMed] [Google Scholar]
- 8.Breitwieser W, Markussen FH, Horstmann H, Ephrussi A. Oskar protein interaction with Vasa represents an essential step in polar granule assembly. Genes Dev. 1996;10:2179–2188. doi: 10.1101/gad.10.17.2179. [DOI] [PubMed] [Google Scholar]
- 9.Markussen FH, Michon AM, Breitwieser W, Ephrussi A. Translational control of oskar generates short OSK, the isoform that induces pole plasm assembly. Development. 1995;121:3723–3732. doi: 10.1242/dev.121.11.3723. [DOI] [PubMed] [Google Scholar]
- 10.Vanzo NF, Ephrussi A. Oskar anchoring restricts pole plasm formation to the posterior of the Drosophila oocyte. Development. 2002;129:3705–3714. doi: 10.1242/dev.129.15.3705. [DOI] [PubMed] [Google Scholar]
- 11.Vanzo N, Oprins A, Xanthakis D, Ephrussi A, Rabouille C. Stimulation of endocytosis and actin dynamics by Oskar polarizes the Drosophila oocyte. Dev Cell. 2007;12:543–555. doi: 10.1016/j.devcel.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Dollar G, Struckhoff E, Michaud J, Cohen RS. Rab11 polarization of the Drosophila oocyte: a novel link between membrane trafficking, microtubule organization and oskar mRNA localization and translation. Development. 2002;129:517–526. doi: 10.1242/dev.129.2.517. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka T, Nakamura A. The endocytic pathway acts downstream of Oskar in Drosophila germ plasm assembly. Development. 2008;135:1107–1117. doi: 10.1242/dev.017293. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen E, Christoforidis S, Uttenweiler-Joseph S, Miaczynska M, Dewitte F, Wilm M, et al. Rabenosyn-5, a novel Rab5 effector, is complexed with hVPS45 and recruited to endosomes through a FYVE finger domain. J Cell Biol. 2000;151:601–612. doi: 10.1083/jcb.151.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weisman LS, Wickner W. Molecular characterization of VAC1, a gene required for vacuole inheritance and vacuole protein sorting. J Biol Chem. 1992;267:618–623. [PubMed] [Google Scholar]
- 16.Tanaka T, Kato Y, Matsuda K, Hanyu-Nakamura K, Nakamura A. Drosophila Mon2 couples Oskar-induced endocytosis with actin remodeling for cortical anchorage of the germ plasm. Development. 2011;138:2523–2532. doi: 10.1242/dev.062208. [DOI] [PubMed] [Google Scholar]
- 17.Jankovics F, Sinka R, Lukácsovich T, Erdélyi M. MOESIN crosslinks actin and cell membrane in Drosophila oocytes and is required for OSKAR anchoring. Curr Biol. 2002;12:2060–2065. doi: 10.1016/s0960-9822(02)01256-3. [DOI] [PubMed] [Google Scholar]
- 18.Polesello C, Delon I, Valenti P, Ferrer P, Payre F. Dmoesin controls actin-based cell shape and polarity during Drosophila melanogaster oogenesis. Nat Cell Biol. 2002;4:782–789. doi: 10.1038/ncb856. [DOI] [PubMed] [Google Scholar]
- 19.Babu K, Cai Y, Bahri S, Yang X, Chia W. Roles of Bifocal, Homer and F-actin in anchoring Oskar to the posterior cortex of Drosophila oocytes. Genes Dev. 2004;18:138–143. doi: 10.1101/gad.282604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang CW, Nashchekin D, Wheatley L, Irion U, Dahlgaard K, Montague TG, et al. Anterior-posterior axis specification in Drosophila oocytes: Identification of novel bicoid and oskar mRNA localisation factors. Genetics. 2011 doi: 10.1534/genetics.111.129312. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singer-Krüger B, Ferro-Novick S. Use of a synthetic lethal screen to identify yeast mutants impaired in endocytosis, vacuolar protein sorting and the organization of the cytoskeleton. Eur. J Cell Biol. 1997;74:365–375. [PubMed] [Google Scholar]
- 22.Jochum A, Jackson D, Schwarz H, Pipkorn R, Singer-Krüger B. Yeast Ysl2p, homologous to Sec7 domain guanine nucleotide exchange factors, functions in endocytosis and maintenance of vacuole integrity and interacts with the Arf-Like small GTPase Arl1p. Mol Cell Biol. 2002;22:4914–4928. doi: 10.1128/MCB.22.13.4914-4928.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Efe JA, Plattner F, Hulo N, Kressler D, Emr SD, Deloche O. Yeast Mon2p is a highly conserved protein that functions in the cytoplasm-to-vacuole transport pathway and is required for Golgi homeostasis. J Cell Sci. 2005;118:4751–4764. doi: 10.1242/jcs.02599. [DOI] [PubMed] [Google Scholar]
- 24.Gillingham AK, Whyte JR, Panic B, Munro S. Mon2, a relative of large Arf exchange factors, recruits Dop1 to the Golgi apparatus. J Biol Chem. 2006;281:2273–2280. doi: 10.1074/jbc.M510176200. [DOI] [PubMed] [Google Scholar]
- 25.Dahlgaard K, Raposo AA, Niccoli T, St. Johnston D. Capu and Spire assemble a cytoplasmic actin mesh that maintains microtubule organization in the Drosophila oocyte. Dev Cell. 2007;13:539–553. doi: 10.1016/j.devcel.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theurkauf WE. Premature microtubule-dependent cytoplasmic streaming in cappuccino and spire mutant oocytes. Science. 1994;265:2093–2096. doi: 10.1126/science.8091233. [DOI] [PubMed] [Google Scholar]
- 27.Zimyanin V, Lowe N, St. Johnston D. An Oskardependent positive feedback loop maintains the polarity of the Drosophila oocyte. Curr Biol. 2007;17:353–359. doi: 10.1016/j.cub.2006.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]