Abstract
Cell wounding is a common event in the life of many cell types, and the capacity of the cell to repair day-to-day wear-and-tear injuries, as well as traumatic ones, is fundamental for maintaining tissue integrity. Cell wounding is most frequent in tissues exposed to high levels of stress. Survival of such plasma membrane disruptions requires rapid resealing to prevent the loss of cytosolic components, to block Ca2+ influx and to avoid cell death. In addition to patching the torn membrane, plasma membrane and cortical cytoskeleton remodeling are required to restore cell function. Although a general understanding of the cell wound repair process is in place, the underlying mechanisms of each step of this response are not yet known. We have developed a model to study single cell wound repair using the early Drosophila embryo. Our system combines genetics and live imaging tools, allowing us to dissect in vivo the dynamics of the single cell wound response. We have shown that cell wound repair in Drosophila requires the coordinated activities of plasma membrane and cytoskeleton components. Furthermore, we identified an unexpected role for E-cadherin as a link between the contractile actomyosin ring and the newly formed plasma membrane plug.
Key words: wound repair, single cell, plasma membrane, cytoskeleton, actin, myosin, E-cadherin, purse string, Drosophila
Introduction
Many cells, including skeletal muscle, myocytes, neurons and epithelial cells from skin and intestine, often find themselves in mechanically and/or chemically challenging environments where disruptions of their plasma membrane occur. For example, skeletal muscle and cardiac myocytes suffer repetitive plasma membrane disruptions, especially when subjected to physical activities such as exercise.1 Frequent cell wounds are also observed in epithelial cells from skin, gut and endothelium, which are exposed to chemical and mechanical stresses on a daily basis.2–4 Traumatic injuries or accidents are often associated with extensive cell damage. For example, a severed nerve must rapidly reseal then remodel to re-innervate its target. Improper plasma membrane repair has also been associated with genetic diseases. Widespread muscle cell wounding is characteristic of patients suffering from different muscular dystrophies, where muscle cells are fragile, easily wounded and lost, with devastating consequences for patients.5 Cell wounding is also prevalent in the fragile skin cells of Epidermolysa bullosa simplex patients, as well as in response to bacterial toxin lesions.6,7 In many cases, cells that suffer from such damages are both essential and irreplaceable for the functioning of the tissue or organism. Therefore, single cell wound repair is an important biological process that is needed to avoid cell death and loss, maintain tissue homeostasis and prevent an excessive inflammatory response.
Over the last decade, research mainly in cultured cells, sea urchin eggs and Xenopus oocytes has elegantly shown a role for the plasma membrane, as well as the underlying cortical cytoskeleton, in the single cell repair response. Here, we discuss how the integration of both the plasma membrane and the associated cytoskeleton responses are important for cell wound repair in light of a new genetic model to study this process, the Drosophila embryo, and its implications in our current understanding of cell wound healing.
The Membrane "Patch" Hypothesis
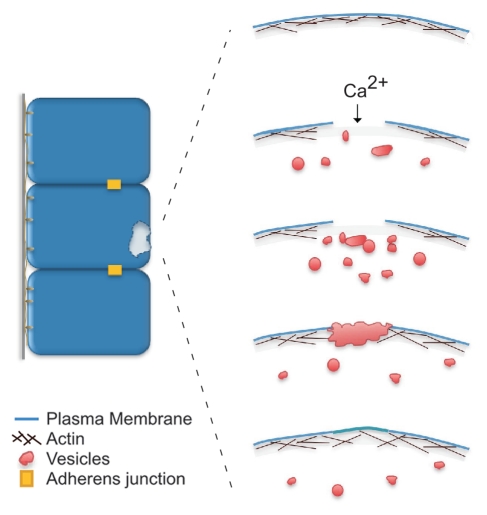
Since membrane lesions are a recurring event in a cell's life, a robust and efficient mechanism must be in place to rapidly repair plasma membrane damage. Our current knowledge of the single cell wound repair process from different model organisms indicates that at least two processes are required to reestablish normal cell function: plasma membrane resealing and cortical cytoskeleton remodeling. Upon disruption of the plasma membrane it has been proposed that a Ca2+ influx is the signal triggering the initial repair response, during which two different membrane fusion events occur: homotypic vesicle-vesicle fusion to create a membrane patch, and vesicle-plasma membrane fusion to link the “patch” to the plasma membrane thereby restoring plasma membrane continuity (Fig. 1).8,9 Immediately upon wounding a rapid and local burst of Ca2+-dependent exocytosis is observed at the wound site in echinoderms and fibroblasts (Fig. 2A).9,10 In support of a role for Ca2+ as the trigger of plasma membrane resealing, blocking the Ca2+ signal by either manipulating the ion concentration in the media or by treatment with tetanus or botulinum toxins (inhibitors of the SNARE protein family and synaptic vesicle endocytosis) results in failure to repair the plasma membrane disruption.8–11
Figure 1.
Schematic diagram of the single cell wound repair process. Upon plasma membrane disruption, a Ca2+ influx triggers internal vesicles to fuse with each other and form a membrane patch. This membrane “patch” fuses with the plasma membrane at specific sites along the periphery of the disruption. Membrane resealing is followed by a process of plasma membrane and cortical cytoskeleton remodeling (adapted from McNeil et al.).21
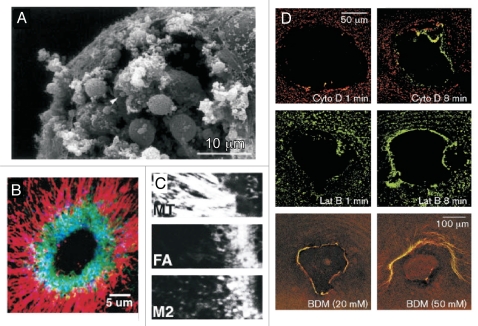
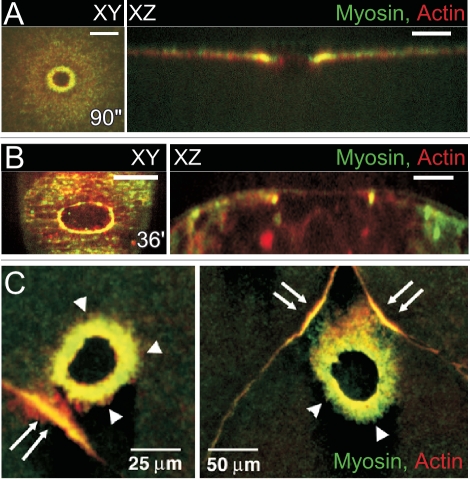
Figure 2.
Plasma membrane and cytoskeleton responses to single cell wounds. (A) Vesicle-vesicle fusions at the wound site. Scanning electron micrographs of the surface of a Sea Urchin egg sheared to produce plasma membrane disruptions. The egg was sheared directly into fixative that contained Ca2+ (10 mM). The disruption site is filled with a large population of vesicles, supporting the idea that Ca2+ induces the recruitment of vesicles to the disruption site. [Figure adapted and reprinted from McNeill and Baker. Cell Tissue Research 2001; 304:141–6, with permission from Springer].33 (B and C) Cytoskeleton response to single cell wounds. Confocal image of a laser wounded Xenopus oocyte showing that a continuous actomyosin ring (actin, green; myosin II, blue) forms during the wound healing process, surrounded by a radial arrangement of microtubules (red). Significantly, the actin (FA) and myosin II (M2) rings are not completely superimposed (C).(Figure adapted and reprinted from Mandato and Bement. Curr Biol 2003; 13:1096–105, with permission from Elsevier).28 (D) An intact cytoskeleton is required for proper cell wound repair. Disruption of the actin and myosin cytoskeleton impairs wound closure. Xenopus oocytes treated with Cytochalasin D (Cyto D), Latrunculin B (Lat B) and BDM shows defects in actin (red) and myosin II (green) recruitment and ring assembly. (Figure adapted and reprinted from Bement et al. Curr Biol 1999; 9:579–87, with permission from Elsevier).26
The membrane “patch” hypothesis explains how large disruptions (>100 −m2), requiring significant membrane replacement, can be rapidly and efficiently repaired. The source of internal vesicles used to assemble the patch varies among the different single cell wound repair models. In sea urchin eggs, yolk granules are used to reseal the plasma membrane damage.11,12 If yolk granules are locally depleted by centrifugation plasma membrane resealing fails.11 Yolk granules also display a Ca2+-dependent fusion activity in vitro. This fusion event takes place in terms of seconds, which correlates well with the dynamics of plasma membrane resealing. Another organelle implicated in cell wound repair is the lysosome. This organelle responds to elevated concentrations of Ca2+ and undergoes homo-typic fusions. In mammalian cells, Ca2+ induced lysosomes to fuse with the plasma membrane and these have been proposed as the source of membrane to form the ‘patch’.13,14 Moreover, inhibition of synaptotagmin VII, a lysosomal membrane protein required for exocytosis, impairs the membrane resealing capacity of fibroblasts and squid axons.14,15 Nevertheless, the speed of lysosome exocytosis in response to an increase in Ca2+ concentration is much slower than would be expected for it to play a role in the resealing response.16 It remains to be seen if lysosomes are the only membrane source involved in cell repair in mammals, or if different cell types utilize other organelle compartments as the source of membrane to reseal plasma membrane disruptions. Finally, work in both sea urchin eggs and fibroblasts suggest that the directional movement of vesicles to the wound is dependent on kinesin and myosin motors.8,17
How vesicles are recruited to the wound site and which protein components are involved in the fast exocytic response evoked by wounding are major questions in the field. To date, only a handful of proteins identified in different model systems have been directly implicated in the repair process. In addition to members of the SNARE family, recent evidence suggests a role for different Ca2+-dependent proteins. Synaptotagmins, a family of proteins involved in the exocytic fusion machinery in lysosomes, are required for membrane resealing in the squid giant axon and fibroblasts.14,15 Functionally blocking synaptotagmins VII and III by introduction of antibodies against the C2A-calcium binding domain inhibits membrane resealing. Significantly, lysosomal membrane is not recruited to the wound area when synaptotagmin VII is inhibited, as shown by the absence of Lamp-1, a lysosomal membrane marker, on the surface of wounded cells, providing additional evidence that lysosomes are a membrane source in cell wound repair.14 Annexins, which promote Ca2+-dependent membrane aggregation and fusion, are also implicated in membrane resealing. In particular annexin A1, normally associated with dysferlin in skeletal muscle, is required for plasma membrane resealing in cultured cells.18 Cytosolic annexin A1 binds to the membrane adjacent to the wound site and may be involved in initiating the homotypic and exocytic membrane fusion events required for the membrane resealing. Additionally, m- and µ-calpain intracellular calcium-dependent proteases are known to facilitate plasma membrane resealing by clearing cortical cytoskeleton remnants at the wound site, a critical step to initiate the vesicle fusion process leading to plasma membrane resealing.19,20
Once the membrane patch is formed and directionally recruited to the wound site, the heterotypic fusion of the patch to the plasma membrane at the wound edge proceeds. It has been recently proposed that this fusion occurs at specific points or “vertices” around the wound perimeter where the fusion protein machineries become concentrated.21 This “vertex” model correlates with the concave crater-like appearance of the wound site in sea urchin eggs after membrane resealing (Fig. 2A).
The Role of the Cortical Cytoskeleton in the Single Cell Wound Repair Response
Rapid resealing of the wound favors cell survival, but additional repairs are required to fully restore normal cell function. A remodeling process follows plasma membrane resealing, during which the membrane patch is replaced and a repopulation of the underlying cortical cytoskeleton takes place. The membrane patch can be envisioned as a temporary structure that must be replaced. The patch is derived from organelle membranes and differs from the plasma membrane in its lipid and protein composition. Indeed, membrane resistance and ion permeability has been shown to be significantly different in wounded cells immediately after healing.22 It is not yet known how cells restore their plasma membrane continuity. Possible mechanisms include the repopulation of the wound membrane plug by membrane diffusion from the surrounding area, or the formation of a new plasma membrane underneath the plug that is subsequently sloughed off as a scab.
The damage caused by wounding extends beyond the superficial plasma membrane: it also affects the underlying cortical cytoskeleton. The role of cytoskeleton components in the initial steps of the wound repair process remains to be fully elucidated. In some cell types, the cortical actin cytoskeleton constitutes a barrier for the vesicle-plasma membrane contact. Destabilization of actin in fibroblasts, neurons and gastric epithelial cells enhances the plasma membrane resealing process;23–25 whereas treatments that stabilize actin in gastric epithelial cells strongly inhibited membrane resealing.25 Thus, a transient disassembly of the cortical cytoskeleton associated with the wound area may be necessary to allow the initial vesicle-plasma membrane docking/contact and initiation of membrane fusion.
In Xenopus oocytes, disruption of the plasma membrane triggers the rapid accumulation of actin and myosin II around the wound (30–60 seconds post-wounding) (Fig. 2B and C).26–28 The assembly of a contractile actomyosin ring is required for wound repair and is dependent on Ca2+ (ref. 26). This ring is dynamic and contracts inward until the wound area is covered. Significantly, actin and myosin II accumulate as concentric rings with myosin II concentrated at the internal edge of the ring and actin dynamically accumulating on the outside, with an overlapping central zone (Fig. 2B).27 The actomyosin ring is also surrounded by a radial array of microtubules (Fig. 2B).28 This array of microtubules is dependent on new microtubule assembly and on the actomyosin ring, and conversely microtubules control the zone of actin assembly and myosin II recruitment at the wound border.28 Pharmacological perturbations of actin, myosin II and microtubules show the requirement of and the specific roles played by each of these cytoskeleton components in mediating cell wound repair (Fig. 2D).26–28 The cytoskeleton response to cell wounds explains not only how the cortical cytoskeleton is re-established, but also suggests an active role for the cytoskeleton in the plasma membrane remodeling process. As indicated by Mandato and Bement,27 a mechanism allowing plasma membrane anchoring/tethering to the cortical cytoskeleton at frequent intervals along the wound explains the correlation between the dynamic inward movement of the contractile actomyosin ring and wound closure.
The Early Drosophila Embryo as a Model for Single-Cell Wound Repair
Recently, we established the early-stage, nuclear cycle 4–6, Drosophila embryo as a genetically tractable model to study single cell repair.29 The Drosophila embryo's first 13 nuclear divisions occur in the absence of cytokinesis making the embryo a large, multi-nucleate single cell (Fig. 3A and B).30 Our study aimed to use the strengths of Drosophila genetics, fluorescent reporters, and the ease of in vivo imaging in the Drosophila embryo to further understand the role of bio-architectural molecules/machineries in single cell repair and to complement the existing single cell wound repair models.
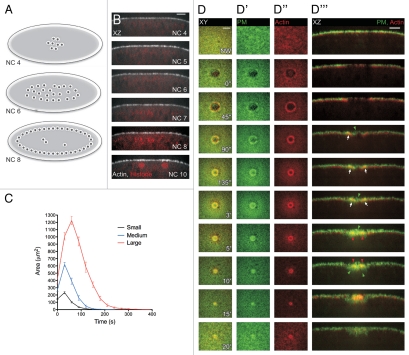
Figure 3.
Single cell wound repair in the early Drosophila embryo. (A) Cartoon of the early Drosophila embryo (Nuclear cycle 4 to 8; NC). The early embryo is a syncytium wherein the nuclei divide in the interior of the embryo without cytokinesis through nuclear cycle 8, thereby forming a large multinucleate cell. (B) Orthogonal view of embryos expressing actin and nuclear markers. Nuclei remain away from the cell periphery until NC10, allowing for the study of wound repair before this time point without the complication of damage to the nuclei. (C) Wound repair curves (area vs. time) of small, medium and large wounds follow a stereotyped response composed of three steps and independent of wound size. Post wounding the area expands, followed by a period of rapid contraction of wound area, and then a slower phase of closure as the wounded area is remodeled. (D–D‴) Time-lapse series following wound repair in embryos expressing plasma membrane and actin markers. Plasma membrane is recruited from the area surrounding the wound and by the trafficking of vesicles to the wound site. By 90″ post wounding, the membrane has formed a plug over the wounded area. Actin is recruited from around the wound and accumulates to form a tight ring around the plasma membrane plug, which progressively contracts until the ring closes 5′ post wounding. Scale bars: XY are 20 µm and XZ 10 µm unless otherwise indicated. (Figure reprinted with permission from Abreu-Blanco et al.; J Cell Biol 2011; DOI:10.1083/jcb.201011018).29
Time-lapse studies of wildtype embryos expressing an actin biomarker revealed that single cell wound repair follows a stereotypical and consistent response composed of three distinct steps: (1) expansion, (2) contraction and (3) closure (Fig. 3C). Upon wounding, the hole rapidly expands until actin is accumulated at the leading edge at which point the wound size begins to rapidly decrease (Fig. 3D″). At the end of the contraction phase, the wound transitions to a slower closure phase during which the underlying cytoskeleton is remodeled.
As observed in other single cell wound repair models, Drosophila embryos rapidly recruit a plasma membrane plug to the wound (Fig. 3D′). This membrane “patch” is formed from plasma membrane surrounding the wound edge, as well as underlying vesicles that traffic to the wound (Fig. 3D‴). In our system, membrane accumulates internally within the actin cable as fast as 30 seconds post-wounding. In addition to actin, myosin II accumulates at the wound edge, forming a contractile actomyosin ring, which is required to mediate wound closure. Significantly, the actin and myosin II rings do not completely overlap: orthogonal cross-sections at the wound show that myosin II is enriched at the leading edge of the ring, followed by a region where both components completely overlap, which in turn is surrounded by an actin rich zone. Embryos in which myosin has been genetically or pharmacologically removed exhibit impaired actin ring formation and an inability to properly close the wound, demonstrating that the purse-string is necessary for repair. Taken together, our data shows that cell wound repair in Drosophila is mediated by the formation of a membrane plug and actomyosin purse-string, indicating that the cell wound repair process in the early Drosophila embryo recapitulate the response observed in other model systems.
E-Cadherin Coordinates Plasma Membrane and Cytoskeleton Responses in Cell Wound Repair
It has been established that both membrane resealing and the actomyosin purse string are important for single-cell repair, however, it is not yet clear how the two processes work together. The vertex model proposes that specific points along the wound periphery serve as fusion initiation sites between the vesicle patch and the wound edge, and sealing of the hole spreads radially from these sites.21 Supporting this model, recent studies in Xenopus embryos suggest that the actomyosin purse-string string is being tethered to the membrane at intervals along the cable by an unknown mechanism.27 In epithelial (multicellular) wound repair, E-Cadherin has been shown to work by linking the actomyosin cable intercellularly, thus mediating the coordinated migration of cells at the wound border (Fig. 4A).31 We asked if E-Cadherin could also be acting as the intermediary between the plasma membrane and actin cytoskeleton in the single cell wound repair process.
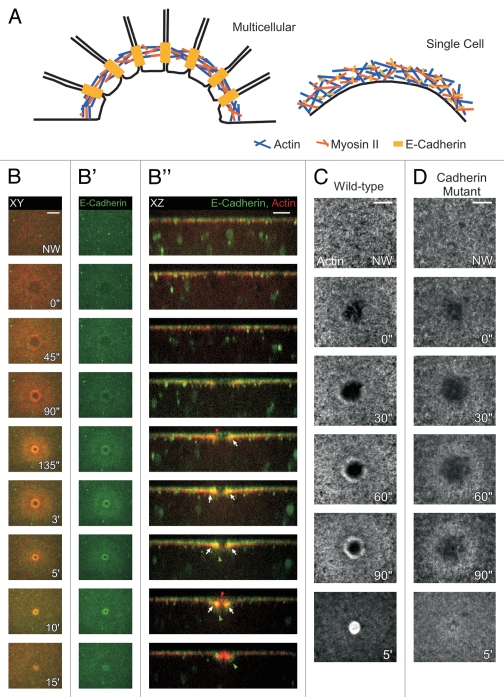
Figure 4.
E-Cadherin mediates the interaction between the plasma membrane and the underlying cytoskeleton in single cell wound repair. (A) Cartoon depicting the actomyosin purse-string in single and multi-cellular repair. In both single and multi-cellular repair, actin and myosin II co-localize to form an actomyosin cable. E-Cadherin co-localizes with this cable in the single cell repair model to tether it to the plasma membrane. In contrast, in multi-cellular repair, E-Cadherin expression is found at cellular junctions along the leading edge to link the actin and myosin II machinery into an intracellular purse-string. (B–B‴) Time-lapse series of E-Cadherin and actin. Membrane-associated E-Cadherin is recruited from the area around the wound and from particles that traffic towards the wound. E-Cadherin co-localizes with the actin ring. (C and D) E-Cadherin mutant embryos fail to assemble an actin cable and show wound healing defects. (C) Surface projection of a wildtype embryo expressing actin. An actin ring is observed 60″ post wounding in wildtype embryos, whereas in E-Cadherin mutants actin forms a diffuse ring and wounds over-expand. E-Cadherin mutant embryos do manage to eventually repair the wound. Scale bars: XY are 20 µm and XZ 10 µm unless otherwise indicated. (Figure reprinted with permission from Abreu-Blanco et al.; J Cell Biol 2011; DOI:10.1083/jcb.201011018).29
We followed in vivo the actin and DE-Cadherin responses during wounding and observed a distinct localization pattern of cadherin at the edge of the wound. We found that DE-Cadherin co-localizes with the actin ring and is excluded from the membrane plug (Fig. 4B-B”). Moreover, when we compared wound repair in wild-type embryos with embryos deficient for E-Cadherin, mutant embryos exhibited wound overexpansion and improper actin cable formation, resulting in impaired wound healing (Fig. 4C and D). This suggests a role for E-Cadherin tethering of the membrane to the actomyosin ring at the wound border, thereby allowing the cable to pull the membrane shut as it contracts. However, as wounds made in embryos mutant for E-Cadherin do manage to close eventually, there are likely other proteins that can tether the membrane to the underlying actomyosin cable and compensate for the cadherin loss in these embryos.
Relationship of the Single Cell and Multicellular Wound Healing Responses
While the single cell wound repair process is fundamental to ensure individual cell survival, it is also an important component of the multicellular wound repair process. When tissues are damaged, some cells will be completely lost while others will suffer non-fatal damage and undergo single cell wound repair long before the tissue has begun to repair itself. Significantly, these repaired cells at the wound border will be the first to signal to their neighbors to initiate the re-epithelialization process as one of the first events in tissue repair. The similarities between the repair mechanisms that heal a hole in single cells and multicellular wounds are striking, considering that both mechanisms operate on massively different scales. Indeed, the emerging picture shows conservation of both machineries and bioarchitectural elements. In both single cell and multicellular repair, an actomyosin cable is formed at the leading edge and is necessary for wounds to heal (Fig. 5A and B). Furthermore, our discovery that E-Cadherin plays a major role in single cell repair was due to comparative studies between single cell and multicellular repair mechanisms. Similarly, in the cellularizing Xenopus embryo, Clark and colleagues examined the single cell response in a multicellular environment: the authors described the recruitment of actin and myosin to the cell-cell junctions nearest to a single cell wound (Fig. 5C), emulating their recruitment to the leading edge of a multicellular wound.32 This result could suggest that the single cell wound may itself act as a cue in a distance dependant manner to trigger the multicellular response in cases where the single cell cannot be repaired. Alternatively, the signal or signals that initiate both single cell and multicellular repair might also be conserved and diffuse from the wound to illicit this response at nearby cell borders. Further studies in single cell wound repair, multicellular wound repair, and the repair of a single cell within a multicellular environment will continue to expand our understanding of how these vital wound repair processes are achieved, and in many cases integrated. It will be interesting to see how the signals, molecules, and pathways involved in single cell and multicellular repair overlap and/or integrate.
Figure 5.
Relationship between single cell and multicellular wound repair. (A) Actin and Myosin II accumulate in a ring surrounding the wound in the early Drosophila embryo. Surface projection and orthogonal view of actin and myosin II show both proteins co-localizing to form an actomyosin ring. [©Abreu-Blanco et al. Originally published in Journal of Cell Biology 2011; DOI: 10.1083/jcb.201011018].29 (B) An actomyosin purse string is observed at the leading edge of multicellular wounds in late Drosophila embryos. Surface projection and orthogonal view of actin and myosin II show both proteins co-localizing to form an actomyosin purse string. (C) Actin and myosin II accumulate at the leading edge of the wound edge as well as at cell junctions near the wound in cellularized Xenopus embryos. This accumulation at the cellular junction is distance dependant and only junctions proximal to the wound will form these secondary accumulations. (Scale bars: XY are 20 µm and XZ 10 µm unless otherwise indicated). (Figure adapted and reprinted from Clark. Curr Biol 2009; 19:1389–95 with permission from Elsevier.)32
Future Prospects
The rapid resealing of the membrane is the immediate and necessary response of the cell in order to not only avoid further damage and eventually death, but to restore cell function. The complexities of this process, as well as its very rapid nature, have contributed to the difficulty of its study. The Drosophila single cell wound repair model provides a new set of tools for the wound repair field. A combination of genetics and live imaging will allow us to better understand the dynamics of this process. There are still many questions surrounding how cells cope with membrane disruptions, particularly in terms of the molecular components and regulatory mechanism governing this emergency response. For example, what other signals besides Ca2+ are involved in the initial resealing response? What is the source of membrane recruited to plug the wound in the different cell types? Does this membrane source matter and how is its mobilization to the wound achieved? What regulates the plasma membrane remodeling? Is the membrane patch remodeled or replaced? The availability of new tools and models, and advances in the imaging and molecular biology fields will help us to answer these fundamental questions.
Acknowledgments
We thank Parkhurst lab members for their interest, advice and comments on the manuscript. This work was supported by NIH grant GM092731 to S.M.P.
References
- 1.Clarke MS, Caldwell RW, Chiao H, Miyake K, McNeil PL. Contraction-induced cell wounding and release of fibroblast growth factor in heart. Circ Res. 1995;76:927–934. doi: 10.1161/01.res.76.6.927. [DOI] [PubMed] [Google Scholar]
- 2.McNeil PL, Ito S. Gastrointestinal cell plasma membrane wounding and resealing in vivo. Gastroenterology. 1989;96:1238–1248. doi: 10.1016/s0016-5085(89)80010-1. [DOI] [PubMed] [Google Scholar]
- 3.McNeil PL, Ito S. Molecular traffic through plasma membrane disruptions of cells in vivo. J Cell Sci. 1990;96:549–556. doi: 10.1242/jcs.96.3.549. [DOI] [PubMed] [Google Scholar]
- 4.Yu QC, McNeil PL. Transient disruptions of aortic endothelial cell plasma membranes. Am J Pathol. 1992;141:1349–1360. [PMC free article] [PubMed] [Google Scholar]
- 5.Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coulombe PA, Hutton ME, Vassar R, Fuchs E. A function for keratins and a common thread among different types of epidermolysis bullosa simplex diseases. J Cell Biol. 1991;115:1661–1674. doi: 10.1083/jcb.115.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilbert RJ. Pore-forming toxins. Cell Mol Life Sci. 2002;59:832–844. doi: 10.1007/s00018-002-8471-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinhardt RA, Bi G, Alderton JM. Cell membrane resealing by a vesicular mechanism similar to neurotransmitter release. Science. 1994;263:390–393. doi: 10.1126/science.7904084. [DOI] [PubMed] [Google Scholar]
- 9.Bi GQ, Alderton JM, Steinhardt RA. Calcium-regulated exocytosis is required for cell membrane resealing. J Cell Biol. 1995;131:1747–1758. doi: 10.1083/jcb.131.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyake K, McNeil PL. Vesicle accumulation and exocytosis at sites of plasma membrane disruption. J Cell Biol. 1995;131:1737–1745. doi: 10.1083/jcb.131.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terasaki M, Miyake K, McNeil PL. Large plasma membrane disruptions are rapidly resealed by Ca2+-dependent vesicle-vesicle fusion events. J Cell Biol. 1997;139:63–74. doi: 10.1083/jcb.139.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNeil PL, Vogel SS, Miyake K, Terasaki M. Patching plasma membrane disruptions with cytoplasmic membrane. J Cell Sci. 2000;113:1891–1902. doi: 10.1242/jcs.113.11.1891. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez A, Webster P, Ortego J, Andrews NW. Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells. J Cell Biol. 1997;137:93–104. doi: 10.1083/jcb.137.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reddy A, Caler EV, Andrews NW. Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell. 2001;106:157–169. doi: 10.1016/s0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- 15.Detrait E, Eddleman CS, Yoo S, Fukuda M, Nguyen MP, Bittner GD, et al. Axolemmal repair requires proteins that mediate synaptic vesicle fusion. J Neurobiol. 2000;44:382–391. doi: 10.1002/1097-4695(20000915)44:4<382::aid-neu2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 16.Jaiswal JK, Chakrabarti S, Andrews NW, Simon SM. Synaptotagmin VII restricts fusion pore expansion during lysosomal exocytosis. PLoS Biol. 2004;2:233. doi: 10.1371/journal.pbio.0020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bi GQ, Morris RL, Liao G, Alderton JM, Scholey JM, Steinhardt RA. Kinesin- and myosin-driven steps of vesicle recruitment for Ca2+-regulated exocytosis. J Cell Biol. 1997;138:999–1008. doi: 10.1083/jcb.138.5.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNeil AK, Rescher U, Gerke V, McNeil PL. Requirement for annexin A1 in plasma membrane repair. J Biol Chem. 2006;281:35202–35207. doi: 10.1074/jbc.M606406200. [DOI] [PubMed] [Google Scholar]
- 19.Mellgren RL, Zhang W, Miyake K, McNeil PL. Calpain is required for the rapid, calcium-dependent repair of wounded plasma membrane. J Biol Chem. 2007;282:2567–2575. doi: 10.1074/jbc.M604560200. [DOI] [PubMed] [Google Scholar]
- 20.Mellgren RL, Miyake K, Kramerova I, Spencer MJ, Bourg N, Bartoli M, et al. Calcium-dependent plasma membrane repair requires m- or mu-calpain, but not calpain-3, the proteasome or caspases. Biochim Biophys Acta. 2009;1793:1886–1893. doi: 10.1016/j.bbamcr.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNeil PL, Kirchhausen T. An emergency response team for membrane repair. Nat Rev Mol Cell Biol. 2005;6:499–505. doi: 10.1038/nrm1665. [DOI] [PubMed] [Google Scholar]
- 22.Fein A, Terasaki M. Rapid increase in plasma membrane chloride permeability during wound resealing in starfish oocytes. J Gen Physiol. 2005;126:151–159. doi: 10.1085/jgp.200509294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie XY, Barrett JN. Membrane resealing in cultured rat septal neurons after neurite transection: evidence for enhancement by Ca(2+)-triggered protease activity and cytoskeletal disassembly. J Neurosci. 1991;11:3257–3267. doi: 10.1523/JNEUROSCI.11-10-03257.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Togo T, Alderton JM, Bi GQ, Steinhardt RA. The mechanism of facilitated cell membrane resealing. J Cell Sci. 1999;112:719–731. doi: 10.1242/jcs.112.5.719. [DOI] [PubMed] [Google Scholar]
- 25.Miyake K, McNeil PL, Suzuki K, Tsunoda R, Sugai N. An actin barrier to resealing. J Cell Sci. 2001;114:3487–3494. doi: 10.1242/jcs.114.19.3487. [DOI] [PubMed] [Google Scholar]
- 26.Bement WM, Mandato CA, Kirsch MN. Wound-induced assembly and closure of an actomyosin purse string in Xenopus oocytes. Curr Biol. 1999;9:579–587. doi: 10.1016/s0960-9822(99)80261-9. [DOI] [PubMed] [Google Scholar]
- 27.Mandato CA, Bement WM. Contraction and polymerization cooperate to assemble and close actomyosin rings around Xenopus oocyte wounds. J Cell Biol. 2001;154:785–797. doi: 10.1083/jcb.200103105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandato CA, Bement WM. Actomyosin transports microtubules and microtubules control actomyosin recruitment during Xenopus oocyte wound healing. Curr Biol. 2003;13:1096–1105. doi: 10.1016/s0960-9822(03)00420-2. [DOI] [PubMed] [Google Scholar]
- 29.Abreu-Blanco MT, Verboon JM, Parkhurst SM. Cell wound repair in Drosophila occurs through three distinct phases of membrane and cytoskeletal remodeling. J Cell Biol. 2011;193:455–464. doi: 10.1083/jcb.201011018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foe VE, Alberts BM. Studies of nuclear and cytoplasmic behaviour during the five mitotic cycles that precede gastrulation in Drosophila embryogenesis. J Cell Sci. 1983;61:31–70. doi: 10.1242/jcs.61.1.31. [DOI] [PubMed] [Google Scholar]
- 31.Wood W, Jacinto A, Grose R, Woolner S, Gale J, Wilson C, et al. Wound healing recapitulates morphogenesis in Drosophila embryos. Nat Cell Biol. 2002;4:907–912. doi: 10.1038/ncb875. [DOI] [PubMed] [Google Scholar]
- 32.Clark AG, Miller AL, Vaughan E, Yu HY, Penkert R, Bement WM. Integration of single and multicellular wound responses. Curr Biol. 2009;19:1389–1395. doi: 10.1016/j.cub.2009.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McNeil PL, Baker MM. Cell surface events during resealing visualized by scanning-electron microscopy. Cell Tissue Res. 2001;304:141–146. doi: 10.1007/s004410000286. [DOI] [PubMed] [Google Scholar]