Abstract
Cytokinesis is the final stage of cell division in which the daughter cells separate. Although a growing body of evidence suggests that cell migration-induced traction forces may be required to provide physical assistance for daughter cells to dissociate during abscission, the role of cell migration in cytokinesis has not been directly elucidated. Recently, we have demonstrated that Crk and paxillin, which are pivotal components of the cell migration machinery, localize to the midbody and are essential for the abscission. These findings provided an important link between the cell migration and cytokinesis machineries and prompted us to dissect the role of cell migration in cytokinesis. We show that cell migration controls the kinetics of cleavage furrowing, midbody extension and abscission and coordinates proper subcellular redistribution of Crk and syntaxin-2 to the midbody after ingression.
Key words: cell migration, cytokinesis, midbody, abscission, cleavage furrow, Crk, paxillin, syntaxin-2, ExoT
Introduction
During cell division and concomitant with mitosis, equal partitioning of cytoplasm and separation of daughter cells occurs by a process known as cytokinesis (reviewed in refs. 1–3). Mammalian cells use a contractile ring, a network of actin and myosin filaments, which is attached to the plasma membrane, to create a cleavage furrow that partitions the dividing cell into two daughter cells during ingression. Recent studies have provided insights into the role of different microtubule structures, such as astral and central spindle microtubules and associated proteins, such as the MKLP1 subfamily protein kinesin, Rho A and MgcRacGAP, in cleavage furrow positioning and ingression during cytokinesis.3
After furrowing is completed, the two daughter cells remain connected through a microtubule-based central spindle and a cytoplasmic bridge, collectively known as the midbody. During abscission, the final stage of cytokinesis, daughter cells resolve the midbody and dissociate. Abscission requires a series of dynamic events, including midbody-targeted vesicle secretion, specialization of plasma membrane domains, disassembly of midbody-associated microtubule bundles and plasma membrane fission (reviewed in refs. 2 and 4). A large number of molecular factors required for abscission have been identified through various genetic and RNAi-based studies in simple model organisms, such as yeast, Dictyostelium, Drosophila and C. elegans, as well as from proteomic studies in mammalian cells.1,2,5–10
A growing body of evidence from the past two decades suggests that migration of daughter cells in opposite directions may also be required for cytokinesis.11–17 However, the role of cell migration in cytokinesis has not been directly elucidated. Recently, we have demonstrated that Crk and paxillin, which are pivotal components of the cell migration machinery,18–21 are essential for abscission.22 We have demonstrated that paxillin is recruited to the midbody after furrow completion where it recruits Crk, which in turn recruits syntaxin 2, all necessary steps for a successful cytokinesis. These findings provided an important link between cell migration and cytokinesis and prompted us to dissect the role of cell migration in cytokinesis.
In this communication, we report that cell migration is essential for cytokinesis. We show that the kinetics of ingression, midbody extension and abscission steps in cytokinesis correlate with the ability of daughter cells to migrate in opposite directions. Moreover, we show that cell migration also functions to coordinate subcellular redistribution of Crk and syntaxin-2, but not paxillin, to the midbody after furrow completion.
Results
Cell migration is required for cytokinesis in adherent cells.
Although cell migration has been suggested to contribute to cytokinesis in dividing cells for nearly two decades,11–13 its role in cytokinesis has not been directly elucidated. The fact that paxillin and Crk play pivotal roles in cell migration23 and cytokinesis22 provided an important link between these two cellular processes and prompted us to study the role of cell migration in cytokinesis.
To examine the contribution of cell migration to cytokinesis, we first examined several β1-integrin blocking antibodies for their ability to inhibit cell migration. We chose β1 integrin because it is the main β integrin subunit expressed in HeLa cells. It forms heterodimers with the α1, α2, α3, α5 and α6 alpha subunits to form integrins that engage the extracellular matrix during cell adhesion and cell migration.24 We determined that addition of MAB17781 monoclonal antibody to scrape-wounded HeLa cells effectively inhibited cell migration and wound closure in a dose-dependent manner, while a control isotype antibody (IgG) had no effect at the highest concentration tested (Fig. 1 and Sup. Vids. 1 and 2).
Figure 1.
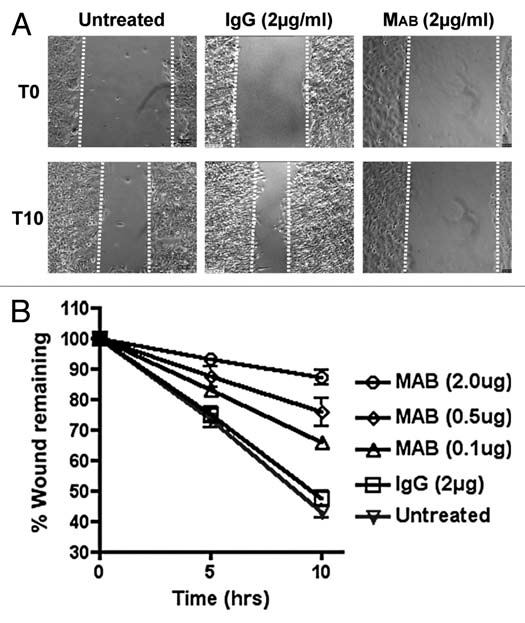
MAB17781 inhibits cell migration. (A) Confluent HeLa cells were scrape-wounded as previously described in reference 37, and the effect of MAB17781 (MAB), a β1-integrin blocking antibody, or IgG control isotype antibody (2 µg/ml) on cell migration was assessed by timelapse videomicroscopy (Sup. Vids. 1 and 2). Shown are representative frames from 0 and 10 hr time-points (T0 and T10 respectively) after wounding and antibody treatment. (B) The effect of MAB17781 antibody on wound closure (% wound remaining) was measured in triplicate samples at 5 and 10 hr post wounding and antibody treatment. The error bars represent the mean ± SEM, indicating that MAB17781 inhibits cell migration in a dose-dependent manner, while the control IgG antibody has no effect.
We then added this antibody to a subconfluent culture of HeLa cells 5 hr post-seeding, so as not to interfere with adhesion of cells to the surface of the well, and assessed the impact of inhibition of cell migration on cytokinesis by flow cytometry 48 hr after antibody addition. The data indicated 80% increase in the G2 sub-population of binucleated cells in the population of cells treated with MAB17781 compared to the control IgG-treated cells (Fig. 2A and B, p < 0.001), supporting the notion that cell migration may be required for cytokinesis.
We have previously shown that protein depletion of CrkI/ II or paxillin by siRNA inhibits cytokinesis.22 Western blotting analyses, however, indicated that inhibition of cell migration by MAB17781 had no effect on cellular levels of CrkI/II or paxillin proteins (Fig. 2C), ruling out the possibility that the MAB17781-mediated cytokinesis block may be due to reduction in the levels of these focal adhesion proteins. Although, integrin signaling has not been implicated in cytokinesis,25,26 there remained a possibility that the MAB17781-induced inhibition of cytokinesis may have resulted from an interference of this antibody with another cellular signaling pathway and not due to its inhibitory effect on cell migration per se. We reasoned that because cell migration requires adhesion to the surface, if the physical act of cell migration is required for cytokinesis, then MAB17781 treatment should only inhibit cytokinesis of adherent cells and not that of non-adherent cells growing in suspension media. On the other hand, if MAB17781-induced inhibition of cytokinesis was due to its interference with another signaling pathway, MAB17781 treatment would likely inhibit cytokinesis in both adherent and non-adherent cells. MAB17781 had no effect on cytokinesis or CrkI/II and paxillin cellular levels in HeLa cells grown in suspension media (Fig. 3), supporting the notion that cell migration is required for cell division. Of note, more cytotoxicity was observed in HeLa cells growing in suspension media, as indicated by increased sub-G1 levels in Figure 3, compared to adherent cells, which remained mostly viable (Fig. 2). This finding is consistent with reports indicating that adhesion to the extracellular matrix promotes cell survival.27,28
Figure 2.
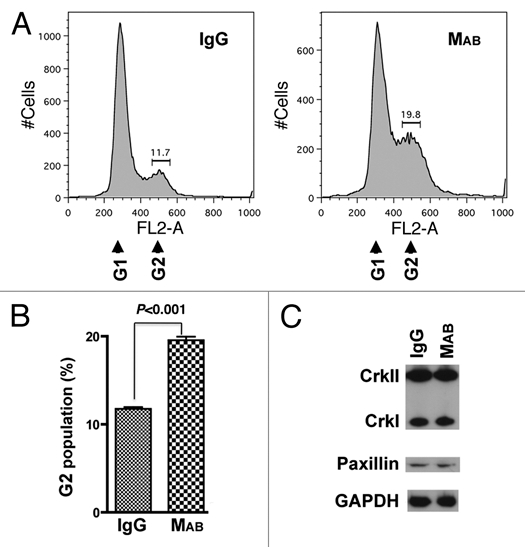
MAB17781 inhibits cytokinesis of adherent HeLa cells. The β1-blocking antibody MAB17781 (MAB) and IgG control isotype antibody were added to a sub-confluent culture of HeLa cells 5 hr post-seeding. (A) Flow cytometry histograms of IgG (control) or MAB-treated cells, 48 hr post-treatment, indicate a rise in the G2 (binucleated cells) subpopulation in the presence of MAB17781. (B) Tabulated results from experiments in (A) done in triplicate and represented as the mean ± SEM (p < 0.001, Student's t-test) have been presented, confirming cytokinesis failure as evidenced by an increase in the G2 subpopulation induced by MAB17781. (C) Western blot analysis of IgG or MAB-treated cells, 48 hr post-treatment, indicate that MAB treatment does not affect steady state levels of CrkII, CrkI or paxillin. GAPDH was used as the loading control.
Figure 3.
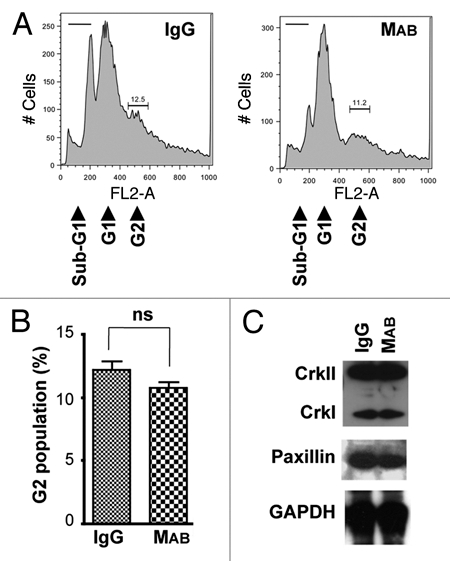
MAB17781 does not inhibit cytokinesis of HeLa cells in suspension. The β1-blocking antibody MAB17781 (MAB) and IgG control isotype antibody were added to HeLa cells grown in suspension media augmented with Z-VAD-fmk (60 µM final concentration) to prevent apoptotic cell death. (A) Flow cytometry histograms of IgG (control) and MAB-treated cells, 48 hr post-treatment, indicate MAB17781 has no effect on cytokinesis as it does not significantly change the G2 (binucleated cells) sub-population. Note that growth in suspension media resulted in an increased cytotoxicity as manifested by a rise in the sub-G1 population. (B) Tabulated results from experiments in (A) done in triplicate and represented as mean ± SEM have been presented. (C) Western blot analysis of IgG and MAB-treated cells, 48 hr post treatment, indicate that MAB treatment does not affect CrkII, CrkI and paxillin protein levels. GAPDH was used as the loading control.
Cell migration is required for multiple steps in cytokinesis.
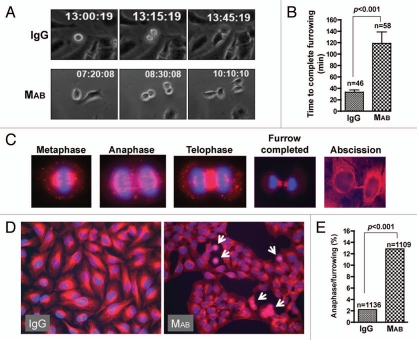
Our data demonstrated that inhibition of cell migration by MAB17781 resulted in failed cell division in adherent cells but could not distinguish whether cell migration was required for mitosis or cytokinesis. We employed time-lapse videomicroscopy to distinguish between these possibilities. While MAB17781 treatment did not appear to affect mitosis, 84% of dividing HeLa cells (n = 33) failed cytokinesis in the presence of MAB17781 (Sup. Vid. 3), compared to IgG-treated cells, which exhibited normal division (n = 44, p < 0.001, Sup. Vid. 4) confirming the importance of cell migration in cytokinesis, and consistent with the results obtained by flow cytometry (Fig. 2). MAB17781 caused cytokinesis failure at two distinct steps. The majority of dividing cells (∼60%) initiated furrowing (ingression) but failed cytokinesis prior to furrow completion (Fig. 4, top parts and Sup. Vid. 5). The remaining cells (∼40%) appeared to complete furrowing, though with a delay kinetically, but were unable to complete abscission (Fig. 4, middle parts and Sup. Vid. 6). No cytokinesis failure was observed in the presence of control IgG (Sup. Vids. 4 and 7). In the cells that managed to complete furrowing, MAB17781 slowed down ingression significantly (Figs. 5A and B) as it took nearly four times longer to complete furrowing in the presence of MAB17781 (118 ± 20 min) than in the presence of the control IgG isotype (33 ± 4 min, p < 0.0001), indicating that cell migration regulated the kinetics of ingression. We confirmed the slowdown in ingression by IF microscopy, using β-tubulin and nuclear staining. We found that of all the dividing cells in asynchronous cultures of untreated or IgG-treated HeLa cell monolayers (n = 1,136), about 2% ± (0.4) were in metaphase through telophase, while the rest (98%) were in a post-furrowing stage (Fig. 5C). We expected the slowdown in furrowing completion in the presence of MAB17781 to result in a significant increase in dividing cells prior to furrowing. In line with this view, the inhibition of cell migration by MAB17781 led to a greater than five-fold increase in cells trapped at these stages (Fig. 5D and E, n = 1,109, p < 0.001). As expected and in line with the flow cytometry and time-lapse videomicroscopy data (Figs. 2 and 4 respectively), the majority of the cells appeared binucleated in the presence of MAB17781 (Fig. 5D).
Figure 4.
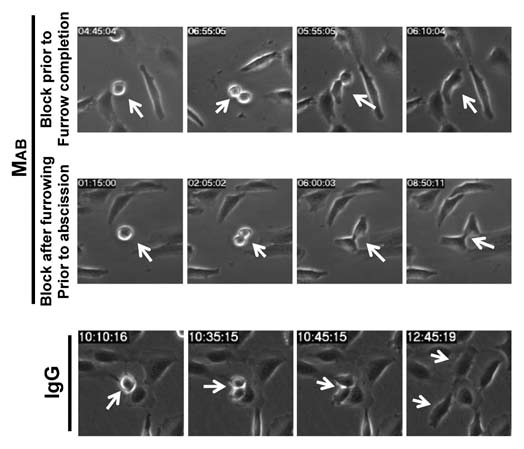
Inhibition of cell migration is sufficient to inhibit cytokinesis. The β1-blocking MAB17781 (MAB) or control IgG isotype antibodies were added to sub-confluent culture of attached HeLa cells 5 hr postseeding. Shown are representative frames from timelapse videomicroscopy, indicating that in the presence of MAB17781, HeLa cells either fail cytokinesis after furrow initiation but prior to furrow completion (Sup. Vid. 5) or after furrow completion but prior to midbody extension and/or other late stages of abscission (Sup. Vid. 6). IgG control antibody had no effect on cytokinesis (Sup. Vid. 7).
Figure 5.
Cell migration regulates the kinetics of ingression. (A) The kinetics of ingression, defined as the time between formation of the metaphase plate until completion of the cleavage furrow, was assessed by timelapse microscopy. The β1-blocking MAB17781 (MAB) or control IgG isotype antibodies were added to sub-confluent culture of attached HeLa cells 5 hr post-seeding. (B) Tabulated results from experiments in (A) and represented as mean ± SEM (p < 0.001, χ2 test) have been presented. The numbers above the column indicate the total number of cells analyzed. (C) Hoechst nuclear dye (blue) and β-tubulin specific antibody (red) were used to show various stages of mitosis/cytokinesis in HeLa cells. (D) Sub-confluent HeLa cells were exposed to MAB17781 or control IgG for 48 hr, fixed and stained for β-tubulin and Hoechst nuclear stain. Note that exposure to MAB17781 prevents cell spreading and culminates in groups of HeLa cells in tight aggregates. (E) Tabulated results from experiments in (D) are presented, indicating that MAB treatment results in a significant increase (p < 0.001, χ2 test) in cells that have accumulated in metaphase through to telophase. The numbers above the column indicate the total number of cells analyzed. These data are in agreement with the results in (A and B) and support the notion that cell migration regulates furrowing kinetics.
Cell migration coordinates the midbody localization of Crk and syntaxin-2 but not paxillin.
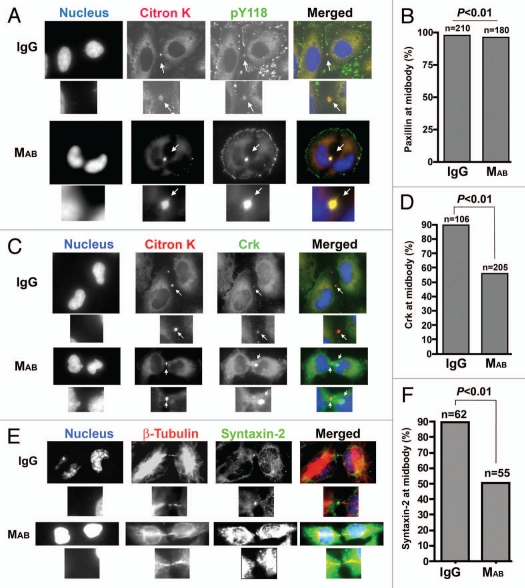
The requirement for cell migration in cytokinesis during cleavage furrowing and the abscission steps prompted us to examine whether cell migration, in addition to providing physical forces during these stages, may also be required for proper positioning and recruitment of molecular components of cytokinesis. We have demonstrated22 that phosphopaxillin localizes to the midbody after furrow completion where it recruits Crk, which in turn mediates the recruitment of syntaxin-2, another component of the terminal stage cytokinetic machinery.7 We used citron K as the marker for dividing cells and IF imaging to investigate the effect of inhibition of cell migration by MAB17781 on phospho-paxillin, Crk and syntaxin-2 localization to the midbody during cytokinesis in cells that were able to complete furrowing. We did not expect citron K localization to be affected by MAB17781, as citron K is recruited to the membrane by RhoA prior to furrow initiation22,29 and MAB17781 treatment did not interfere with furrow initiation (Figs. 4 and 5A and Sup. Vids. 3, 5 and 6). Addition of MAB17781 to subconfluent HeLa cells did not affect phospho-paxillin midbody localization (Fig. 6A and B) but prevented Crk (n = 205) co-localization with citron K at the midbody in over 40% of cells examined (Fig. 6C and D, p < 0.01). In contrast, Crk-midbody staining was absent in 10.5% (n = 106) of IgG-treated cells. As expected, syntaxin-2 was not observed at the midbody in 59% (n = 62) of cells treated with MAB17781, despite longer exposure times for the syntaxin-2 panel, compared to 12.5% (n = 55) in IgG-treated cells (Fig. 6E and F, p < 0.01). Interestingly, in MAB17781-treated cells, Crk appeared as aggregates in close proximity to citron K (Fig. 6C). These results indicated that interference with cell migration adversely affect the delivery of Crk and syntaxin-2 to the midbody during cytokinesis.
Figure 6.
Cell migration is required for proper midbody localization of Crk and syntaxin-2 but not paxillin. Subconfluent HeLa cells were treated with MAB17781 or IgG control antibody, fixed and stained for citron K (red), Hoechst nuclear dye (blue) and either pY118-paxillin (green) in (A) or Crk (green), which interacts with both isoforms of Crk, in (C). In (E) HeLa cells were stained with Hoechst nuclear dye (blue), β-tubulin (red) and syntaxin-2 (green). The fraction of cells with paxillin (B), Crk (D) or syntaxin-2 (E) at the midbody were tabulated from multiple fields by IF microscopy. Localization of Crk and syntaxin-2 but not paxillin to the midbody was significantly inhibited (p < 0.01, χ2 test) in MAB17781-treated cells. The numbers above the column indicate the total number of cells analyzed. Note that Crk appears as aggregates near citron K at the midbody in (C). Also of note, the syntaxin-2 part in MAB17781-treated cells (E) is overexposed to show the absence of this protein at the midbody more clearly.
Discussion
Although cell migration has been suggested to contribute to cytokinesis in dividing cells for nearly two decades,11–17 its role in cytokinesis has not been directly elucidated. For the first time, we demonstrate that cell migration is indeed required for cytokinesis of mammalian cells. We show that blocking cell migration using a β1-integrin blocking antibody, MAB17781, while having no effect on the intracellular levels of Crk or paxillin, results in cytokinesis failure at multiple steps in adherent cells. Our data indicate that, in addition to providing daughter cells with physical assistance during ingression, midbody extension and abscission, cell migration coordinates the midbody recruitment of late cytokinesis components, Crk and syntaxin-2.
It has been generally appreciated that constriction of the actomyosin ring, mediated by RhoA and its recruitment of citron K, ROCK and myosin II, is the main driving force needed to cleave a cell into two during cytokinesis.1,30 We now show that cell migration is also essential for this process. Inhibition of cell migration by MAB17781, while having no impact on the recruitment of RhoA and citron K (Fig. 6 and data not shown), or their activity with respect to furrow initiation,29 caused cytokinesis failure prior to furrow completion (Fig. 4 and Sup. Vids. 3 and 5). Moreover, ingression was slowed down by nearly six-fold in dividing cells that were able to complete furrowing under the conditions where cell migration was inhibited (Fig. 5A and B). This slowdown manifested as a greater than five-fold accumulation of mitotic cells prior to furrow completion (Fig. 5C–E, p < 0.001). Collectively, these data indicate that cell migration is also necessary for progression of furrowing. Moreover, very few cells managed to extend their midbodies after furrow completion or dissociation; rather they appeared to remain trapped in the abscission step (Sup. Vids. 3 and 6), indicating that cell migration is also required for these steps in cytokinesis. Exactly how cell migration impacts progression through furrowing during cytokinesis remains to be determined. The fact that in the presence of MAB1771, dividing cells are able to initiate furrowing (Sup. Vids. 3, 5 and 6) indicate that the factors involved in central spindle and contractile ring3 assembly are recruited and functioning properly at least until furrow initiation. Supporting this interpretation, we find RhoA and citron K to be localized to the cell cortex during furrow initiation (Fig. 6 and data not shown). We postulate that cleavage furrowing in adherent cells may require physical assistance, which is provided by cell migration. In line with this interpretation, traction forces have been shown to be generated during ingression in dividing human fibroblasts.11
Our data further indicate that in addition to a physical contribution, cell migration is also necessary to coordinate the timely subcellular redistribution and midbody localization of at least Crk and syntaxin-2 (Fig. 6) and perhaps other late molecular components of the cytokinetic machinery after furrow completion. The finding that Crk fails to colocalize with citron K and phosphopaxillin in the presence of MAB17781 supports this notion and further confirms the hypothesis that paxillin's localization to the midbody and function in cytokinesis is upstream of Crk.22 It remains to be determined how the migration of daughter cells regulates the timely recruitment of Crk to the midbody during cytokinesis. The finding that Crk appears as aggregates near the midbody under conditions where cell migration is blocked (Fig. 6) suggests that Crk may be shuttled to the midbody by specialized vesicles or structures in the membrane. Consistent with this view, it has now been established that new membrane is added to expanding daughter cells during cytokinesis and that these membranes have a distinct lipid and protein composition from the rest of the plasma membrane.31–33 This may reflect a requirement for both increased surface area during cytokinesis and for the coordinated delivery of important cytokinetic components, such as Crk, to the correct spatial coordinates required during cytokinesis. Cell migration, by the virtue of rapid membrane remodeling, is likely involved in facilitating cargo trafficking through the membrane to the midbody during cytokinesis.
The data reported here have important implications for cancer, particularly with respect to metastatic cancer, as they suggest that cells may have different molecular requirements for division depending on whether they are growing as adherent cells, as in solid tumors or planktonically, when they metastasize. In line with this notion, Lee et al.13 have reported that Dictostelium, deficient in RasGAP1, is defective in cytokinesis only when grown in suspension medium but not when allowed to adhere to the surface. It is thus imperative to assess the efficacy of chemotherapeutic treatment in adhesion-dependent and independent growth models.
Materials and Methods
Antibodies.
Antibodies were obtained from the following sources: CrkI/II, paxillin and pY118-paxillin (BD Transduction Laboratories, San Jose, CA); β-tubulin (Sigma, St. Louis, MO); RhoA, citron K and IgG1 (Santa Cruz Biotechnology, Santa Cruz, CA); syntaxin-2 for IF (Synaptic Systems, Goettingen, Germany) and western blotting (Calbiochem, San Diego, CA); MAB17781 antibody (R&D Systems, Minneapolis, MN); GAPDH (Chemicon International, Temecula, CA); HRP-conjugated goat anti-mouse and rabbit IgG (Jackson ImmunoResearch; West Grove, PA).
Growth and maintenance of epithelial cell lines.
HeLa cells were grown and maintained in Minimal Essential-Eagle medium (MEM) supplemented with 10% heat-inactivated FBS (Gibco BRL), L-glutamine (0.292 g/liter), glucose (1.0 g/liter) and NaHCO3 (2.2 g/liter) and incubated at 37°C in the presence of 5% CO2 at a density of 1.0 × 106 cells/well (24-well dish) to obtain confluent monolayers for wound healing experiments and 2.0 × 104 cells/well (24-well dish) to achieve subconfluence. For experiments involving IF videomicroscopy, cells were grown in the same media without phenol red for 3 days prior to seeding. Cells were then seeded in the same media without phenol red and augmented with Z-VAD-fmk (R&D Systems, Minneapolis, MN) at 60 µM to block cell death and with propidium iodide (PI) at 7 µg/ml (Sigma, Saint Louis, MO) to identify dying cells.
Static and live microscopy.
All static and live imaging were performed as described previously in reference 22 and 34. For static microscopy, 48 hr after antibody treatment, the attached cells were fixed with 10% Trichloroacetic acid (TCA; Fisher Scientific, Fair Lawn, NJ) for 10 min at room temperature, washed three times with PBS containing glycine (30 mM), permeabilized with PBS containing 0.2% Triton X-100 for 15 min at 37°C and blocked with PBS containing 0.7% fish scale gelatin for 1 hr at 37°C. The primary antibodies were added to the blocking solution and incubated overnight at 4°C. All antibodies were added at 1:50 dilution except the mouse monoclonal anti-β-tubulin antibody, which was added at 1:200 dilution. Cells were washed three times (10 min at 37°C incubation each) after which the secondary antibodies (all at 1:400 dilution) and Hoechst 33342 nuclear stain (at 5 µg/ml from Molecular Probes, Eugene, OR) were added to the same blocking solution and incubated for 1 hour at 37°C. After washing three times with PBS (5 min each), the coverslips were dipped in ddH2O and mounted using Vectashield anti-fading mounting solution (Vector Laboratories, Burlingame, CA). Images were captured at a 1,000× magnification under oil immersion with a Zeiss Axio Observer Z1 Motorized Inverted Research Microscope equipped with an environmental chamber and optimized for immunofluorescent static and live cell imaging studies. Video images were captured every 5 min for phase contrast imaging.
FACS analyses.
1.0 × 106 HeLa cells were seeded in 6-well dishes or grown in suspension in Ultra Low Attachment dishes (Fisher) which were pre-coated with Poly-HEME (Aldrich Chemicals) as described in reference 35. Z-VAD-fmk at 60 µM was added to prevent apoptotic cell death. 5 hr after seeding, MAB17781 or IgG control isotype antibodies were added at 2 µg/ml concentration. 48 hr after treatment, adherent cells and non-adherent cells were collected and cell cycle analyses were performed by flow cytometry, using Flo Jo software as described previously in reference 36.
Western blot analyses.
HeLa cells were seeded into 6-well dishes at 1.0 × 106 cells/well density to achieve subconfluence. 5 hr after seeding, MAB17781 or IgG control isotype antibodies were added at a concentration of 2 µg/ml. 48 hr after antibody treatment, the rounded non-adherent cells contained in the media and washes were collected by centrifugation at 1,000 rpm for 5 min. The cell pellet and remaining adherent cells were lysed with 150 µl of 1% Triton X-100 in PBS with Complete Mini Protease Inhibitor Cocktail (Roche, Indianapolis, IN) at room temperature for 20 min. The lysis mixture was centrifuged at 14,000 rpm for 15 min at room temperature. The supernatant was combined with 50 µl of 4X SDS-PAGE sample buffer. A 20 µl volume of the sample was electrophoresed on an 8% SDS-polyacrylamide gel. Proteins were transferred to polyvinylidene difluoride membranes and immunoblotted for the indicated proteins with antibodies to Crk (1:2,500 dilution), paxillin (1:500 dilution) and syntaxin-2 (1:500 dilution). Duplicate membranes were immunoblotted with anti-GAPDH antibody (1:10,000 dilution) as the loading control. HRP-conjugated goat anti-mouse or rabbit IgG (1:5,000 dilution) was used to visualize the proteins.
Statistical analysis.
Statistical analyses were based on either the two-tailed Student's t-test and represented as mean ± SEM or the Chi-square that was used to determine pair-wise statistical significance. p < 0.05 was taken as significant.
Acknowledgements
We would like to thank members of the Shafikhani and Engel labs for their advice and suggestions. This work was supported by the NIH grants F32 AI054056 (to S.S.), Rush Startup funds (to S.S.) and R01 AI42806 (to J.E.).
Supplementary Material
References
- 1.Barr FA, Gruneberg U. Cytokinesis: placing and making the final cut. Cell. 2007;131:847–860. doi: 10.1016/j.cell.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Glotzer M. The molecular requirements for cytokinesis. Science. 2005;307:1735–1739. doi: 10.1126/science.1096896. [DOI] [PubMed] [Google Scholar]
- 3.D'Avino PP, Savoian MS, Glover DM. Cleavage furrow formation and ingression during animal cytokinesis: a microtubule legacy. J Cell Sci. 2005;118:1549–1558. doi: 10.1242/jcs.02335. [DOI] [PubMed] [Google Scholar]
- 4.Guizetti J, Gerlich DW. Cytokinetic abscission in animal cells. Semin Cell Dev Biol. 2010 doi: 10.1016/j.semcdb.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Somma MP, Fasulo B, Cenci G, Cundari E, Gatti M. Molecular dissection of cytokinesis by RNA interference in Drosophila cultured cells. Mol Biol Cell. 2002;13:2448–2460. doi: 10.1091/mbc.01-12-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skop AR, Liu H, Yates J, 3rd, Meyer BJ, Heald R. Dissection of the mammalian midbody proteome reveals conserved cytokinesis mechanisms. Science. 2004;305:61–66. doi: 10.1126/science.1097931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Low SH, Li X, Miura M, Kudo N, Quinones B, Weimbs T. Syntaxin 2 and endobrevin are required for the terminal step of cytokinesis in mammalian cells. Dev Cell. 2003;4:753–759. doi: 10.1016/s1534-5807(03)00122-9. [DOI] [PubMed] [Google Scholar]
- 8.Gromley A, Jurczyk A, Sillibourne J, Halilovic E, Mogensen M, Groisman I, et al. A novel human protein of the maternal centriole is required for the final stages of cytokinesis and entry into S phase. J Cell Biol. 2003;161:535–545. doi: 10.1083/jcb.200301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gromley A, Yeaman C, Rosa J, Redick S, Chen CT, Mirabelle S, et al. Centriolin anchoring of Exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell. 2005;123:75–87. doi: 10.1016/j.cell.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 10.Montagnac G, Echard A, Chavrier P. Endocytic traffic in animal cell cytokinesis. Curr Opin Cell Biol. 2008;20:454–661. doi: 10.1016/j.ceb.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Burton K, Taylor DL. Traction forces of cytokinesis measured with optically modified elastic substrata. Nature. 1997;385:450–454. doi: 10.1038/385450a0. [DOI] [PubMed] [Google Scholar]
- 12.Fukui Y. Toward a new concept of cell motility: cytoskeletal dynamics in amoeboid movement and cell division. Int Rev Cytol. 1993;144:85–127. doi: 10.1016/s0074-7696(08)61514-4. [DOI] [PubMed] [Google Scholar]
- 13.Lee S, Escalante R, Firtel RA. A Ras GAP is essential for cytokinesis and spatial patterning in Dictyostelium. Development. 1997;124:983–996. doi: 10.1242/dev.124.5.983. [DOI] [PubMed] [Google Scholar]
- 14.Kanada M, Nagasaki A, Uyeda TQ. Adhesiondependent and contractile ring-independent equatorial furrowing during cytokinesis in mammalian cells. Mol Biol Cell. 2005;16:3865–3872. doi: 10.1091/mbc.E05-03-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angers-Loustau A, Cote JF, Charest A, Dowbenko D, Spencer S, Lasky LA, et al. Protein tyrosine phosphatase-PEST regulates focal adhesion disassembly, migration and cytokinesis in fibroblasts. J Cell Biol. 1999;144:1019–1031. doi: 10.1083/jcb.144.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee CC, Putnam AJ, Miranti CK, Gustafson M, Wang LM, Vande Woude GF, et al. Overexpression of sprouty 2 inhibits HGF/SF-mediated cell growth, invasion, migration and cytokinesis. Oncogene. 2004;23:5193–5202. doi: 10.1038/sj.onc.1207646. [DOI] [PubMed] [Google Scholar]
- 17.Tsubakimoto K, Matsumoto K, Abe H, Ishii J, Amano M, Kaibuchi K, et al. Small GTPase RhoD suppresses cell migration and cytokinesis. Oncogene. 1999;18:2431–2440. doi: 10.1038/sj.onc.1202604. [DOI] [PubMed] [Google Scholar]
- 18.Panetti TS. Tyrosine phosphorylation of paxillin, FAK and p130CAS: Effects on cell spreading and migration. Front Biosci. 2002;7:143–150. doi: 10.2741/A771. [DOI] [PubMed] [Google Scholar]
- 19.Brown MC, Turner CE. Paxillin: Adapting to change. Physiol Rev. 2004;84:1315–1339. doi: 10.1152/physrev.00002.2004. [DOI] [PubMed] [Google Scholar]
- 20.Feller SM. Crk family adaptors-signalling complex formation and biological roles. Oncogene. 2001;20:6348–6371. doi: 10.1038/sj.onc.1204779. [DOI] [PubMed] [Google Scholar]
- 21.Meng F, Lowell CA. A beta1 integrin signaling pathway involving Src-family kinases, Cbl and PI-3 kinase is required for macrophage spreading and migration. EMBO J. 1998;17:4391–4403. doi: 10.1093/emboj/17.15.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shafikhani SH, Mostov K, Engel J. Focal adhesion components are essential for mammalian cell cytokinesis. Cell Cycle. 2008;7:2868–2876. doi: 10.4161/cc.7.18.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feller SM, Posern G, Voss J, Kardinal C, Sakkab D, Zheng J, Knudsen BS. Physiological signals and oncogenesis mediated through Crk family adapter proteins. J Cell Physiol. 1998;177:535–552. doi: 10.1002/(SICI)1097-4652(199812)177:4<535::AID-JCP5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 24.Riikonen T, Vihinen P, Potila M, Rettig W, Heino J. Antibody against human alpha1beta1 integrin inhibits HeLa cell adhesion to laminin and to type I, IV and V collagens. Biochem Biophys Res Commun. 1995;209:205–212. doi: 10.1006/bbrc.1995.1490. [DOI] [PubMed] [Google Scholar]
- 25.Cabodi S, Di Stefano P, Leal Mdel P, Tinnirello A, Bisaro B, Morello V, et al. Integrins and signal transduction. Adv Exp Med Biol. 2010;674:43–54. doi: 10.1007/978-1-4419-6066-5_5. [DOI] [PubMed] [Google Scholar]
- 26.Juliano RL. Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins and immunoglobulin-superfamily members. Annu Rev Pharmacol Toxicol. 2002;42:283–323. doi: 10.1146/annurev.pharmtox.42.090401.151133. [DOI] [PubMed] [Google Scholar]
- 27.Cho SY, Klemke RL. Extracellular-regulated kinase activation and CAS/Crk coupling regulate cell migration and suppress apoptosis during invasion of the extracellular matrix. J Cell Biol. 2000;149:223–236. doi: 10.1083/jcb.149.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Almeida EA, Ilic D, Han Q, Hauck CR, Jin F, Kawakatsu HS, et al. Matrix survival signaling: From fibronectin via focal adhesion kinase to c-Jun NH(2)-terminal kinase. J Cell Biol. 2000;149:741–754. doi: 10.1083/jcb.149.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madaule P, Eda M, Watanabe N, Fujisawa K, Matsuoka T, Bito H, et al. Role of Citron kinase as a target of the small GTPase Rho in cytokinesis. Nature. 1998;394:491–494. doi: 10.1038/28873. [DOI] [PubMed] [Google Scholar]
- 30.Matsumura F. Regulation of myosin II during cytokinesis in higher eukaryotes. Trends Cell Biol. 2005;15:371–377. doi: 10.1016/j.tcb.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Echard A. Membrane traffic and polarization of lipid domains during cytokinesis. Biochem Soc Trans. 2008;36:395–399. doi: 10.1042/BST0360395. [DOI] [PubMed] [Google Scholar]
- 32.Albertson R, Riggs B, Sullivan W. Membrane traffic: A driving force in cytokinesis. Trends Cell Biol. 2005;15:92–101. doi: 10.1016/j.tcb.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 33.O'Halloran TJ. Membrane traffic and cytokinesis. Traffic. 2000;1:921–926. [PubMed] [Google Scholar]
- 34.Garrity-Ryan L, Shafikhani S, Balachandran P, Nguyen L, Oza J, Jakobsen T, et al. The ADP ribosyltransferase domain of Pseudomonas aeruginosa ExoT contributes to its biological activities. Infect Immun. 2004;72:546–558. doi: 10.1128/IAI.72.1.546-558.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frisch SM, Francis H. Disruption of epithelial cellmatrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shafikhani S, Morales C, Engel J. The Pseudomonas aeruginosa type III secreted toxin ExoT is necessary and sufficient to induce apoptosis in epithelial cells. Cell Micro. 2008;10:994–1007. doi: 10.1111/j.1462-5822.2007.01102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.