Abstract
Studies suggest that the affective response is impaired in both schizophrenia and adolescent offspring of schizophrenia patients. Adolescent offspring of patients are developmentally vulnerable to impairments in several domains, including affective responding, yet the bases of these impairments and their relation to neuronal responses within the limbic system are poorly understood. The amygdala is the central region devoted to the processing of emotional valence and its sub-nuclei including the baso-lateral and centro-medial are organized in a relative hierarchy of affective processing. Outputs from the centro-medial nucleus converge on regions involved in the autonomous regulation of behavior, and outputs from the basolateral nucleus modulate the response of reward processing regions. Here using fMRI we assessed the intra-amygdala response to positive, negative, and neutral valenced faces in a group of controls (with no family history of psychosis) and offspring of schizophrenia parents (n=44 subjects in total). Subjects performed an affective continuous performance task during which they continually appraised whether the affect signaled by a face on a given trial was the same or different from the previous trial (regardless of facial identity). Relative to controls, offspring showed reduced activity in the left centro-medial nucleus to positively (but not negatively or neutral) valenced faces. These results were independent of behavioral/cognitive performance (equal across groups) suggesting that an impaired affective substrate in the intra-amygdala response may lie at the core of deficits of social behavior that have been documented in this population.
Keywords: Development, Affective Response, Vulnerability, Centro-medial Nucleus, Baso-lateral nucleus, Reward
1. Introduction
The affective bases of psychiatric disorders continue to be a central question in clinical neuroscience research. Disorders such as schizophrenia, and of mood and anxiety have been associated with impaired affective appraisal and regulation, and resultant impairments in social function and interaction . With regards to schizophrenia specifically, retrospective studies of large scale cohorts have documented widespread impairments in social interaction and function during (pre-morbid) adolescence . These results indicate the emergence of social impairments during critical stages of development in the illness. Social interactions in part rely on intact cortico-limbic function, suggesting that functional alterations particularly in limbic circuitry may impair the appraisal of social cues, and by consequence social behavior.
Impairments in social interaction have also been documented in healthy adolescent offspring of schizophrenia patients. Moreover, estimated incidence rates of schizophrenia-related psychosis or otherwise unspecified psychosis range in offspring of schizophrenia patients (SCZ-Off) range from eight to 20 percent . Though the rates of conversion to the narrowly defined clinical phenotype of schizophrenia may be relatively low, the incidence of Axis I psychopathology is relatively high in this population . In addition, studies of brain function and structure suggest that compared to controls with no family history of psychiatric illness, schizophrenia offspring show widespread deficits . Whereas longitudinal studies that quantify rates of conversion to schizophrenia are lacking, these cross-sectional data indicate that SCZ-Off are vulnerable to developmentally mediated deficits of structural and functional biology which in turn may predispose them to become manifestly ill later in life.
Prospective studies examining the offspring of schizophrenia patients find increased incidence of anhedonia, social withdrawal, affective flattening, poor global adjustment, and poor social competence . Additionally, this population shows a high occurrence (20–50 percent) of schizotaxia disorder, characterized by negative symptoms and neuropsychological dysfunction . Altered limbic system activity may play a crucial role in the neurobiology of the hypothesized affective and social deficits in this population. In particular, impaired responses in key limbic structures during affective appraisal may negatively impact the response to affective facial cues. Finally, given the exquisite developmental expansion that is needed to subserve the normal development of emotional appraisal and processing , offspring who are characterized by developmental derailment may also show altered developmental patterns in their appraisal of stimuli.
The observed affect deficits in SCZ-Off are generally consistent with studies in schizophrenia itself. The altered appraisal of social stimuli is a recognized impairment among schizophrenia patients, and while a complete understanding of the neural bases of these abnormalities is still emerging, studies on emotional face processing in patients suggest state-independent, diminished amygdala activation compared to controls . However, the amygdala is not a functionally homogenous structure. Studies have identified functionally distinguishable sub-regions with separable efferents and afferents to other regions of the brain . Understanding how the intra-amygdala circuit responds in risk, vulnerability and disease may greatly enhance the understanding of how the affective response contributes to behavior and psychiatric illness.
1.1. The emotional centers of the brain: The intra-amygdala circuit
The amygdala is an essential emotional center of the brain which drives emotional processing and output, and amygdala dysfunction underlies emotional and conduct disorders . Functional neuroimaging and PET studies show that both positive and negative emotions drive amygdala activity , yet how the amygdala influences behavioral responses to affect is yet not entirely resolved. Work in the basic neuroscience of emotion has revealed reciprocal influences between the amygdala and other cortical and sub-cortical centers, and these influences may offer rich models linking amygdala function, social behavior and psychiatric disorders. Thus, just as cortical areas influence processing within amygdala nuclei , projections from the amygdala to cortical areas as well as autonomic and reward centers in the cortex and brainstem may in turn influence behavioral responses to emotional stimuli or situations.
Specific patterns of sensory information transmission within amygdala subregions suggest a relative segregation of intra-amygdala function. After information from the sensory cortices enters the amygdala through the lateral nucleus (located in the baso-lateral area [BA]) , adaptive filtering occurs. The lateral nucleus transmits information to the basal and central nuclei , which further process information via intra- and inter-divisional nuclear projections . The basolateral nucleus has been most recently associated with the experience of anxiety , and extensively projects to ventral regions of the frontal cortex, as well as reward related regions such as the nucleus accumbens . Further, the central (or centro-medial) nucleus receives information from all other nuclei, and serves as a key site for signal projections to the cortex, the brainstem and hypothalamic autonomic centers, and may be a critical site for signal integration and output affecting behavior . Figure 1 provides a conceptual model of perceptual information flow from sensory cortices to the amygdala, affective information flow within key amygdala nuclei, and the translation of affective evaluation/response in the amygdala to regions involved in autonomous behavioral regulation and response. As the model implies, the response of key sub-regions within the amygdala such as the basolateral and centro-medial nuclei to affectively valenced stimuli may be crucial in mediating behavioral responses. Combining fMRI data with probabilistic maps of the intra-amygdala nuclei may allow the estimation of, and separation of activity within the intra-amygdala circuit.
Figure 1.
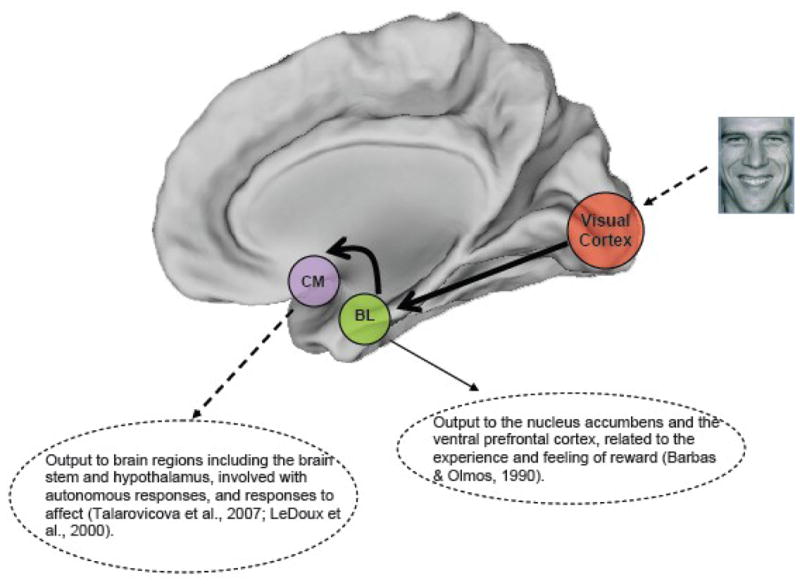
The figure depicts a conceptual model of affective information flow from sensory to cortical regions via intra-amygdala interactions. As noted in the text, adaptive filtering within the amygdala pathways results in relative specialization and specific patterns of information flow. The lateral nucleus (not depicted) transmits information to the basal and central nuclei , the outputs from which have specific roles related to positively (presumably rewarding) and negatively (presumably aversive) valenced stimuli. The basolateral nucleus most recently associated with anxiety , extensively projects to ventral regions of the frontal cortex, as well as reward related regions such as the nucleus accumbens . The centromedial nucleus receives information from all other nuclei, serving as a key site for signal projections to the cortex, the brainstem and hypothalamic autonomic centers.
1.2 Cyto-architectonic maps to assess the intra-amygdala circuit
Recent advances in quantitative cytoarchitectonic mapping facilitate the identification of intra-amygdala activity in vivo by associating brain activation to their cytoarchitectonic origins using a probabilistic atlas . The details have been extensively documented elsewhere . In brief these techniques rely on observer-independent characterization of architectonics in human post-mortem brains followed by an MRI-based probabilistic quantitation of how these cytoarchitectonic regions are mapped into stereotactic space . The ensuing cytoarchitectonic probability maps quantify both location and spatial variability of the respective areas by identifying spatial regions of high convergence against regions of low convergence across sampled post-mortem brains. Figure 2 (left) shows bilateral probabilistic distributions of the baso-lateral and centro-medial nuclei in stereotactic space . The map depicts the considerable spatial variability across samples (see adjacent probability scale). Corresponding maximum probability maps (right, shown for the left hemisphere) are derived by assigning each voxel of the reference space to the most likely anatomical area at the respective position, yielding a continuous, non-overlapping summary based on individual probabilistic maps . This emerging field of combining cytoarchitectonic maps with fMRI has resulted in advances in the mapping of the somatosensory system and activity in Broca’s area (Brodmann areas 44 & 45) and the amygdala , yet the current application is, to our knowledge, the first to estimate intra-amygdala activity differences in developmentally vulnerable populations.
Figure 2.
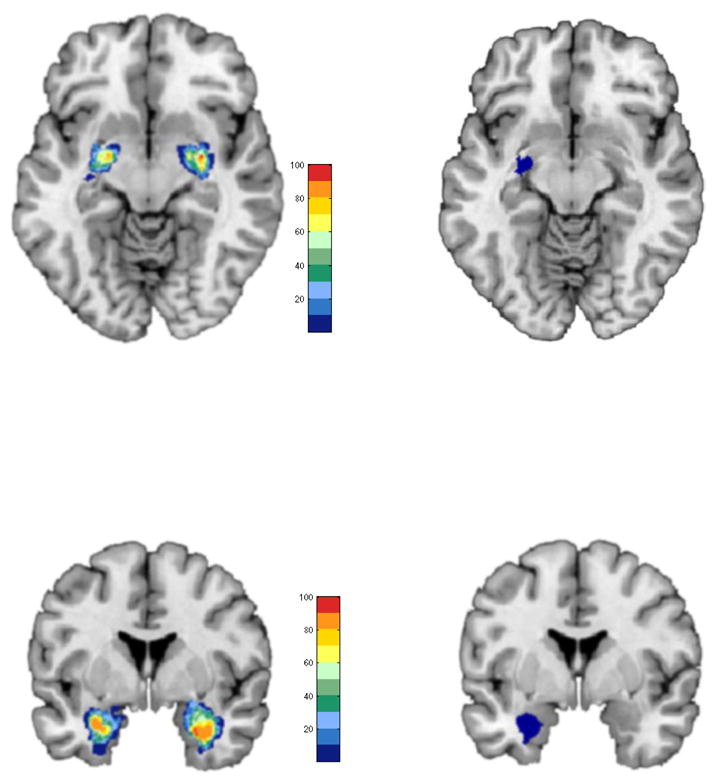
The relationship between quantified spatial probability maps (left) of the centro-medial (top) and baso-lateral (bottom) and corresponding maximum probability maps is depicted in single axial and coronal sections. Note that the maximum probability maps are a subset of the spatial probability maps and reflect maximum spatial probabilities on the accompanying color bars.
Altered limbic system activity may play a crucial role in the neurobiology of the hypothesized affective and social deficits in young offspring of schizophrenia patients . In particular, impaired responses in key limbic structures may result in abnormal signaling to regions modulating behavior, thereby negatively impacting social interaction. It is plausible that limbic impairments precede the manifestation of behavioral impairments and therefore are a leading indicator of behavioral problems that may follow . We used fMRI to investigate the amygdala response, and the response of individual amygdala nuclei to the appraisal of affectively valenced faces in a group of adolescent SCZ-Off and controls (HC) with no family history of psychiatric illness (to the 2nd degree). Given longitudinal and cross-sectional evidence of progressive deficits in function in SCZ-Off , we also assessed age-related changes in the amygdala response through adolescence in each of the groups.
2. Methods
2.1 Subjects
Forty four subjects gave informed consent or assent to participate in the fMRI studies. MRI and behavioral protocols were approved by the Human Investigative Committee (HIC) of Wayne State University. Subjects received monetary compensation for their participation. The twenty five controls (HC; age:10–19, mean=14.9 yrs; 8 females) had no family history of psychiatric illness to the 2nd degree. Nineteen schizophrenia offspring had at least one parent with schizophrenia (SCZ-Off; age:8–19, mean=14.3 yrs; 7 females). Subjects were recruited from the greater Detroit area through advertisements and through in patient services at the University Physicians Center, Wayne State University School of Medicine. Rule outs were achieved through telephone and personal interview, and screening questionnaires, to ascertain if subjects had a history of psychotic illness in first-degree relatives. Diagnoses for parents of offspring were reached using the Structured Clinical Interview for DSM-IV schizophrenia . All HC and SCZ-Off were clinically evaluated using the Schedule for Affective Disorders and Schizophrenia-Child Version and the SCID. Assessments were administered by a trained interviewer (U.R., A.J.). Demographic information including Full Scale IQ are depicted in Table 1. Four SCZ-Off were diagnosed with disorders (which were not exclusionary criteria) including Separation Anxiety Disorder (n=1), Attention Deficit Hyperactivity Disorder hyperactive type (n=1), Attention Deficit Hyperactivity Disorder NOS (n=1) and Social phobia (n=1).
Table 1.
Demographic info including FSIQ, age and gender distributions for the HC and SCZ-Off groups.
| SCZ-Off (n=19) | HC (n=25) | |
|---|---|---|
| Gender (M/F) | 12/7 | 17/8 |
| Age range (yrs) | 8–19 | 10–19 |
| Mean age (± sd) | 14.3 ± 3.1 | 14.9± 2.8 |
| Mean FSIQ (± sd) | 93.8 ± 13.9 | 93.1 ± 15.9 |
2.2 Behavioral Task
Subjects performed a continuous affective task. During the task faces expressing varying emotions were presented (3s/stimulus) in pseudo-random order with a randomly jittered inter-stimulus interval (3–5 s. in 0.5s increments) . Eighty normatively rated photographs depicting positive (happy), negative (angry, fearful, sad) and neutral emotions were used. The subject had to indicate whether the emotion expressed by a face on a given trial was the same as on the preceding trial (irrespective of identity). The task was designed to ensure that subjects appraised the attribute of choice (expressed emotion) without forcing a choice of a verbal label to attach to the expressed emotion. Control stimuli were created by inverting and pixelating face pictures to provide a baseline to assess the effects of visual stimulation. A total of 96 trials were used in the paradigm (80 faces and 16 control stimuli). Stimuli were not repeated. A schematic of the task is presented in Figure 3.
Figure 3.
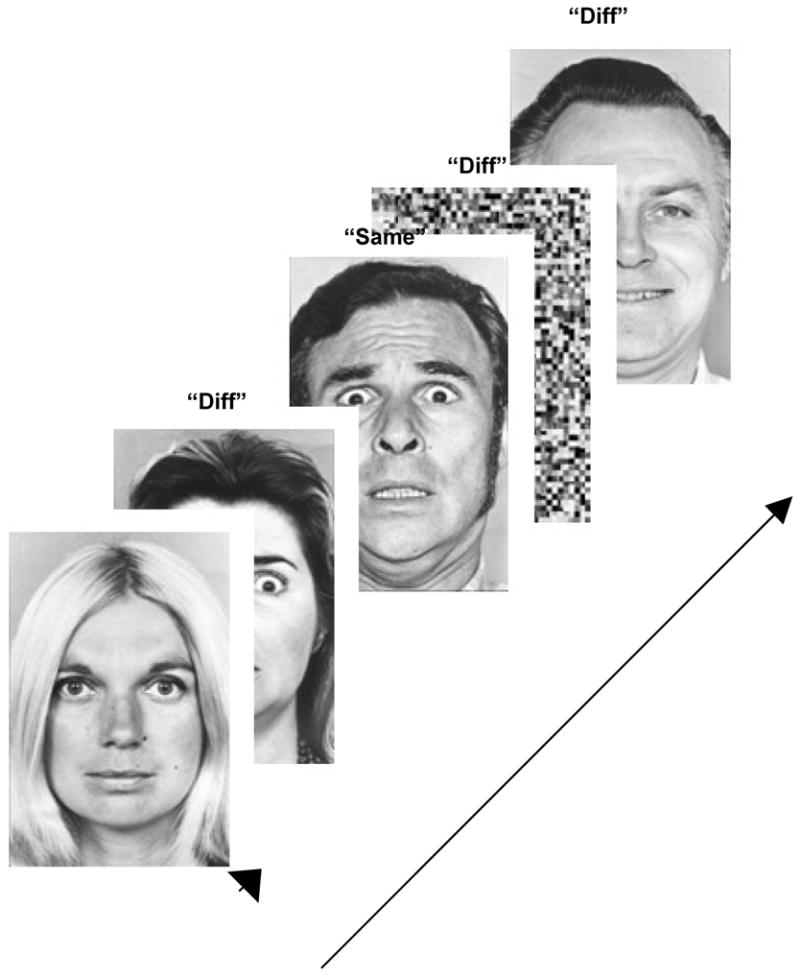
The task is schematically depicted. The appropriate responses to the individual stimuli are labeled for the sake of exposition. fMRI activity to events (faces, small arrow) was assessed (see text for details) and contrasted between HC and SCZ-Off.
2.3 Functional MRI
Gradient echo EPI was acquired over an 11.5 minute scan; (TR: 2s, TE: 30 ms, matrix: 64x64, 24 slices, FOV: 240 mm, voxel size 3.8x3.8x4.0 mm) using a full body Bruker MedSpec 4.0T system. Functional images were preprocessed using SPM5. Raw images were realigned with the AC-PC orientation. Subsequently images in the series were co-registered with the first image and correction for susceptibility-by-movement interactions. Following spatial realignment the images were then normalized to a standard EPI template (Montreal Neurological Institute). Individual stimulus presentations were modeled as 3 s box-car events convolved with a canonical hemodynamic reference wave form.
2.3.1 Intra-amygdala analyses
The centro-medial and baso-lateral amygdala nuclei were identified on the bases of maximum probability maps derived from probabilistic cytoarchitectonic maps (Fig 2, right). To minimize the influence of smoothing and therefore maximize the effective spatial resolution of the fMRI data within these small sub-regions , BOLD was extracted from unsmoothed normalized images. The resultant data reflect estimated signal change to the stimuli of interest (positively, neutral and negatively valenced faces) in those regions of interest.
2.3.2 Activation analyses within SPM
For random field analyses, normalized images were smoothed with an 8 mm full width at half maximum (FWHM) isotropic Gaussian kernel. Individual contrasts were employed to examine main effects of valence (Positive, Neutral, Negative vs. Distorted images). First level contrasts from each subject were submitted to second level random effects analyses where individual contrasts were used to identify intra-group activity in HC and SCZ-Off.
3. Results
3.1 Behavioral Results
Discrimination sensitivity was assessed using d’ . An analyses of covariance with group as the single factor and age and gender as covariates revealed no significant differences in d’ between the groups, F1,40=.38, p>.5, indicating that overall discrimination performance between HC and SCZ-Off was comparable (HC: mean=2.47, sd=.71; SCZ-Off: mean=2.31, sd=.69). To assess potential differences in response bias (biased toward responding “same” or biased toward responding “different”) between groups, average measures of the bias criterion metric c were compared. Again, no differences were observed, F1,40=.41, p>.5, with groups showing a slight bias to respond “same” (HC: mean=.42, sd=.29; SCZ-Off: mean=.49, sd=.38). The analyses of behavioral data demonstrate that the cognitive component of the appraisal task was comparable, with both HC and SCZ-Off evincing comparable discrimination sensitivity and responses biases.
3.2 fMRI Results
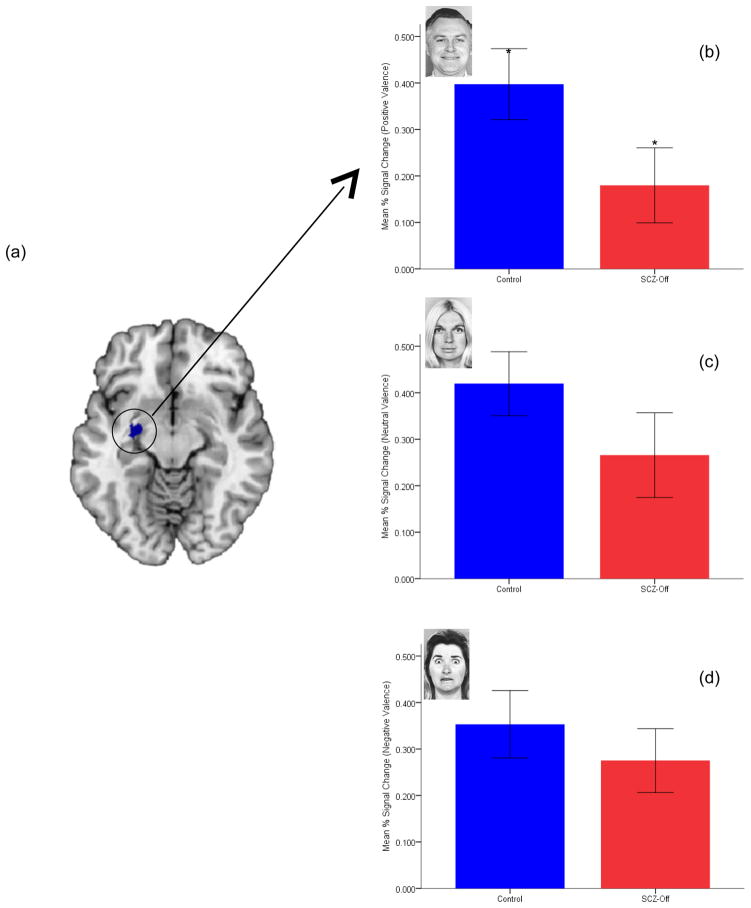
Average BOLD extracted from unsmoothed images of the baso-lateral and centro-medial nuclei were analyzed in an omnibus repeated measures analyses of covariance. For the overall analyses, in addition to the single between groups factor (HC, SCZ-Off), three repeated measures factors were used: Valence of the appraised face (Positive, Negative, Neutral), Region of Interest (BL, CM) and hemisphere (left, right). Age and gender were used as covariates. The omnibus analyses permitted the assessment of significant interactions involving Group, Valence or Region of Interest that may identify specificity of differences between groups as a function of either the valence of the appraised face and/or the region of interest. Two higher order interactions reached significance, specifically Group x Hemisphere, F1,40=3.91, p<.05, MSe=.12 and Hemi x Valence x Group, F1,40=3.94, p<.05, MSe=.02. Each of the significant interactions evinced moderate effect sizes (η2=.09 each). Individual contrasts revealed that the three way interaction resulted from a reduction in activity in the left CM in SCZ-Off relative to HC for positively valenced faces, t42=1.97, p<.05. No other effects reached significance. To investigate the influence on the results of SCZ-Off with co-morbid non-psychotic diagnoses (n=4), data were reanalyzed excluding these subjects. Resultant analyses revealed marginal changes in the significance of the individual interactions: Group x Hemisphere, F1,36=4.55, MSe=.12, p<.05, and Hemi x Affect x Group, F1,36=3.06, MSe=.01, p<.05 (one-tailed), with respective effect sizes of η2=.11 and η2=. 08. The responses in the left CM across groups and valence for the entire data set are depicted in Figure 4.
Figure 4.
BOLD differences in left centro-medial amygdala (maximum probability map depicted in (a) are shown for positive (b), neutral (c) and negative (d) valenced faces. A progressive difference in activation is observed between HC and SCZ-Off, with increased positive valence reflecting a potentially anhedonic response (see Discussion) to positive valence in SCZ-Off (*, p<.05). Error bars are ± sem.
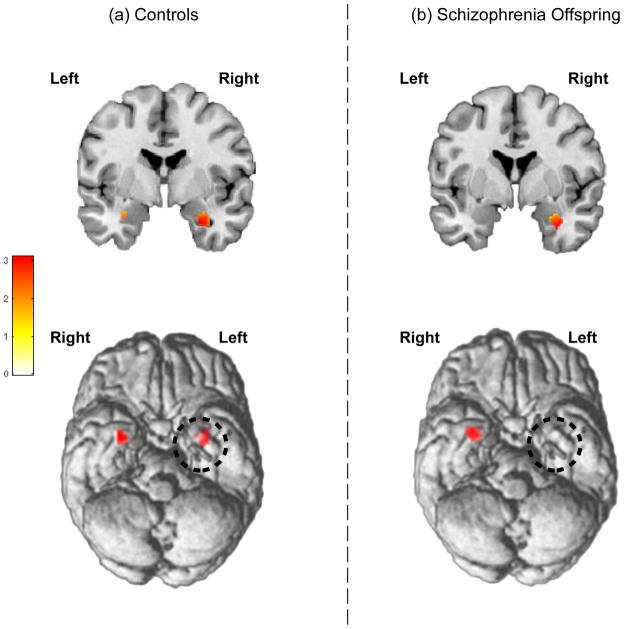
Intra-group analyses of first level contrast maps (Positive > Distorted) revealed significant bilateral amygdala activation in HC but not SCZ-Off. Figure 5a depicts bilateral significance peaks (visualized at p<.05) in the amygdala on a coronal section and ventral surface view of the brain for HC. By comparison (Fig 5b), amygdala activation in SCZ-Off was observed only in the right hemisphere (consistent with the signal change analyses, Fig 4).
Figure 5.
Based on the analyses of signal intensity (Fig 4), intra-group analyses using SPM’s random fields approach were conducted to assess bi- or uni-lateral amygdala activity in HC and SCZ-Off. (a) In HC, bilateral peaks in the amygdala response to positively valenced faces were observed: Right: t42=3.11, kE=46, pFWE<.05, x=30, y=−2, z=−26; Left: t42=2.56, kE=20, p<.007, x=−30, y=−4, z=−22. (b) By comparison, in SCZ-Off activation was only observed in the right amygdala, t42=3.71, kE=60, pFWE<.01, x=30, y=−2, z=−26. Inter-group differences assessed with this approach were not significant.
3.2.1 Age-related changes in HC and SCZ-Off
Because developmental derailment may in part underlie many of the vulnerabilities observed in offspring , we explored the relationship between age and estimated BOLD in the left CM separately for each valence (neutral, negative and positive) and group. The relationship between age and BOLD was modeled using locally weighted polynomial regression (LOESS). Unlike classical methods which use a priori defined functions to fit the entire range of data, curve fitting in LOESS involves iterative fitting of polynomials to each individual data point using weighted least squares. The regression function for the data series is only completed after regression function values have been computed for each of the data points, and the resultant curve fit therefore does not possess a single mathematical form (such as linear, quadratic or cubic). Weighted nearest neighbor kernel functions are employed to weigh the neighbors of the current data point in the analyses based on distance, with nearest neighbors weighted more than points further away in the entire data set.
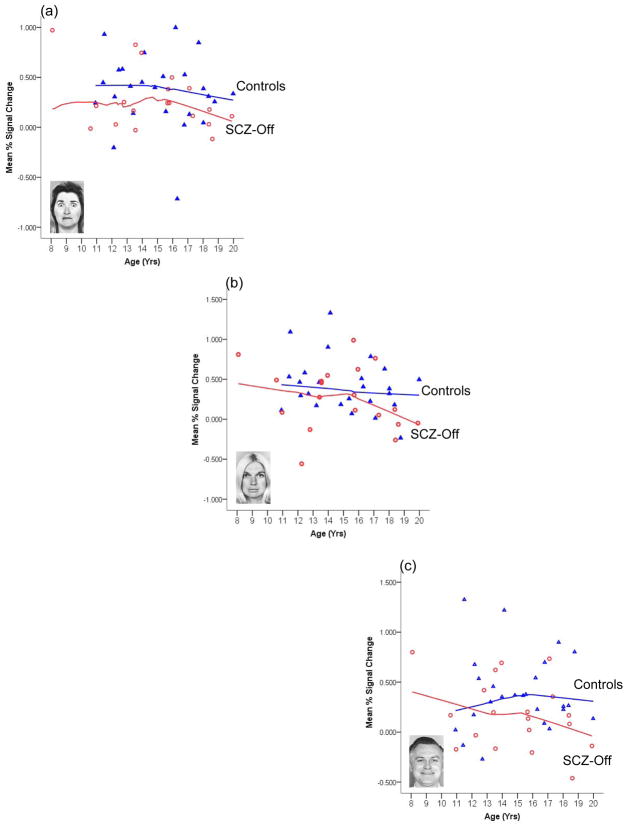
We employed the Epanechnikov kernel function the form of which strikes an optimal balance between weighting nearest versus distant neighbors of the data point, thereby minimizing the asymptotic mean integrated square error for the fit . To minimize effects of differential sampling density of values around different ages, all points in the series were used in the application of the kernel at each point (maximal “band width”). Figure 6 depicts LOESS fits to BOLD from the CM nucleus as a function of age for each valence and group.
Figure 6.
LOESS curve fits relating age with activity in the left CM for each valence and region’s response in SCZ-Off and HC. Whereas age-related trends to negative valence (a) are comparable, increasing positive valence results in decreases in the age-related response in SCZ-Off but not HC (b and c respectively). These results suggest that in older SCZ-Off, positive stimuli evoke less of a response in the CM and may provide evidence of increased impairment in the affective response closer to the typical age of onset of most psychiatric disorders.
As seen, LOESS curve fits assessing the relationship between age and the CM response vary greatly as a function of valence, with the form of the LOESS fits for SCZ-Off markedly differing from HC for the neutral (b) and positive valence (c), but not negative valence (a). In SCZ-Off, the CM nucleus appears increasingly hypo-responsive with age for the neutral and positive faces. These results indicate that more appetitive or rewarding faces, lead to decreased response of the CM in older SCZ-Off. Thus, complex interactions between age-mediated responses to valence may underlie observed differences in the intra-amygdala response between SCZ-Off and HC.
4. Discussion
Using a novel continuous affective appraisal task we assessed behavioral performance and the response of intra-amygdala regions to faces signaling positive, negative or neutral valence in adolescent offspring of schizophrenia patients, and controls. Three general results emerged. First, task performance was comparable between offspring and controls, indicating that cognitive appraisal was intact. Secondly, offspring showed a significantly reduced response to positively valenced faces, specifically in the left centro-medial nucleus of the amygdala (Figures 4 & 5). Thirdly age-related differences in the response of the CM were observed in HC and SCZ-Off groups. In general, SCZ-Off evinced age-related decreases in CM responsivity to more rewarding or appetitive stimuli (Figure 6). The decreased response may be related to a decrease in the salience of positively valenced stimuli, which may decrease progressively through adolescence that is as SCZ-Off approach the modal age of onset of disorders such as schizophrenia.
4.1 Amygdala response and salience
In addition to its role in affective function, the amygdala plays an important role in the detection of novelty and the assignment of salience to stimuli . Consistent with this idea, disorders of mood and anxiety which are likely to result in the attachment of disordered salience to affective stimuli do lead to variations in the amygdala response. For example, in pediatric mood disorders, studies suggest hypersensitivity of the amygdala , particularly to negatively valenced faces signaling fear or threat . Similarly the amygdala is hyper-sensitive in patients with anxiety disorders , and its activity is positively correlated with measures of state anxiety . In contrast, patients with depression show reduced amygdala sensitivity to positive faces , with the degree of anhedonia mediating reduced responses . Recent studies have further established the role of the amygdala in mediating responses to positive stimuli or outcomes. Under conditions in which attention is to be directed at faces, the amygdala appears more responsive to positive than negatively valenced faces , and traits such as optimism which are likely to involved particularly high salience for positively valenced stimuli or outcomes, are associated with increased amygdala responsivity . The reduced salience for positive stimuli has recently been documented in schizophrenia. In particular, greater anhedonia in schizophrenia has been associated with reduced amygdala activity to positively valenced images , suggesting a basis of our observed results in the schizophrenia spectrum.
4.2 Amygdala response and behavior
The observed hypo-functionality of the centro-medial nucleus to positively valenced faces may be particularly relevant, given its role in transmitting amygdala information to other cortical and sub-cortical structures. In addition to influencing autonomic centers, the centro-medial nucleus also projects to the Nucleus Basalis of Meynert which modulates attention particularly to salient stimuli . Reduced activity within the CM nucleus may reflect a graded response mediated by stimulus salience that subsequently modulates the attentional response, to or away from the stimulus. While it remains unclear how positive or appetitive stimuli affect attention, prospective studies of developmentally vulnerable offspring of schizophrenia patients, have documented an increased incidence of attention dysfunction in childhood . Thus, reduced salience for positive stimuli may result in reduced responses and consequently reduced attention to them. Reduced attention to rewarding stimuli may reflect reduced appetitive characteristics of stimuli to SCZ-Off, and may underlie behavioral deficits that are seen in this population including social withdrawal and affective flattening . These in turn echo effects observed in adult schizophrenia patients.
A putative mechanism for centro-medial hypoactivation may also involve the influence of intercalated nuclei. The intercalated nuclei inhibit centro-medial nuclei via basal nuclear projections ; these nuclei receive orbitofrontal projections which gate information flow between the basal and the central nucleus . This pathway may provide a basis for the widely hypothesized modulatory role of the prefrontal cortex on amygdala activity, with the maintenance of social cues depending on interactions between the amygdala, obitofrontal cortex, resulting in an integration of emotion and memory, and socially relevant information . Disruption in these interactions may underlie disorders in the mood and schizophrenia spectrum, and may be latent in vulnerable populations.
Studies on animals have provided more direct links between amygdala impairment (via lesions) and social behavior. Amygdala lesions impact social behavior differentially depending on the period in development when the lesion is incurred . For instance, while lesions in adult monkeys reduce their reaction to environmental stimuli, without affecting social functioning, in infant monkeys, amygdala lesions impair social behavior including increased fearful behavior in dyadic social interactions and more uninhibited social behavior . In humans, the role of the amygdala in interpreting social cues in faces may be critical in this process. Because the recognition of social cues is an important prerequisite for appropriate social interaction, reduced amygdala responsivity to positive valence may impair signal transmission to the brain’s behavioral centers. Thus, hypo- (or hyper-) activity within the amygdala in response to facial affect may provide a latent and emergent deficit in emotional processing that could lead to an inability to access affective content, consequently manifesting as social dysfunction.
The mechanism by which reduced amygdala responses to positively valenced stimuli translates into impaired social behavior, is plausibly complex. Normal social development is in part dependent on the ability to successfully process both aversive and appetitive cues in different modalities and translate such information into contextually appropriate behavioral responses . At present, the neurobiological bases of various disorders featuring social impairment remain obscure. However, populations with extreme social deficits such as those in the autism spectrum evince psychological deficits and limbic system impairment in the evaluation of affective valence . Schizophrenia offspring are not in the autism spectrum, yet there is an increased incidence of behavioral impairments in this population. Prospective studies provide evidence of an increased incidence of conduct and adjustment disorders that circumstantially imply impairments in underlying limbic system neurocircuitry . The age-related analyses (Figure 6) provide suggestive evidence that the CM nucleus in older SCZ-Off is less responsive to positive or appetitive stimuli than in their younger counterparts, reflecting growing emotional vulnerability within the limbic system as the typical age of onset of psychiatric disorders approaches. This in turn may suggest increased vulnerability in this population to stressors that alter normal adolescent neuronal remodeling . Social dysfunction is also a recognized feature of the prodromal phase of schizophrenia evidenced by studies in “ultra high risk” populations . fMRI studies of amygdala function in this group are lacking, though studies suggest that amygdala volumes between ultra high risk subjects and controls are not different . Juxtaposing these negative volumetric findings against our offspring study, we suggest that fMRI may be more sensitive than morphometry in assessing the state of the vulnerable or at-risk “social” brain.
4.3 Conclusions and Limitations
These results are a preliminary extension of emerging techniques (stereotactic mapping) toward an understanding of intra-amygdala differences in function in a developmentally vulnerable population (SCZ-Off). As such, the interpretation of the results is constrained by known limitations in fMRI signal sensitivity , spatial limitations and noise , and the size (n=19) and nature (cross-sectional) of the SCZ-Off sample. Further, there is no precedent or context from which to interpret our results; while the literature on the activity of the amygdala in different psychiatric populations is too vast to summarize, to our knowledge only a handful of studies have sought to differentiate amygdala sub-units to assess their functional specificity in vivo and none have applied these methods to the study of clinical or developmentally vulnerable populations. While the left hemispheric specificity of our results are generally consistent with morphometric studies documenting reduced volumes of left amygdala structure in either offspring of schizophrenia patients , or individuals at high risk of developing schizophrenia , there is no clear evolutionary bases for a left hemispheric specificity for processing affective facial expressions . Finally, it will be important in future studies to closely examine the relationship between intra-amygdala activity and clinical function in prodromal or early course patient populations, and to study modulatory interactions between the frontal cortex and amygdala nuclei from the framework of systems biology . Pending these extensions, our results document limbic system impairments in the amygdala in adolescent schizophrenia offspring, and provide a plausible framework for emergent models of limbic system dysfunction as they relate to altered processes of development and vulnerability.
Acknowledgments
We thank R. Rajarathinem, A. Amirsadri, L. Haddad, A. Jenrow and R. Marciano for assistance in subject recruitment and characterization. We also thank Jeffrey Stanley and Mary Phillips for helpful discussions, and Valentina Gumenyuk, Karthik Sundaresan and Serguei Fedorov for assistance in experimental design and programming.
Funding Sources. This research was supported by grants from the National Institute of Mental Health (MH68680) and the Children’s Research of Michigan (CRCM) to VAD. TB was supported by the Joseph Young Jr. Fund. The funding sources had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Contributions. TB analyzed the data and developed the conceptual model. PP collected data and performed preprocessing and analyses. EM assisted in study design. SE was involved in data analyses and interpretation. MK was involved in study design and theoretical discussions. UR was involved in subject assessment and theoretical discussions. CZ was involved in subject assessment and theoretical discussions. VD supervised study design, data collection and analyses and interpretation.
Conflicts of Interest. None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R, Baron-Cohen S, Tranel D. Impaired recognition of social emotions following amygdala damage. J Cogn Neurosci. 2002;14:1264–1274. doi: 10.1162/089892902760807258. [DOI] [PubMed] [Google Scholar]
- Aleman A, Kahn RS. Strange feelings: do amygdala abnormalities dysregulate the emotional brain in schizophrenia? Prog Neurobiol. 2005;77:283–298. doi: 10.1016/j.pneurobio.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Behniea H, Kelly JL. Topographic organization of projections from the amygdala to the visual cortex in the macaque monkey. Neuroscience. 2003a;118:1099–1120. doi: 10.1016/s0306-4522(02)01001-1. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Capitanio JP, Jourdain M, Mason WA, Mendoza SP, Prather M. The amygdala: is it an essential component of the neural network for social cognition? Neuropsychologia. 2003b;41:235–240. doi: 10.1016/s0028-3932(02)00154-9. [DOI] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl) 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Amunts K, Weiss PH, Mohlberg H, Pieperhoff P, Eickhoff S, Gurd JM, Marshall JC, Shah NJ, Fink GR, Zilles K. Analysis of neural mechanisms underlying verbal fluency in cytoarchitectonically defined stereotaxic space--the roles of Brodmann areas 44 and 45. Neuroimage. 2004;22:42–56. doi: 10.1016/j.neuroimage.2003.12.031. [DOI] [PubMed] [Google Scholar]
- Amunts K, Zilles K. Advances in cytoarchitectonic mapping of the human cerebral cortex. Neuroimaging Clin N Am. 2001;11:151–169. vii. [PubMed] [Google Scholar]
- Ashwin C, Baron-Cohen S, Wheelwright S, O'Riordan M, Bullmore ET. Differential activation of the amygdala and the 'social brain' during fearful face-processing in Asperger Syndrome. Neuropsychologia. 2007;45:2–14. doi: 10.1016/j.neuropsychologia.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Ball T, Rahm B, Eickhoff SB, Schulze-Bonhage A, Speck O, Mutschler I. Response properties of human amygdala subregions: evidence based on functional MRI combined with probabilistic anatomical maps. PLoS ONE. 2007;2:e307. doi: 10.1371/journal.pone.0000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, De Olmos J. Projections from the amygdala to basoventral and mediodorsal prefrontal regions in the rhesus monkey. J Comp Neurol. 1990;300:549–571. doi: 10.1002/cne.903000409. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams SC. The amygdala theory of autism. Neurosci Biobehav Rev. 2000;24:355–364. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, Williams SC. Social intelligence in the normal and autistic brain: an fMRI study. Eur J Neurosci. 1999;11:1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Young AW. Understanding the recognition of facial identity and facial expression. Nat Rev Neurosci. 2005;6:641–651. doi: 10.1038/nrn1724. [DOI] [PubMed] [Google Scholar]
- Cannon M, Caspi A, Moffitt TE, Harrington H, Taylor A, Murray RM, Poulton R. Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder: results from a longitudinal birth cohort. Arch Gen Psychiatry. 2002;59:449–456. doi: 10.1001/archpsyc.59.5.449. [DOI] [PubMed] [Google Scholar]
- Cannon M, Jones P, Gilvarry C, Rifkin L, McKenzie K, Foerster A, Murray RM. Premorbid social functioning in schizophrenia and bipolar disorder: similarities and differences. Am J Psychiatry. 1997;154:1544–1550. doi: 10.1176/ajp.154.11.1544. [DOI] [PubMed] [Google Scholar]
- Cleveland WS, Devlin SJ. Locally-Weighted Regression: An Approach to Regression Analysis by Local Fitting. J Am Stat Assoc. 1988;83:596–610. [Google Scholar]
- Cohen J. Statistical power analysis for behavioral sciences. 2. Laurence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Diwadkar V, Sweeney J, Boarts D, Montrose D, Keshavan M. Oculomotor delayed response abnormalities in young offspring and siblings at risk for schizophrenia. CNS Spectr. 2001a;6(11):899–903. doi: 10.1017/s109285290000095x. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA, Montrose DM, Dworakowski D, Sweeney JA, Keshavan MS. Genetically predisposed offspring with schizotypal features: an ultra high-risk group for schizophrenia? Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:230–238. doi: 10.1016/j.pnpbp.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA, Prasad KM, Keshavan MS. Approaches for adolescents with an affected family member with schizophrenia. Curr Psychiatry Rep. 2004;6:296–302. doi: 10.1007/s11920-004-0080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA, Sweeney JA, Boarts D, Montrose DM, Keshavan MS. Oculomotor delayed response abnormalities in young offspring and siblings at risk for schizophrenia. CNS Spectr. 2001b;6:899–903. doi: 10.1017/s109285290000095x. [DOI] [PubMed] [Google Scholar]
- Dowd EC, Barch DM. Anhedonia and emotional experience in schizophrenia: neural and behavioral indicators. Biol Psychiatry. 2010;67:902–911. doi: 10.1016/j.biopsych.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin RH, Cornblatt BA, Friedmann R, Kaplansky LM, Lewis JA, Rinaldi A, Shilliday C, Erlenmeyer-Kimling L. Childhood precursors of affective vs. social deficits in adolescents at risk for schizophrenia. Schizophr Bull. 1993;19:563–577. doi: 10.1093/schbul/19.3.563. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K. Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuroimage. 2006a;32:570–582. doi: 10.1016/j.neuroimage.2006.04.204. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Lotze M, Wietek B, Amunts K, Enck P, Zilles K. Segregation of visceral and somatosensory afferents: an fMRI and cytoarchitectonic mapping study. Neuroimage. 2006b;31:1004–1014. doi: 10.1016/j.neuroimage.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Ekman P, Oster H. Facial expressions of emotion. Ann Rev Psychology. 1979;30:527–554. [Google Scholar]
- Erlenmeyer-Kimling L. Neurobehavioral deficits in offspring of schizophrenic parents: Liability indicators and predictors of illness. Am J Med Genet. 2000;97:65–71. doi: 10.1002/(sici)1096-8628(200021)97:1<65::aid-ajmg9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Erlenmeyer-Kimling L, Cornblatt B. High-risk research in schizophrenia: A summary of what has been learned. J Psychiatr Res. 1987;21:451–463. doi: 10.1016/0022-3956(87)90087-2. [DOI] [PubMed] [Google Scholar]
- Etkin A, Klemenhagen KC, Dudman JT, Rogan MT, Hen R, Kandel ER, Hirsch J. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44:1043–1055. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Evans AC, Collins DL, Holmes CJ. Computational approaches to quantifying human neuroanatomical variability. In: Toga AW, Mazziotta JC, editors. BRAIN MAPPING The Methods. Academic Press, Inc; San Diego: 1996. pp. 343–361. [Google Scholar]
- Fakra E, Salgado-Pineda P, Delaveau P, Hariri AR, Blin O. Neural bases of different cognitive strategies for facial affect processing in schizophrenia. Schizophr Res. 2008;100:191–205. doi: 10.1016/j.schres.2007.11.040. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Green AI, Seidman LJ, Tsuang MT. “Schizotaxia”: clinical implications and new directions for research. Schizophr Bull. 2001;27:1–18. doi: 10.1093/oxfordjournals.schbul.a006849. [DOI] [PubMed] [Google Scholar]
- First MD, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured clinical interview for DSM–IV Axis II personality disorders. Biometrics Research Department, NYSPI; New York: 1997. [Google Scholar]
- Freedman LR, Rock D, Roberts SA, Cornblatt BA, Erlenmeyer-Kimling L. The New York High-Risk Project: attention, anhedonia and social outcome. Schizophr Res. 1998;30:1–9. doi: 10.1016/s0920-9964(97)00132-1. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Noll DC, Eddy WF. Estimating test-retest reliability in functional MR imaging. I: Statistical methodology. Mag Reson Med. 1997;38:497–507. doi: 10.1002/mrm.1910380319. [DOI] [PubMed] [Google Scholar]
- Gur RE, McGrath C, Chan RM, Schroeder L, Turner T, Turetsky BI, Kohler C, Alsop D, Maldjian J, Ragland JD, Gur RC. An fMRI study of facial emotion processing in patients with schizophrenia. Am J Psychiatry. 2002;159:1992–1999. doi: 10.1176/appi.ajp.159.12.1992. [DOI] [PubMed] [Google Scholar]
- Hans SL, Auerbach JG, Auerbach AG, Marcus J. Development from birth to adolescence of children at-risk for schizophrenia. J Child Adolesc Psychopharmacol. 2005;15:384–394. doi: 10.1089/cap.2005.15.384. [DOI] [PubMed] [Google Scholar]
- Hastie T, Tibshirani R, Friedman J. The elements of statistical learning, data mining, inference and prediction. Springer Verlag; New York: 2001. [Google Scholar]
- Henry JD, Green MJ, de Lucia A, Restuccia C, McDonald S, O'Donnell M. Emotion dysregulation in schizophrenia: reduced amplification of emotional expression is associated with emotional blunting. Schizophr Res. 2007;95:197–204. doi: 10.1016/j.schres.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Herba C, Phillips M. Annotation: Development of facial expression recognition from childhood to adolescence: behavioural and neurological perspectives. J Child Psychol Psychiatry. 2004;45:1185–1198. doi: 10.1111/j.1469-7610.2004.00316.x. [DOI] [PubMed] [Google Scholar]
- Insel TR, Fernald RD. How the brain processes social information: searching for the social brain. Annu Rev Neurosci. 2004;27:697–722. doi: 10.1146/annurev.neuro.27.070203.144148. [DOI] [PubMed] [Google Scholar]
- Josephs O, Henson RN. Event-related functional magnetic resonance imaging: modelling, inference and optimization. Philos Trans R Soc Lond B Biol Sci. 1999;354:1215–1228. doi: 10.1098/rstb.1999.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58:843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Keshavan M, Montrose DM, Rajarethinam R, Diwadkar V, Prasad K, Sweeney JA. Psychopathology among offspring of parents with schizophrenia: Relationship to premorbid impairments. Schizophr Res. 2008 doi: 10.1016/j.schres.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Dick E, Mankowski I, Harenski K, Montrose DM, Diwadkar V, DeBellis M. Decreased left amygdala and hippocampal volumes in young offspring at risk for schizophrenia. Schizophr Res. 2002;58:173–183. doi: 10.1016/s0920-9964(01)00404-2. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, Montrose DM, Rajarethinam R, Sweeney JA. Premorbid indicators and risk for schizophrenia: a selective review and update. Schizophr Res. 2005;79:45–57. doi: 10.1016/j.schres.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, Montrose DM, Stanley JA, Pettegrew JW. Premorbid characterization in schizophrenia: the Pittsburgh High Risk Study. World Psychiatry. 2004;3:163–168. [PMC free article] [PubMed] [Google Scholar]
- Killcross S, Robbins TW, Everitt BJ. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature. 1997;388:377–380. doi: 10.1038/41097. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Whalley H, Kestelman JN, Abukmeil SS, Byrne M, Hodges A, Rimmington JE, Best JJ, Owens DG, Johnstone EC. Magnetic resonance imaging of brain in people at high risk of developing schizophrenia. Lancet. 1999;353:30–33. doi: 10.1016/S0140-6736(98)06244-8. [DOI] [PubMed] [Google Scholar]
- Ledoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2002:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotional memory: in search of systems and synapses. Ann N Y Acad Sci. 1993;702:149–157. doi: 10.1111/j.1749-6632.1993.tb17246.x. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Pelletier JG, Paz R, Pare D. Prefrontal control of the amygdala. J Neurosci. 2005;25:7429–7437. doi: 10.1523/JNEUROSCI.2314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK. The neural basis of the blood-oxygen-level-dependent functional magnetic resonance imaging signal. Philos Trans R Soc Lond B Biol Sci. 2002;357:1003–1037. doi: 10.1098/rstb.2002.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoPresti ML, Schon K, Tricarico MD, Swisher JD, Celone KA, Stern CE. Working memory for social cues recruits orbitofrontal cortex and amygdala: a functional magnetic resonance imaging study of delayed matching to sample for emotional expressions. J Neurosci. 2008;28:3718–3728. doi: 10.1523/JNEUROSCI.0464-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection Theory: A Users Guide. Lawrence Erlbaum Associates; Mahwah, NJ: 2005. [Google Scholar]
- Masterman DL, Cummings JL. Frontal-subcortical circuits: the anatomic basis of executive, social and motivated behaviors. J Psychopharmacol. 1997;11:107–114. doi: 10.1177/026988119701100203. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Is there an amygdala and how far does it extend? An anatomical perspective. Ann N Y Acad Sci. 2003;985:1–21. doi: 10.1111/j.1749-6632.2003.tb07067.x. [DOI] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, Chen G, McClure-Tone EB, Ernst M, Pine DS. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65:568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. An fMRI study of the neural correlates of incidental versus directed emotion processing in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:308–319. doi: 10.1097/CHI.0b013e3181948fc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Wager TD, Taylor SF, Liberzon I. Functional neuroimaging studies of human emotions. CNS Spectr. 2004;9:258–266. doi: 10.1017/s1092852900009196. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Phillips LK, Seidman LJ. Emotion Processing in Persons at Risk for Schizophrenia. Schizophr Bull. 2008 doi: 10.1093/schbul/sbn085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Williams L, Senior C, Bullmore ET, Brammer MJ, Andrew C, Williams SC, David AS. A differential neural response to threatening and non-threatening negative facial expressions in paranoid and non-paranoid schizophrenics. Psychiatry Res. 1999;92:11–31. doi: 10.1016/s0925-4927(99)00031-1. [DOI] [PubMed] [Google Scholar]
- Pine DS. Research review: a neuroscience framework for pediatric anxiety disorders. J Child Psychol Psychiatry. 2007;48:631–648. doi: 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Amaral DG. Organization of the intrinsic connections of the monkey amygdaloid complex: projections originating in the lateral nucleus. J Comp Neurol. 1998;398:431–458. doi: 10.1002/(sici)1096-9861(19980831)398:3<431::aid-cne9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, Fromm SJ, Pine DS, Leibenluft E. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci U S A. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleicher A, Palomero-Gallagher N, Morosan P, Eickhoff SB, Kowalski T, de Vos K, Amunts K, Zilles K. Quantitative architectural analysis: a new approach to cortical mapping. Anat Embryol (Berl) 2005;210:373–386. doi: 10.1007/s00429-005-0028-2. [DOI] [PubMed] [Google Scholar]
- Scouten A, Papademetris X, Constable RT. Spatial resolution, signal-to-noise ratio, and smoothing in multi-subject functional MRI studies. Neuroimage. 2006;30:787–793. doi: 10.1016/j.neuroimage.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Sharot T, Riccardi AM, Raio CM, Phelps EA. Neural mechanisms mediating optimism bias. Nature. 2007;450:102–105. doi: 10.1038/nature06280. [DOI] [PubMed] [Google Scholar]
- Shim G, Kang DH, Chung YS, Yoo SY, Shin NY, Kwon JS. Social functioning deficits in young people at risk for schizophrenia. Aust N Z J Psychiatry. 2008;42:678–685. doi: 10.1080/00048670802203459. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Kim H, Johnstone T, Alexander AL, Whalen PJ. Human amygdala responses during presentation of happy and neutral faces: correlations with state anxiety. Biol Psychiatry. 2004;55:897–903. doi: 10.1016/j.biopsych.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Stephan KE. On the role of general system theory for functional neuroimaging. J Anat. 2004;205:443–470. doi: 10.1111/j.0021-8782.2004.00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, Williams SC, Phillips ML. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry. 2005;57:201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front Hum Neurosci. 2009;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velakoulis D, Wood SJ, Wong MT, McGorry PD, Yung A, Phillips L, Smith D, Brewer W, Proffitt T, Desmond P, Pantelis C. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry. 2006;63:139–149. doi: 10.1001/archpsyc.63.2.139. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Revised. Psychological Corporation; New York: 1987. [Google Scholar]
- Weierich MR, Wright CI, Negreira A, Dickerson BC, Barrett LF. Novelty as a dimension in the affective brain. Neuroimage. 2010;49:2871–2878. doi: 10.1016/j.neuroimage.2009.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens TD. Elementary Signal Detection Theory. Oxford University Press; Oxford, UK: 2001. [Google Scholar]
- Williams LM, Felmingham K, Kemp AH, Rennie C, Brown KJ, Bryant RA, Gordon E. Mapping frontal-limbic correlates of orienting to change detection. Neuroreport. 2007;18:197–202. doi: 10.1097/WNR.0b013e328010ff80. [DOI] [PubMed] [Google Scholar]
- Williams MA, McGlone F, Abbott DF, Mattingley JB. Differential amygdala responses to happy and fearful facial expressions depend on selective attention. Neuroimage. 2005;24:417–425. doi: 10.1016/j.neuroimage.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Witthaus H, Mendes U, Brune M, Ozgurdal S, Bohner G, Gudlowski Y, Kalus P, Andreasen N, Heinz A, Klingebiel R, Juckel G. Hippocampal subdivision and amygdalar volumes in patients in an at-risk mental state for schizophrenia. J Psychiatry Neurosci. 2010;35:33–40. doi: 10.1503/jpn.090013. [DOI] [PMC free article] [PubMed] [Google Scholar]