Abstract
Objective:
Patients with hepatitis C virus (HCV) are advised to refrain from alcohol consumption. A questionnaire was developed to measure concepts associated with alcohol use for individuals with HCV.
Method:
Subjects with HCV (N = 527) completed a telephone survey. Eligible respondents had screened negative for current abuse/dependence disorders (Alcohol Use Disorders Identification Test [AUDIT] ≤ 10). Measures of personality, self-efficacy, knowledge, readiness, coping styles, stigma, and symptoms were examined for associations with alcohol use.
Results:
Factor analysis supported a measurement structure of 105 items in 35 subdomains. A total of 26 subdomains had significant bivariate associations with alcohol use. Higher self-efficacy for resisting drinking in social situations was associated with lower alcohol use (r = −.68, p < .001), as was knowledge of alcohol and HCV (r = −.27, p < .001). Although agreeableness and marital status are typically associated with lower current drinking in samples of those with alcohol use problems, in our study agreeableness (β= .13, p < .01) and marital status (β = .08, p < .05) were modestly associated with higher current drinking. The final multivariate R2 was .55.
Conclusions:
The pattern of associations suggests the importance of the social aspects of drinking for drinking decisions. Existing brief interventions will need to be tailored to a contextualized psychosocial model for medical patients with HCV and AUDIT scores ≤ 10 to optimize effectiveness. Such future interventions should emphasize the potential medical hazards of drinking for persons with HCV, the maintenance of social relationships in the absence of alcohol use, and strategies for building confidence for resisting drinking in specific situations.
Alcohol reduction is an important self-management health behavior for individuals with hepatitis C virus (HCV; Centers for Disease Control and Prevention [CDC], 2010). The purpose of this study was to identify concepts and measures associated with alcohol use and reduction for drinkers with HCV who are not drinking at levels that would likely result in a diagnosis of alcohol abuse or dependence. We hypothesized that some of the psychosocial factors would have prominent and novel relationships to alcohol use.
HCV is a significant health problem, affecting an estimated 4 million people in the United States alone (Armstrong et al., 2006). It is typically recommended that patients with HCV abstain from alcohol use (Blixen et al., 2008; CDC, 2010), both because of concerns about long-term liver damage and because alcohol use is an impediment to treatment readiness, initiation, and success (Dieperink et al., 2010). Although not fully contraindicated for HCV treatment (Dieperink et al., 2010), low levels of alcohol use have been shown to adversely affect HCV-infected patients by impeding immune response, aggravating histological lesions, and increasing fibrosis, thereby increasing the likelihood of developing cirrhosis and/or hepatic cancer/hepatoma (Hézode et al., 2003; Pessione et al., 1998; Szabo et al., 2006; Westin et al., 2002). There has been little study of factors associated with alcohol reduction in individuals with medical conditions in which abstinence is recommended because of the medical condition rather than an alcohol-related problem. A better understanding of these factors could facilitate tailored interventions that improve outcomes.
Our study is unique in focusing on drinkers who are unlikely to have a diagnosable alcohol problem but who are faced with a medical reason to quit drinking. Although one or two drinks on most days is generally not hazardous for most community drinkers, that level (30 grams per day or more) is likely hazardous for the majority of people with HCV (Rigamonti et al., 2003). Levels of alcohol use that have been shown to convey health benefits on community drinkers are hazardous for those with HCV. Persons with HCV and other medical conditions are often advised to reduce or abstain from alcohol use.
Alcohol reduction for patients with HCV who have been advised to reduce or stop drinking can be viewed as a form of patient adherence. Models for understanding adherence generally incorporate a broad range of psychosocial variables, including health beliefs, illness knowledge, perceived disease severity, self-efficacy, coping styles, and patient-physician relationship (DiMatteo, 2004). Motivation for change and the associated concepts of decisional balance have been studied in at least 48 health-related behaviors (Hall and Rossi, 2008).
We report herein on the results of a multiyear mixed methods research project—Alcohol Reduction in Medical Illnesses (ARIMI): HCV as Prototype—designed to identify factors predictive of the likelihood of alcohol reduction among drinkers at a low likelihood of clinical alcohol problems who have a diagnosis of HCV. We used a mixed methods research approach that included substantial input from individuals with HCV as well as a thorough review of existing instruments measuring concepts that the literature on adherence to alcohol reduction in other populations suggested might be relevant.
This study integrates principles from two theoretical traditions in the study of health behavior change: the Transtheoretical Model of Behavior Change (Prochaska and DiClemente, 1992) and the Theory of Reasoned Action (Ajzen, 1991; Fishbein, 1979; Fishbein and Yzer, 2003). The Transtheoretical Model describes health behavior change as a process in which individuals have varied levels of success in their progression through discrete stages. Our prior qualitative work is consistent with the stage of change framework in demonstrating multiple diverse alcohol use trajectories for patients diagnosed with HCV (Stoller et al., 2006).
The Theory of Reasoned Action holds that one of the most powerful and proximal determinants of behavior is a person's own assessment of what he or she expects to do (intention) and confidence (self-efficacy) in his or her ability to do it (Fishbein and Yzer, 2003). Intention is related to positive and negative evaluations of the behavior, perceptions of social pressure from others, and perceived ease or difficulty in performing the behavior.
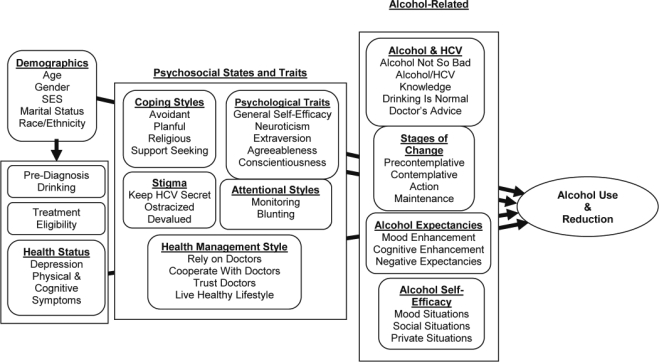
Both of these theories emphasize the importance of patient knowledge, attitudes, belief systems about their disease, and coping strategies. Because little work has been done on the perceptions of patients with HCV about alcohol consumption, our prior work used qualitative methods to elucidate patients’ knowledge, attitudes, and beliefs (Blixen et al., 2008; Stoller et al., 2006, 2009). Our conceptual model integrates these HCV and alcohol-specific concepts with state and trait-like psychosocial characteristics and is presented in Figure 1. The figure should be seen as a cross-sectional heuristic for understanding the group of factors potentially predictive of alcohol reduction at the time of HCV diagnosis rather than a causal, life course model for understanding alcohol use. Alcohol use and reduction are seen as being influenced most proximally by alcohol-related concepts, including alcohol and illness knowledge, readiness to change, expectations about drinking alcohol, and confidence in the ability to resist drinking alcohol.
Figure 1.
Conceptual model of alcohol reduction for patients newly diagnosed with chronic hepatitis C virus (HCV) infection. SES = socioeconomic status.
These proximal factors are preceded in the model by intra-individual psychosocial states and traits such as coping styles and psychological traits, which can affect alcohol use through direct and indirect pathways. Individuals with HCV consider their health status when evaluating the pros and cons of drinking alcohol (Stoller et al., 2009). Current health status is conceptualized as a variable that can contribute to health behavior decision-making; individuals with tangible, firsthand evidence of illness consequences in the form of symptoms may alter their drinking behavior.
This cross-sectional study uses regression analysis to examine the associations between a large array of psychosocial concepts and self-reported current drinking and alcohol reduction. We anticipate that the alcohol-related psychosocial concepts in Figure 1 will have the largest bivariate and multivariate associations with current drinking and alcohol reduction.
Method
This study included human subjects and was conducted with approval from the MetroHealth Institutional Review Board.
The selection of concepts and measures for the development of the instrument reported here is grounded in the ARIMI team's past qualitative research (Blixen et al., 2008; Stoller et al., 2006, 2009), which included focus groups and in-depth interviews. Building on the qualitative results, we first assembled a list of concepts that could have a potential relationship with alcohol use for patients chronically infected with HCV. A comprehensive review of the literature on alcohol reduction and health behavior change was conducted to identify existing instruments measuring these concepts. A total of 758 survey items in research articles and books were collected (Stoller et al., 2009). Prior development of items and scales predicting alcohol reduction has been largely confined to concepts thought to be relevant in populations of those diagnosed with alcohol abuse or dependence (Allen and Wilson, 2003). In the process of refining our item pool, each survey item was examined carefully and edited when necessary to ensure relevance to our study population.
A panel of survey design experts familiar with alcohol reduction and scale construction reviewed the item pool, and some questions were modified to improve internal validity. The panel reviewed items using a binning and winnowing approach (Cella et al., 2007; DeWalt et al., 2007). We authored new questions when existing survey items could not be found to measure specific concepts (Stoller et al., 2009). This entire process yielded 139 items for potential inclusion in the questionnaire.
Cognitive interviews were conducted with three HCV patients to further verify the appropriateness and ease of comprehension of the potential items for patients with HCV (Willis, 2005), which led to the rewriting of survey questions that were confusing.
Measures
The 139 items in the initial HCV and alcohol use questionnaire measured 10 broad conceptual domains, with a total of 35 subdomains plus demographic questions. The domains and subdomains are depicted in Figure 1. Many items from public domain sources were altered to improve accessibility for telephone interviewing of patients with low education and literacy. The full set of items and response choices is freely available from the authors on request.
Integrating items from several secondary sources required some changes in response categories to minimize the heterogeneity of response categories in the overall instrument. Our method of item selection and revision was nearly identical to that used concurrently by the Patient Reported Outcomes Measurement Information System (DeWalt et al., 2007).
Alcohol expectancies
Alcohol expectancies measure the pros and cons of drinking and were adapted from the Drinking Expectancy Questionnaire. Respondents were asked to rate what happens when they drink alcohol. We tested six mood enhancement items (calm down, feel happy, improves mood, have fun, feel outgoing, and open up), four cognitive enhancement items (more mentally alert, sharpens mind, perform tasks better, get better ideas), and four negative expectancy items (disappointed in self, feel sick, bad tempered, nagged by family) (Young and Knight, 1989; Young and Oei, 1996).
Stages of change
We operationalized the stages of change in the Transtheoretical Model as interrelated concepts (Prochaska and DiClemente, 1992), adapting 16 items from the Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES) to measure four stages of change: precontemplative, contemplative, action, and maintenance (Miller and Tonigan, 1996).
Alcohol self-efficacy
Eleven items from the Drinking-Refusal Self-Efficacy Questionnaire were used to assess the likelihood that patients would resist drinking alcohol in a number of situations (Young and Oei, 1996; Young et al., 1991), particularly mood situations (when you feel angry, upset, down, uptight), social situations (drinking at a party, a bar, with friends, on holidays), and private situations (by yourself, when you first arrive home).
Alcohol and hepatitis C virus
Prior qualitative studies led to the development of 26 items potentially predictive of alcohol use (Stoller et al., 2009) in four main subdomains: knowledge of the hazards of alcohol use for people with HCV, a general attitude that drinking is bad for people with HCV, the belief that drinking is a normal behavior, and the extent of doctors’ advice to quit drinking.
Coping style and attentional styles
Our survey included 13 questions measuring four coping styles (avoidant, planful, support seeking, and religious; Carver, 1997) and two attentional styles (monitoring and blunting). Patients who have higher monitoring scores want to know more, and those who score higher on blunting want less (Miller, 1987). We used a single scenario from the Miller Behavioral Style Scale, consisting of four monitoring and four blunting items (Miller, 1987).
Personality traits and general self-efficacy
We included 12 questions from the Big Five Inventory designed to screen patients for four core personality traits: extraversion, agreeableness, conscientiousness, and neuroticism (Benet-Martinez and John, 1998). We also included seven questions measuring general self-efficacy (Schroeder and Schwarzer, 2005; Schwarzer and Jerusalem, 1995).
Health management styles
The existing literature and our own qualitative work with HCV patients suggested that patients’ adherence is partially determined by aspects of the patient–doctor relationship (Blixen et al., 2008; Vermeire et al., 2001). Eleven items were included to measure health management styles, including reliance on doctors, cooperates with doctors, trusts doctors, and lives a healthy lifestyle (Fiscella et al., 1999; Hulka et al., 1970; Millon et al., 1979; Zyzanski et al., 1974). Reliance is the degree to which patients relied on themselves as opposed to healthcare providers in caring for their health.
Perceived stigma
Stigma of “invisible” diseases such as HIV/AIDS and HCV is multidimensional (Fife and Wright, 2000; Joachim and Acorn, 2000; Pryor et al., 1999). We included seven items intended to measure both internal and external subdimensions of stigma.
Health status
We measured health status using self-reported depression and the frequency of physical and cognitive symptoms in the past month. The six depression items were adapted from the Patient Health Questionnaire–9 (Kroenke et al., 2001). Qualitative work and expert consultation led to the development of items measuring the frequency of nine HCV-related symptoms: nausea, soreness of muscles or joints, lack of appetite, abdominal pain, difficulty sleeping, trouble with memory, brain fog, difficulty reasoning, and difficulty concentrating.
Alcohol use and reduction measures
All subjects were administered a structured telephone interview that included the 139 candidate items and screening for current alcohol use with the Alcohol Use Disorders Identification Test (AUDIT; Babor et al., 2001). The 10-item AUDIT includes three questions on consumption (the AUDIT-C) and seven on the impact of alcohol use. The AUDIT has been shown to have good sensitivity and specificity in medical and general populations. Those with AUDIT scores greater than 10 were not eligible for the study, a cutoff that has been previously validated in medical populations (Bohn et al., 1995; Saunders et al., 1993). In our bivariate and multivariate models of alcohol consumption, we use AUDIT-C, a valid tool that excludes the impact and life interference questions that are mostly relevant to individuals with alcohol abuse or dependency (Bush et al., 1998). Analyses were replicated using AUDIT as the dependent variable (available from the authors on request) with no change in findings. The other primary outcome is a Yes/No self-report item: “Have you quit drinking?”
In addition, the four-item CAGE questionnaire was used to screen patients for a history of alcohol use disorders (a score of 2 or higher) (Ewing, 1984). As secondary outcomes, respondents were also asked to retrospectively report quantity and frequency of their drinking immediately after their HCV diagnosis and the yes/no item, “Have you had even one drink containing alcohol since being diagnosed with HCV?”
Sample
The cross-sectional sample consisted of 527 patients with HCV who consumed alcohol at a level that would not usually be considered harmful. To be eligible for the study, patients had to be at least 21 years of age, aware of their HCV diagnosis, had not achieved sustained virological response, were not HIV-positive, were not pregnant, had consumed at least one drink containing alcohol in their lifetime, and had an AUDIT ≤ 10. Subjects were screened for eligibility from a randomized list of 2,931 potential subjects (consisting of the entire population of HCV patients seen at an urban safety-net medical center in the 2 years before recruitment) until we completed a sampling grid designed to ensure variability by race/ethnicity. Letters were sent to 1,191 potential patients informing them that they would be called. Of these patients, 783 were reached by telephone and met eligibility criteria. We completed 577 interviews for a participation rate of 74%. HCV diagnosis was determined from clinical data in the electronic medical record (diagnosis of chronic HCV infection on the problem list). Fifty patients who were newly diagnosed with HCV (less than 3 months before being contacted) were excluded from the current analysis because they were eligible to be included in an ongoing prospective cohort study. Demographic characteristics of the sample are summarized in Table 1.
Table 1.
Sample characteristics (N = 527)
| Variable | n (%) |
| Female | 235 (44.6) |
| Education < high school | 133 (25.2) |
| Income < $25,000 per year | 386 (73.2) |
| White | 225 (42.7) |
| Hispanic | 46 (8.7) |
| Black | 222 (42.1) |
| Other racial groups | 30 (5.7) |
| Married | 200 (38.0) |
| Had treatment to clear HCV | 244 (46.3) |
| CAGE > 2 | 208 (39.5) |
| Even one drink since HCV | 347 (65.8) |
| Quit drinking | 387 (73.4) |
| AUDIT = 0 | 314 (59.6) |
| AUDIT 1–3 | 108 (20.5) |
| AUDIT 4–6 | 58 (11.0) |
| AUDIT 7–10 | 47 (8.9) |
| Provider drinking advice | |
| Total abstinence | 336 (63.6) |
| No advice | 84 (15.9) |
| On special occasions | 40 (7.6) |
| Drink moderately | 36 (6.8) |
| Couldn't remember | 31 (5.9) |
| M (SD) | |
| Age (years) | 49.9 (8.0) |
| Neighborhood income ($) | 32,675 (15,520) |
| Years since HCV diagnosis | 4.3 (3.6) |
| AUDIT | 1.6 (2.7) |
| Q & F drinking | 2.3 (1.8) |
| Q & F before HCV diagnosis | 6.4 (2.8) |
| Q & F immediately after HCV diagnosis | 2.6 (3.0) |
Notes: HCV = hepatitis C virus; AUDIT = Alcohol Use Disorders Identification Test; Q & F = quantity and frequency.
Although the majority of subjects in the study reported having quit drinking (73.4%), 65.8% had consumed at least one drink since their HCV diagnosis, and 40.4% had consumed at least one drink in the 12 months before being interviewed. In prior work, we examined this inconsistency using qualitative and quantitative data, which both suggested that HCV patients have varied drinking trajectories. Laypeople's definitions of quitting may be descriptive of quitting a prior heavier drinking pattern rather than descriptive of a new pattern of total abstinence (Perzynski et al., 2006; Stoller et al., 2006).
Analytic procedures
Exploratory factor analysis and confirmatory factor analysis (CFA) were used to test the measurement structure and internal validity of each concept. Concepts and items that demonstrated poor reliability or internal validity were eliminated. We tested models ranging from 15 to 35 factors, and the 35-factor solution was best supported by logic, theory, and the empirical evidence.
We conducted domain- and subdomain-level CFA using Amos 7.0 (Arbuckle, 2009). Our decisions whether to accept or reject a given CFA model were informed by Hu and Bentler's (1999) Two-Index Presentation Strategy. Standards of model fit were standardized root mean residual of .09 or lower, Tucker–Lewis index of .95 or higher, root mean square error of approximation of .06 or lower, and comparative fit index of .95 or higher. After establishing the final measurement models with CFA, Cronbach's α was used to assess reliability (Nunnelly, 1978).
Bivariate analyses were conducted using Pearson and Spearman correlations. Nested ordinary least squares multiple regression analysis was conducted to examine the effects of the subdomains on alcohol consumption (AUDIT-C). Linear logistic regression analysis was used to examine the effects of the subdomains on quitting drinking. The bootstrap procedure was used to calculate confidence intervals and examine potential bias in all model parameters (Cirincione and Gurrieri, 1997; Efron, 1982). Missing values were handled using multiple imputation.
Results
Measurement modeling and reliability results
As shown in Table 2, statistical fit of the final measurement models was excellent for nearly all of the conceptual domains, with the exception of attentional styles. The attentional styles measures were nevertheless retained as indices of the number of affirmative monitoring and blunting responses.
Table 2.
Confirmatory factor analysis model fit statistics for conceptual domains (N = 527)a
| Variable | χ2 | df | RMSEA | CFI | TLI | SRMR |
| Alcohol expectancies | 94.66 | 41 | .050 | .97 | .96 | .047 |
| Stages of change | 80.89 | 38 | .046 | .97 | .95 | .038 |
| Alcohol self-efficacy | 152.41 | 41 | .072 | .98 | .97 | .027 |
| Alcohol and HCV | 63.43 | 38 | .036 | .98 | .97 | .038 |
| Coping styles | 124.93 | 48 | .055 | .96 | .93 | .042 |
| Attentional styles | 45.77 | 19 | .052 | .90 | .82 | .048 |
| Psychological traits | 112.15 | 55 | .044 | .97 | .94 | .037 |
| Health management style | 18.80 | 14 | .026 | .99 | .99 | .022 |
| Stigma of HCV | 11.84 | 6 | .043 | .99 | .98 | .029 |
| Health status | 165.34 | 81 | .044 | .97 | .97 | .033 |
Notes: RMSEA = root mean square error of approximation; CFI= comparative fit index; TLI = Tucker–Lewis index; SRMR = standardized root mean residual; HCV = hepatitis C virus.
All CFA models are conditionally independent, with no cross loadings or correlated residuals.
Table 2 presents only the final model fit statistics. A total of 34 items were eliminated because of poor variability, low primary factor loadings, high secondary factor loadings, or poor reliability.
Descriptive statistics and reliability estimates are shown in Table 3. Eleven scales had excellent reliability (>.80), 15 had adequate reliability (.60–.80), 4 had borderline reliability of .55 to .60, and 5 had poor reliability. The vast majority of the subdomains are deemed sufficiently reliable. This reliability was achieved with survey items using brief, easy-to-understand statements and a conservative number of items per concept.
Table 3.
Descriptive statistics and reliability coefficients for HCV/AUQ subdomains (N = 527)
| Variable | No. of items | Cronbach's α | M | SD | No. of imputed missing values |
| Alcohol expectancies | |||||
| Mood enhancement | 5 | .860 | 3.56 | 1.29 | 0 |
| Cognitive enhancement | 2 | .825 | 2.14 | 1.00 | 0 |
| Negative | 4 | .706 | 3.63 | 0.91 | 0 |
| Stages of change | |||||
| Precontemplative | 2 | .761 | 3.44 | 1.60 | 0 |
| Contemplative | 3 | .746 | 4.93 | 1.21 | 0 |
| Action | 3 | .809 | 4.48 | 1.37 | 1 |
| Maintenance | 3 | .505 | 3.92 | 1.26 | 1 |
| Alcohol self-efficacy | |||||
| Mood situations | 4 | .931 | 1.63 | 0.94 | 0 |
| Social situations | 4 | .937 | 2.04 | 1.24 | 0 |
| Private situations | 3 | .828 | 1.37 | 0.66 | 0 |
| Alcohol and HCV | |||||
| Alcohol not so bad | 2 | .610 | 5.01 | 0.89 | 0 |
| HCV/alcohol knowledge | 3 | .784 | 5.41 | 0.62 | 1 |
| Reasons to drink | 3 | .770 | 2.74 | 1.31 | 0 |
| Doctor's advice | 2 | .592 | 5.11 | 0.81 | 3 |
| Coping styles | |||||
| Avoidant | 3 | .645 | 1.87 | 0.89 | 1 |
| Planful | 3 | .711 | 3.96 | 0.78 | 0 |
| Religious | 2 | .846 | 3.72 | 1.25 | 1 |
| Support seeking | 4 | .826 | 3.14 | 0.99 | 1 |
| Attentional styles | |||||
| Monitoring | 4 | .533 | 0.72 | 0.27 | 0 |
| Blunting | 4 | .459 | 0.38 | 0.27 | 1 |
| Psychological traits | |||||
| General self-efficacy | 3 | .693 | 4.71 | 0.75 | 0 |
| Neuroticism | 3 | .730 | 3.09 | 1.16 | 0 |
| Extraversion | 2 | .699 | 4.03 | 1.06 | 0 |
| Agreeableness | 2 | .588 | 4.76 | 0.85 | 0 |
| Conscientiousness | 3 | .560 | 4.71 | 0.87 | 0 |
| Health management style | |||||
| Reliance on doctors | 2 | .421 | 4.01 | 0.85 | 0 |
| Cooperate with doctors | 2 | .701 | 4.98 | 0.86 | 0 |
| Trust doctors | 2 | .473 | 4.13 | 1.09 | 0 |
| Live healthy lifestyle | 2 | .765 | 3.89 | 0.99 | 0 |
| Stigma of HCV | |||||
| Keep HCV secret | 2 | .883 | 3.01 | 1.47 | 0 |
| Ostracized | 2 | .778 | 2.28 | 1.04 | 14 |
| Devalued | 2 | .550 | 2.59 | 1.16 | 0 |
| Health status | |||||
| Depression | 6 | .827 | 18.64 | 3.21 | 10 |
| Physical symptoms | 5 | .740 | 2.34 | 0.89 | 0 |
| Cognitive symptoms | 4 | .855 | 2.14 | 0.99 | 0 |
| 35 subdomains | 105 |
Notes: HCV = hepatitis C virus; AUQ = alcohol use questionnaire.
Bivariate results
Table 4 presents correlations between each of the subdomains and the primary and secondary drinking outcomes. Of the 35 subdomains, 26 had statistically significant associations with at least one of the alcohol use measures. The strongest and most consistent pattern of associations was found for the three alcohol self-efficacy subdomains. As expected, subdomains associated with quitting drinking are inversely associated with having had even one drink since HCV diagnosis.
Table 4.
Bivariate associations of subdomains with five alcohol use measures (N = 527)
| Variable | Primary outcomes |
Secondary outcomes |
||||||
| AUDIT-C |
Quit drinking? |
Post-diagnosis drinking |
Even one drink? |
|||||
| r | p | ρ | p | r | p | ρ | P | |
| Alcohol expectancies | ||||||||
| Mood enhancement | −.06 | .197 | .08 | .052 | .11 | .010 | −.11 | .010 |
| Cognitive enhancement | −.06 | .171 | .04 | .301 | −.04 | .373 | −.08 | .060 |
| Negative | −.27 | <.001 | .36 | <.001 | −.01 | .846 | −.15 | .001 |
| Stages of change | ||||||||
| Precontemplative | −.06 | .165 | .21 | <.001 | .07 | .094 | −.05 | .252 |
| Contemplative | −.14 | .001 | .23 | <.001 | .02 | .693 | −.11 | .009 |
| Action | .01 | .864 | .02 | .634 | −.01 | .726 | .04 | .382 |
| Maintenance | −.17 | <.001 | .24 | <.001 | .01 | .804 | −.11 | .009 |
| Alcohol self-efficacy | ||||||||
| Mood situations | −.59 | <.001 | .58 | <.001 | −.19 | <.001 | −.45 | <.001 |
| Social situations | −.68 | <.001 | .66 | <.001 | −.22 | <.001 | −.55 | <.001 |
| Private situations | −.54 | <.001 | .53 | <.001 | −.10 | .025 | −.36 | <.001 |
| Alcohol and HCV | ||||||||
| Alcohol is bad | −.33 | <.001 | .29 | <.001 | −.11 | .009 | −.29 | <.001 |
| HCV & alcohol knowledge | −.27 | <.001 | .25 | <.001 | −.07 | .074 | −.22 | <.001 |
| Drinking is normal | −.10 | .028 | .21 | <.001 | .11 | .015 | −.16 | <.001 |
| Doctor's advice | −.15 | <.001 | .19 | <.001 | −.10 | .035 | −.18 | <.001 |
| Coping styles | ||||||||
| Avoidant | −.04 | .376 | .07 | .085 | −.02 | .692 | .03 | .481 |
| Planful | −.23 | <.001 | .15 | <.001 | −.14 | .001 | −.19 | <.001 |
| Religious | −.25 | <.001 | .20 | <.001 | −.01 | .806 | −.09 | .054 |
| Support seeking | −.14 | .001 | .10 | .026 | −.02 | .618 | −.12 | .009 |
| Attentional styles | ||||||||
| Monitoring | −.03 | .446 | −.04 | .382 | −.04 | .375 | −.06 | .162 |
| Blunting | −.01 | .773 | .03 | .447 | .00 | .936 | .03 | .517 |
| Psychological traits | ||||||||
| General self-efficacy | −.01 | .834 | .00 | .999 | −.12 | .007 | −.12 | .007 |
| Neuroticism | −.01 | .756 | .04 | .250 | .10 | .022 | .09 | .041 |
| Extraversion | .08 | .083 | −.05 | .202 | .00 | .989 | −.01 | .838 |
| Agreeableness | .08 | .054 | −.10 | .019 | −.03 | .559 | −.02 | .705 |
| Conscientiousness | .10 | .026 | −.04 | .331 | −.03 | .515 | −.02 | .631 |
| Health management style | ||||||||
| Reliance on doctors | −.02 | .677 | .03 | .600 | −.04 | .348 | −.06 | .162 |
| Cooperate with doctors | −.26 | <.001 | .25 | <.001 | −.13 | .004 | −.21 | <.001 |
| Trust doctors | −.02 | .630 | .04 | .288 | −.06 | .143 | −.14 | .002 |
| Live healthy lifestyle | −.20 | .000 | .17 | <.001 | −.08 | .051 | −.16 | <.001 |
| Stigma of HCV | ||||||||
| Keep HCV secret | .04 | .331 | −.04 | .338 | −.01 | .783 | .04 | .384 |
| Ostracized | −.04 | .304 | .12 | .007 | .06 | .150 | .04 | .408 |
| Devalued | −.04 | .308 | .07 | .088 | .03 | .507 | .02 | .651 |
| Health status | ||||||||
| Depression & vitality | .06 | .476 | −.05 | .054 | −.07 | .021 | −.01 | .360 |
| Physical symptoms | −.02 | .663 | .07 | .117 | .10 | .023 | .06 | .145 |
| Cognitive symptoms | −.11 | .013 | .15 | .000 | .06 | .139 | −.01 | .893 |
Notes: AUDIT-C = Alcohol Use Disorders Identification Test consumption questions; HCV = hepatitis C virus.
Alcohol expectancies.
A higher score on the negative expectancies subdomain, the cons of drinking, was associated with lower AUDIT-C scores, a higher likelihood of having quit drinking, and a lower likelihood of having had even one drink.
Stages of change.
Scores on the precontemplative, contemplative, and maintenance domains were all associated with having quit drinking.
Alcohol and hepatitis C virus.
Thinking that alcohol is bad for people with HCV was moderately associated with lower alcohol consumption (r = −.33, p < .001) and quitting drinking (ρ = .29, p < .001), as was better knowledge of how alcohol influences HCV progression (r = −.27, p < .001; ρ = .5, p < .001). Drinking normalcy had a small but significant inverse association with current alcohol consumption (r = −.10, p < .05) and was also associated with quitting drinking (ρ = .21, p < .001). Advice from a doctor to reduce drinking alcohol after HCV infection was associated with lower alcohol consumption (r = −.15, p < .001) and a lower likelihood of having had even one drink since HCV diagnosis (ρ = −.18, p < .001).
Alcohol self-efficacy.
Having higher levels of task-specific self-efficacy was strongly associated with lower current alcohol consumption in mood (r = −.59, p < .001), social (r = −.68, p < .001) situations, and private situations (r = −.54, p < .001). Similar associations were found with quitting drinking.
Coping styles.
Planful, religious, and support-seeking coping styles were all associated with lower current alcohol use (r = −.23, p < .001; r = −.25, p < .001; and r = −.15, p < .01, respectively).
Attentional styles.
Monitoring and blunting were not associated with any of the alcohol use or reduction measures.
Psychological traits and general self-efficacy.
In general, psychological trait factors had small associations with the alcohol use measures. Higher levels of agreeableness were associated with not quitting drinking (ρ = −.10, p < .05). General self-efficacy was associated with lower postdiagnosis drinking and abstinence after HCV diagnosis (r = −.12, p < .01; ρ = −.12, p < .01).
Health management style.
Two dimensions of health management style had an association with alcohol consumption: cooperation with doctors (r = −.26, p < .001) and living a healthy lifestyle (r = −.23, p < .001). Higher trust in doctors was related to a higher likelihood of not having a single drink after HCV diagnosis (ρ = −.14, p < .01).
Perceived stigma.
The three stigma subdomains were primarily not associated with the alcohol use measures, with the exception being that feeling ostracized was associated with quitting drinking (ρ = .12, p < .01).
Health status.
Depression was not associated with any of the alcohol use measures. Physical symptoms were associated with post-diagnosis drinking, whereas cognitive symptoms were associated with lower current alcohol consumption and not quitting drinking.
Multivariate results
Results of nested ordinary least squares and logistic regression analysis for three models with outcomes AUDIT-C and “quit drinking” are presented in Tables 5 and 6, respectively. The analysis in Table 5 was repeated with the full AUDIT score as the outcome, and the results were nearly identical. The three nested models reflect the conceptual model in Figure 1: (a) sociodemographics and health status; (b) sociodemographics, health status, psychosocial states and traits; and (c) sociodemographics, health status, psychosocial states and traits, and alcohol-related concepts.
Table 5.
Nested ordinary least squares regression analysis, dependent variable = AUDIT-Ca (N = 527)
| Variable | Model 1 |
Model 2 |
Model 3 |
|||
| β | p | β | p | β | p | |
| Married or with partner | .09 | .061 | .09 | .045 | .08 | .028 |
| Cognitive symptoms | −.19 | .009 | −.12 | .025 | −.04 | .362 |
| Extraversion | .10 | .038 | .01 | .780 | ||
| Agreeableness | .13 | .005 | .04 | .220 | ||
| Conscientiousness | .11 | .100 | .06 | .145 | ||
| Avoidant | −.04 | .392 | −.07 | .047 | ||
| Planful | −.11 | .037 | −.04 | .369 | ||
| Religious | −.21 | <.001 | −.09 | .026 | ||
| Cooperate with doctors | −.17 | .002 | −.06 | .155 | ||
| Trust doctors | .07 | .110 | .06 | .091 | ||
| Live healthy lifestyle | −.12 | .020 | −.01 | .770 | ||
| Mood situations | −.09 | .149 | ||||
| Social situations | −.46 | <.001 | ||||
| Private situations | −.09 | .051 | ||||
| Alcohol is bad | −.07 | .055 | ||||
| HCV and alcohol knowledge | −.10 | .014 | ||||
| R2 | .03 | .20 | .55 | |||
| Adjusted R2 | .01 | .15 | .51 | |||
Notes: AUDIT-C = Alcohol Use Disorders Identification Test consumption questions; HCV = hepatitis C virus.
Control variables with small magnitude, nonsignificant relationships are omitted from the table. Models 1–3 omit age, gender, household income, neighborhood income, education level, marital status, race/ethnicity, physical symptoms, and depression. Models 2 and 3 omit general self-efficacy, neuroticism, support seeking, monitoring, blunting, reliance on doctors, and stigma. Model 3 omits drinking is normal, precontemplative, contemplative, action, maintenance, mood enhancement, cognitive enhancement, negative expectancies, and CAGE.
Table 6.
Nested logistic regression analysisa: Have you quit drinking?
| Variable | Model 1 |
Model 2 |
Model 3 |
|||
| OR | [95% CI] | OR | [95% CI] | OR | [95% CI] | |
| Cognitive symptoms | 1.47 | [1.11, 1.94] | 1.54 | [1.12, 2.11] | ||
| Agreeableness | 0.62 | [0.46, 0.84] | ||||
| Religious | 1.39 | [1.13, 1.70] | ||||
| Monitoring | 2.50 | [1.04, 5.90] | ||||
| Cooperate with doctors | 1.75 | [1.28, 2.40] | 1.88 | [1.15, 3.09] | ||
| Live healthy lifestyle | 1.34 | [1.01, 1.76] | ||||
| Social situations | 3.44 | [2.03, 5.78] | ||||
| HCV and alcohol knowledge | 2.27 | [1.09, 4.74] | ||||
| Drinking is normal | 0.53 | [0.35, 0.81] | ||||
| Action | 1.61 | [1.08, 2.44] | ||||
| Negative expectancies | 2.30 | [1.38, 3.84] | ||||
| Nagelkerke R2 | .04 | .22 | .72 | |||
Notes: OR = odds ratio; CI = confidence interval; HCV = hepatitis C virus.
Models 1–3 for the logistic regression here contain the identical set of independent variables to the ordinary least squares regression reported in Table 5; independent variables without significant associations are omitted in this table.
Being married or living with a partner was associated with higher alcohol consumption, and having more cognitive symptoms was associated with lower alcohol consumption. The effect of cognitive symptoms was reduced by the addition of psychosocial states and traits in Model 2 and further by the addition of alcohol-related concepts in Model 3.
Religious and planful coping styles, cooperation with doctors, and living a healthy lifestyle were associated with lower alcohol consumption in Model 2, whereas agreeable-ness and extraversion were associated with higher alcohol consumption. Total variance explained in Model 2 was 20%. The effects of all the psychosocial states and traits in Model 2 were substantially reduced by the addition of alcohol-related concepts in Model 3.
Both alcohol self-efficacy (social and private situations) and knowledge of the risks of drinking alcohol for people with HCV were associated with lower alcohol consumption in Model 3. Although the effects of marital status and religious coping were partially reduced, these variables still had significant effects in Model 3. Total variance in alcohol consumption explained in Model 3 increased to 55%.
Table 6 presents results for significant associations between subdomains and the dichotomous item, “Have you quit drinking?” The variables in Model 3 do an exceptional job of classification, explaining 72% of the variance (pseudo R2 = .72, c statistic = .960). Similar to Model 3 with AUDIT-C as the dependent variable, alcohol self-efficacy for social situations had the highest magnitude association (odds ratio = 3.44).
Discussion
Our study found that multiple psychosocial concepts were associated with alcohol use and retrospective self-reports of alcohol reduction for medical patients with chronic HCV infection and AUDIT scores of 10 or less. These results diverge from studies of alcohol reduction in other populations, and these specific findings might be used in the design of future interventions for medical patients faced with a need to reduce or eliminate alcohol consumption.
Our analysis lends preliminary support to the conceptual model presented in Figure 1 in which alcohol use is associated with psychosocial states and traits as well as alcohol and illness-related knowledge, attitudes, and beliefs. The final model explained an estimated 55% of the variation in current alcohol use and 72% of the variation in reports of quitting drinking after HCV diagnosis, suggesting that study concepts and measures may have predictive value for identifying HCV patients most at risk of continuing to consume alcohol after HCV diagnosis.
Self-efficacy for social situations was found to have the single strongest magnitude of association with both current alcohol use and alcohol cessation since HCV diagnosis. Our research is consistent with the study by Oei et al. (2007), which found that task-specific rather than general self-efficacy is associated with alcohol use among drinkers without clinically diagnosed drinking problems.
Cognitive symptoms of HCV were associated with reduced alcohol consumption. Differences in cognitive symptoms are most likely not caused by differences in alcohol use history and are likely to be related to the etiologic course of HCV (Perry et al., 2008). We suspect that individuals experiencing cognitive symptoms are more likely to internalize the seriousness of their chronic HCV infection, altering their decisional balance and leading them to reduce their drinking. Based on our results, we postulate that better informing patients how alcohol exacerbates HCV infection and disease progression and persuading patients that drinking alcohol is hazardous (both through information and tangible symptoms) may lead to reduced alcohol consumption.
The research literature on agreeableness in general populations and populations of those diagnosed with alcohol use disorders has most often found that lower agreeableness is associated with a higher likelihood of alcoholism and alcohol-related problems in both adults and college students (Laursen et al., 2002; Loukas et al., 2000; Ruiz et al., 2003; Walton and Roberts, 2004). Although no prior studies have focused on the relationship between alcohol use and agreeableness in people not drinking at a very high level, one prior study found that abstinence is associated with higher agreeableness in the first year of college (McAdams and Donnellan, 2009). Our results contrast with these prior studies; higher agreeableness was associated with higher alcohol consumption and a lower likelihood of quitting drinking. This result is consistent with the conclusions of van Schoor et al. (2008), who found that, in situations where peers were drinking more, those who had high agreeableness scores were likely to drink more. The effects of psychological traits like agreeableness seem to be conditional on levels of current and prior drinking, the particulars of drinking situations, and drinking self-efficacy for those situations.
Our results show a pattern of associations that suggest the centrality of the social aspects of drinking alcohol. Prior studies have often ignored the social aspects of drinking such as celebration, relaxation, and camaraderie (Heath, 2007). Corroborating previously reported results, feeling ostracized because of HCV infection was associated with quitting drinking (Stoller et al., 2009). Having HCV may lead people to either avoid situations in which alcohol is consumed, or they may feel routinely left out of celebrations and social gatherings where others are drinking. Although the magnitude of the stigma association is small, the importance is further illuminated when considering the very strong multivariate association between alcohol consumption and self-efficacy for drinking in social situations. A guideline such as “zero alcohol consumption is best for persons with HCV infection” misses the fact that adherence to this recommendation may translate into fewer pleasurable social interactions or may run contrary to a person's agreeable personality. Psychosocial context plays an important role in shaping individual-level alcohol use decisions.
Our study also provides insight into the relationship between marital status and alcohol use. In the general population, married individuals tend to drink less over the course of their lives (Karlamangla et al., 2006). Individuals in a stable marriage have lower rates of alcohol dependence than the never married; those who have been married and divorced have a lower rate of alcohol dependence than those who were never married (Dick et al., 2006). In contrast, our study found that being currently married is associated with drinking more alcohol among those with HCV not drinking at a high level. Thus, although marital status generally protects against alcohol use disorders, married individuals with a medical diagnosis like HCV may be less likely to quit drinking when advised to do so.
This study has some key limitations. The study population consisted of HCV patients from a single urban, safety-net care setting in the Great Lakes region. Development of the measurement model for this study was conducted on a single sample, and more work is needed to verify the measurement structure. The primary outcomes, alcohol consumption and quitting drinking, were measured by self-report; although bias and inaccuracy are possible, self-reports of alcohol use are practical and used routinely for clinical decision making (Del Boca and Darkes, 2003). Generalizability of our findings is further limited because subjects in this research study were given assurances of the confidentiality of their responses; elicitation of drinking behavior in a clinical context does not carry such assurances.
This study included many psychosocial concepts and was cross-sectional. Thus, our confidence in the relationships and the ability to infer a causal connection between concepts are limited until future studies can be conducted. Future work in this area should seek predictive validity by examining whether the concepts found to have an association in the current study are able to predict actual within-subject reductions in alcohol use over time in a sample of patients recently diagnosed with HCV and currently drinking at a low or moderate level.
Adaptation of brief interventions used previously in outpatient settings could be a cost-effective solution for reducing alcohol consumption and improving health outcomes for patients with HCV and other medical conditions (Fleming et al., 2004). Some principles effective for those with diagnosed alcohol abuse or dependence may translate well to medical patients who need to reduce consumption, but a one-size-fits-all approach is unlikely to be successful. In combination, our results lead us to suggest three potentially important features of future research on interventions for medical patients faced with a need to reduce alcohol consumption: (a) further education of patients on the hazards of drinking with their medical condition, including the potential exacerbation of symptoms; (b) emphasis on the maintenance of healthy social relationships in the absence of alcohol use; and (c) focus on building strategies and confidence for resisting drinking in specific settings identified by a particular patient.
Acknowledgments
The authors are grateful to Eleanor Stoller, Ph.D., for her work and contributions in earlier study phases and to Kyle Kercher, Ph.D., for his expertise in methodological consultation.
Footnotes
This research was supported by National Institute on Alcohol Abuse and Alcoholism Grant 1R01AA13302 (to Neal V. Dawson).
References
- Ajzen I. The theory of planned behavior. Organizational Behavior and Human Decision Processes. 1991;50:179–211. [Google Scholar]
- Allen JP, Wilson VB, editors. Assessing alcohol problems: A guide for clinicians and researchers. 2nd ed. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2003. Retrieved from http://pubs.niaaa.nih.gov/publications/Assesing%20Alcohol/index.htm. (NIH Publication No. 03–3745) [Google Scholar]
- Arbuckle JL. Chicago: SPSS; 2009. Amos (Version 18.0.0) [Computer Program] [Google Scholar]
- Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Annals of Internal Medicine. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. The alcohol use disorders identification test: Guidelines for use in primary care. Geneva, Switzerland: World Health Organization, Department of Mental Health and Substance Dependence; 2001. [Google Scholar]
- Benet-Martínez V, John OP. Los Cinco Grandes across cultures and ethnic groups: Multitrait-multimethod analyses of the Big Five in Spanish and English. Journal of Personality and Social Psychology. 1998;75:729–750. doi: 10.1037//0022-3514.75.3.729. [DOI] [PubMed] [Google Scholar]
- Blixen CE, Webster NJ, Hund AJ, Perzynski AT, Kanuch SW, Stoller EP, Dawson NV. Communicating about alcohol consumption to nonharmful drinkers with hepatitis C: Patient and provider perspectives. Journal of General Internal Medicine. 2008;23:242–247. doi: 10.1007/s11606-007-0483-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): Validation of a screening instrument for use in medical settings. Journal of Studies on Alcohol. 1995;56:423–432. doi: 10.15288/jsa.1995.56.423. [DOI] [PubMed] [Google Scholar]
- Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA the Ambulatory Care Quality Improvement Project (ACQUIP) The AUDIT alcohol consumption questions (AUDIT-C): An effective brief screening test for problem drinking. Archives of Internal Medicine. 1998;158:1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- Carver CS. You want to measure coping but your protocol's too long: Consider the brief COPE. International Journal of Behavioral Medicine. 1997;4:92–100. doi: 10.1207/s15327558ijbm0401_6. [DOI] [PubMed] [Google Scholar]
- Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, Rose M the PROMIS Cooperative Group. The Patient-Reported Outcomes Measurement Information System (PROMIS): Progress of an NIH Roadmap Cooperative Group during its first two years. Medical Care. 2007;45:S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Hepatitis C: General Information. 2010. Retrieved from http://www.cdc.gov/hepatitis/hcv/pdfs/hepcgeneralfactsheet.pdf. [Google Scholar]
- Cirincione C, Gurrieri GA. Research methodology: Computer-intensive methods in the social sciences. Social Science Computer Review. 1997;15:83–97. [Google Scholar]
- Del Boca FK, Darkes J. The validity of self-reports of alcohol consumption: State of the science and challenges for research. Addiction, 98, Supplement s2. 2003:1–12. doi: 10.1046/j.1359-6357.2003.00586.x. [DOI] [PubMed] [Google Scholar]
- DeWalt DA, Rothrock N, Yount S, Stone AA the PROMIS Cooperative Group. Evaluation of item candidates: The PROMIS qualitative item review. Medical Care. 2007;45:S12–S21. doi: 10.1097/01.mlr.0000254567.79743.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Agrawal A, Schuckit MA, Bierut L, Hinrichs A, Fox L, Begleiter H. Marital status, alcohol dependence, and GABRA2: Evidence for gene-environment correlation and interaction. Journal of Studies on Alcohol. 2006;67:185–194. doi: 10.15288/jsa.2006.67.185. [DOI] [PubMed] [Google Scholar]
- Dieperink E, Ho SB, Heit S, Durfee JM, Thuras P, Willenbring ML. Significant reductions in drinking following brief alcohol treatment provided in a hepatitis C clinic. Psychosomatics. 2010;51:149–156. doi: 10.1176/appi.psy.51.2.149. [DOI] [PubMed] [Google Scholar]
- DiMatteo MR. Variations in patients’ adherence to medical recommendations: A quantitative review of 50 years of research. Medical Care. 2004;42:200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- Efron B. The jackknife, the bootstrap and other resampling plans. Philadelphia, PA: Society for Industrial and Applied Mathematics; 1982. [Google Scholar]
- Ewing JA. Detecting alcoholism. The CAGE questionnaire. Journal of the American Medical Association. 1984;252:1905–1907. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- Fife BL, Wright ER. The dimensionality of stigma: A comparison of its impact on the self of persons with HIV/AIDS and cancer. Journal of Health and Social Behavior. 2000;41:50–67. [PubMed] [Google Scholar]
- Fiscella K, Franks P, Clancy CM, Doescher MP, Banthin JS. Does skepticism towards medical care predict mortality? Medical Care. 1999;37:409–414. doi: 10.1097/00005650-199904000-00010. [DOI] [PubMed] [Google Scholar]
- Fishbein M. A theory of reasoned action: Some applications and implications. Nebraska Symposium on Motivation. 1979;27:65–116. [PubMed] [Google Scholar]
- Fishbein M, Yzer MC. Using theory to design effective health behavior interventions. Communication Theory. 2003;13:164–183. [Google Scholar]
- Fleming M, Brown R, Brown D. The efficacy of a brief alcohol intervention combined with %CDT feedback in patients being treated for type 2 diabetes and/or hypertension. Journal of Studies on Alcohol. 2004;65:631–637. doi: 10.15288/jsa.2004.65.631. [DOI] [PubMed] [Google Scholar]
- Hall KL, Rossi JS. Meta-analytic examination of the strong and weak principles across 48 health behaviors. Preventive Medicine. 2008;46:266–274. doi: 10.1016/j.ypmed.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Heath DB. Why we don't know more about the social benefits of moderate drinking. Annals of Epidemiology. 2007;17:S71–S74. [Google Scholar]
- Hézode C, Lonjon I, Roudot-Thoraval F, Pawlotsky J-M, Zafrani E-S, Dhumeaux D. Impact of moderate alcohol consumption on histological activity and fibrosis in patients with chronic hepatitis C, and specific influence of steatosis: A prospective study. Alimentary Pharmacology & Therapeutics. 2003;17:1031–1037. doi: 10.1046/j.1365-2036.2003.01546.x. [DOI] [PubMed] [Google Scholar]
- Hu L-T, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6:1–55. [Google Scholar]
- Hulka BS, Zyzanski SJ, Cassel JC, Thompson SJ. Scale for the measurement of attitudes toward physicians and primary medical care. Medical Care. 1970;8:429–435. doi: 10.1097/00005650-197009000-00010. [DOI] [PubMed] [Google Scholar]
- Joachim G, Acorn S. Stigma of visible and invisible chronic conditions. Journal of Advanced Nursing. 2000;32:243–248. doi: 10.1046/j.1365-2648.2000.01466.x. [DOI] [PubMed] [Google Scholar]
- Karlamangla A, Zhou K, Reuben D, Greendale G, Moore A. Longitudinal trajectories of heavy drinking in adults in the United States of America. Addiction. 2006;101:91–99. doi: 10.1111/j.1360-0443.2005.01299.x. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen B, Pulkkinen L, Adams R. The antecedents and correlates of agreeableness in adulthood. Developmental Psychology. 2002;38:591–603. doi: 10.1037//0012-1649.38.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukas A, Krull JL, Chassin L, Carle AC. The relation of personality to alcohol abuse/dependence in a high-risk sample. Journal of Personality. 2000;68:1153–1175. doi: 10.1111/1467-6494.00130. [DOI] [PubMed] [Google Scholar]
- McAdams KK, Donnellan MB. Facets of personality and drinking in first-year college students. Personality and Individual Differences. 2009;46:207–212. [Google Scholar]
- Miller SM. Monitoring and blunting: Validation of a questionnaire to assess styles of information seeking under threat. Journal of Personality and Social Psychology. 1987;52:345–353. doi: 10.1037//0022-3514.52.2.345. [DOI] [PubMed] [Google Scholar]
- Miller WR, Tonigan JS. Assessing drinkers’ motivations for change: The Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES) Psychology of Addictive Behaviors. 1996;10:81–89. [Google Scholar]
- Millon T, Green CJ, Meagher RB. The MBHI: A new inventory for the psychodiagnostician in medical settings. Professional Psychology. 1979;10:529–539. [Google Scholar]
- Nunnelly JC. Psychometric theory. 2nd ed. New York, NY: McGraw-Hill; 1978. [Google Scholar]
- Oei TP, Hasking P, Phillips L. A comparison of general self-efficacy and drinking refusal self-efficacy in predicting drinking behavior. American Journal of Drug and Alcohol Abuse. 2007;33:833–841. doi: 10.1080/00952990701653818. [DOI] [PubMed] [Google Scholar]
- Perry W, Hilsabeck RC, Hassanein TI. Cognitive dysfunction in chronic hepatitis C: A review. Digestive Diseases and Sciences. 2008;53:307–321. doi: 10.1007/s10620-007-9896-z. [DOI] [PubMed] [Google Scholar]
- Perzynski AT, Kanuch S, Webster NJ, McCormick R, Stoller EP, Blixen C, Dawson NV. Is quitting drinking the same as abstinence? Evidence from a mixed methods study of non-dependent drinkers with hepatitis C. Cambridge, MA: Presented at the 28th Annual Meeting of the Society for Medical Decision Making; 2006. [Google Scholar]
- Pessione F, Degos F, Marcellin P, Duchatelle V, Njapoum C, Martinot-Peignoux M, Rueff B. Effect of alcohol consumption on serum hepatitis C virus RNA and histological lesions in chronic hepatitis C. Hepatology. 1998;27:1717–1722. doi: 10.1002/hep.510270635. [DOI] [PubMed] [Google Scholar]
- Prochaska JO, DiClemente CC. Stages of change in the modification of problem behaviors. Progress in Behavior Modification. 1992;28:183–218. [PubMed] [Google Scholar]
- Pryor JB, Reeder GD, Landau S. A social-psychological analysis of HIV-related stigma: A two-factor theory. American Behavioral Scientist. 1999;42:1193–1211. [Google Scholar]
- Rigamonti C, Mottaran E, Reale E, Rolla R, Cipriani V, Capelli F, Albano E. Moderate alcohol consumption increases oxidative stress in patients with chronic hepatitis C. Hepatology. 2003;38:42–49. doi: 10.1053/jhep.2003.50275. [DOI] [PubMed] [Google Scholar]
- Ruiz MA, Pincus AL, Dickinson KA. NEO PI-R predictors of alcohol use and alcohol-related problems. Journal of Personality Assessment. 2003;81:226–236. doi: 10.1207/S15327752JPA8103_05. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Schroder KE, Schwarzer R. Habitual self-control and the management of health behavior among heart patients. Social Science & Medicine. 2005;60:859–875. doi: 10.1016/j.socscimed.2004.06.028. [DOI] [PubMed] [Google Scholar]
- Schwarzer R, Jerusalem M. Generalized self-efficacy scale. In: Weinman J, Wright S, Johnston M, editors. Measures in health psychology: A user's portfolio. Causal and control beliefs. Windsor, UK: NFER-Nelson; 1995. pp. 35–37. [Google Scholar]
- Stoller EP, Hund AJ, Webster NJ, Blixen CE, Perzynski AT, McCormick RA, Dawson NV. Alcohol consumption within the context of hepatitis C: A qualitative study of non-problematic drinkers. Alcohol and Alcoholism. 2006;41:546–552. doi: 10.1093/alcalc/agl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoller EP, Webster NJ, Blixen CE, McCormick RA, Hund AJ, Perzynski AT, Dawson NV. Alcohol consumption decisions among nonabusing drinkers diagnosed with hepatitis C: An exploratory sequential mixed methods study. Journal of Mixed Methods Research. 2009;3:65–86. doi: 10.1177/1558689808326119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Aloman C, Polyak SJ, Weinman SA, Wands J, Zakhari S. Hepatitis C infection and alcohol use: A dangerous mix for the liver and antiviral immunity. Alcoholism: Clinical and Experimental Research. 2006;30:709–719. doi: 10.1111/j.1530-0277.2006.00083.x. [DOI] [PubMed] [Google Scholar]
- van Schoor G, Bot SM, Engels RC. Alcohol drinking in young adults: The predictive value of personality when peers come around. European Addiction Research. 2008;14:125–133. doi: 10.1159/000130416. [DOI] [PubMed] [Google Scholar]
- Vermeire E, Hearnshaw H, Van Royen P, Denekens J. Patient adherence to treatment: Three decades of research. A comprehensive review. Journal of Clinical Pharmacy and Therapeutics. 2001;26:331–342. doi: 10.1046/j.1365-2710.2001.00363.x. [DOI] [PubMed] [Google Scholar]
- Walton KE, Roberts BW. On the relationship between substance use and personality traits: Abstainers are not maladjusted. Journal of Research in Personality. 2004;38:515–535. [Google Scholar]
- Westin J, Lagging LM, Spak F, Aires N, Svensson E, Lindh M, Wejstål R. Moderate alcohol intake increases fibrosis progression in untreated patients with hepatitis C virus infection. Journal of Viral Hepatitis. 2002;9:235–241. doi: 10.1046/j.1365-2893.2002.00356.x. [DOI] [PubMed] [Google Scholar]
- Willis GB. Cognitive interviewing: A tool for improving questionnaire design. Thousand Oaks, CA: Sage; 2005. [Google Scholar]
- Young R.McD, Knight RG. The Drinking Expectancy Questionnaire: A revised measure of alcohol-related beliefs. Journal of Psychopathology and Behavioral Assessment. 1989;11:99–112. [Google Scholar]
- Young R.McD, Oei TPS, Crook GM. Development of a drinking self-efficacy questionnaire. Journal of Psychopathology and Behavioral Assessment. 1991;13:1–15. [Google Scholar]
- Young RS, Oei TP. Drinking Expectancy Profile: A test manual. Brisbane, Australia: BRTC, University of Queensland; 1996. [Google Scholar]
- Zyzanski SJ, Hulka BS, Cassel JC. Scale for the measurement of “satisfaction” with medical care: Modifications in content, format and scoring. Medical Care. 1974;12:611–620. doi: 10.1097/00005650-197407000-00007. [DOI] [PubMed] [Google Scholar]