Abstract
BACKGROUND
Calcium removal from the medium promptly reduces human sperm motility and induces a Na+-dependent depolarization that is accompanied by an increase in intracellular sodium concentration ([Na+]i) and a decrease in intracellular calcium concentration ([Ca2+]i). Sodium loading activates a Na+/K+-ATPase.
METHODS
Membrane potential (Vm) and [Ca2+]i were simultaneously detected in human sperm populations with the fluorescent probes diSC3(5) and fura 2. [Na+]i and was measured independently in a similar fashion using sodium-binding benzofuran isophthalate. Motility was determined in a CASA system, ATP was measured using the luciferin-luciferase assay, and cAMP was measured by radioimmunoassay.
RESULTS
Human sperm motility reduction after calcium removal is related to either Na+-loading or Na+-dependent depolarization, because, under conditions that inhibit the calcium removal-induced Na+-dependent depolarization and [Na+]i increase, sperm motility was unaffected. By clamping sperm Vm with valinomycin, we found that the motility reduction associated with the calcium removal was related to sodium loading, and not to membrane potential depolarization. Mibefradil, a calcium channel blocker, markedly inhibited the Na+-dependent depolarization and sodium loading, and also preserved sperm motility. In the absence of calcium, both ATP and cAMP concentrations were decreased by 40%. However ATP levels were unchanged when calcium removal was performed under conditions that inhibit the calcium removal-induced Na+-dependent depolarization and [Na+]i increase.
CONCLUSIONS
Human sperm motility arrest induced by external calcium removal is mediated principally by sodium loading, which would stimulate the Na+/K+-ATPase and in turn deplete the ATP content.
Keywords: calcium, human sperm motility, Na, K-ATPase, ATP, Catsper
Introduction
Motility is required for sperm to reach the oocyte. In humans, and other mammals, sperm motility is initiated after ejaculation, allowing sperm to pass through the female tract (Darszon et al., 2007). Mammalian sperm are unable to fertilize oocytes immediately after ejaculation, yet they acquire this capacity through a process called ‘capacitation’, while residing for a certain time in the female genital tract. This complex process allows sperm to undergo the acrosome reaction in response to the oocyte zona pellucida glycoprotein ZP3, and concomitantly, to hyperactivate their motility (Suarez and Pacey, 2006). These processes can be reproduced in vitro, i.e. in sperm separated from seminal plasma and suspended in physiological medium. Non-capacitated purified sperm in physiological medium display an ‘initial motility’ consisting of a straightforward pattern produced by a near symmetrical flagellar beat (Suarez and Pacey, 2006; Darszon et al., 2007). In the presence of bicarbonate and bovine serum albumin (BSA), that lead to activation of soluble adenylyl cyclase (sAC) and subsequent PKA activation, sperm develop a ‘hyperactivated motility’ characterized by an increased flagellar bend increases, which results in more asymmetrical whip-like movements. Hyperactivation leads to erratic motility patterns that viscous medium, such as that present in the female tract, which generate a forceful straightforward motility which sperm require to encounter the oocyte (Suarez and Pacey, 2006).
It has been reported that ‘initial’ and ‘hyperactivated’ motility depend on external Ca2+, though their relative sensitivity to this cation is different (Suarez and Ho, 2003). Hyperactivation requires the presence of Catsper1–4 (Ren et al., 2001; Jin et al., 2007), a calcium channel uniquely present in the sperm flagella that is pHi and moderately voltage-dependent. In the absence of external calcium, Catsper functions as a voltage-independent sodium channel, as shown by Carlson et al. (2009) in mouse sperm and by Lishko and Kirichok (2010) in human sperm. Thus, an increase in intraflagellar calcium produced by calcium influx through Catsper1–4 may be associated with the strong flagellar beating typical of hyperactivation. On the other hand, in the absence of external calcium, mouse (Jin et al., 2007), hamster (Feng et al., 1988) and human sperm become motionless (Aaberg et al., 1989). Interestingly, demembranated sperm are able to swim in the absence of external calcium (Feng et al., 1988), indicating that the motility decrease observed in intact sperm in Ca2+-free medium does not directly depend on intracellular calcium but does depend on plasma membrane mechanisms. In this respect, it is worth noting that, in Ca2+-free media, motility decreases significantly faster in wild-type sperm than in sperm from Catsper null mice (Jin et al., 2007). Recently Strunker et al. (2011) and Lishko et al. (2011) showed that CatSper mediates progesterone-induced Ca2+ entry. These studies revealed that the T-type voltage-gated Ca2+ channel blockers (NNC55-0396 and mibefradil) significantly impaired the progesterone-induced Ca2+ response and the pH-evoked Ca2+ responses attributed to CatSper activity.
In this context, earlier reports had shown that external calcium removal induces a Na+-dependent depolarization in mouse (Espinosa and Darszon, 1995) and human sperm (González-Martínez, 2003). Human sperm Vm changes from around –50 mV to values close to 0 mV (González-Martínez, 2003). This depolarization is accompanied by increases in intracellular sodium concentration ([Na+]i) from 5 mM to values much greater than 35 mM occurring within a few minutes (Torres-Flores et al., 2008a) Both the Na+-dependent depolarization and the [Na+]i increase are inhibited by millimolar magnesium, suggesting that the putative channel has an external regulatory binding site for divalent cations (González-Martínez, 2003; Torres-Flores et al., 2008a). Intracellular sodium loading would stimulate the electrogenic activity generated by Na+/K+-ATPase, while replenishment of external calcium restores the channel to its low-conductance state in which the plasma membrane potential is mainly determined by the electrogenic pump, causing an ouabain-sensitive hyperpolarization (González-Martínez, 2003; Torres-Flores et al., 2008a). In this context, we note that the Catsper channels present in the sperm plasma membrane contribute to resting calcium concentration, and allow substantial sodium influx in the absence of external calcium (Ren et al., 2001; González-Martínez, 2003; Jin et al., 2007; Qi et al., 2007; Torres-Flores et al., 2008a).
In this work we show that the reduction in sperm motility in calcium-deficient medium is not directly related to a decrease in [Ca2+]i, but is related to the resulting sodium influx. We also report that, mibefradil, a calcium channel blocker, antagonizes the sodium-dependent depolarization and sodium influx and also blocks the inhibitory effect of calcium removal on sperm motility. Hypotheses are discussed around the effect of sodium loading on the inhibition of sperm motility.
Methods
Materials and media
Diisopropylthiodicarbo-cyanine iodide [diSC3(5)], fura 2-AM and sodium-binding benzofuran isophthalate (SBFI-AM) were obtained from Molecular Probes. The other reagents were obtained from SIGMA or Merck. HEPES-buffered human sperm medium (+Na+) (Suarez et al., 1986) had the following composition (in mM): NaCl 117.5, KCl 8.6, CaCl2 2.5, NaH2PO4 0.3, MgCl2 0.49, Na-Pyruvate 0.3, Na-lactate 19, glucose 2, and 25 HEPES-Na (pH 7.6). In some experiments, 117.5 mM NaCl was substituted with 117.5 mM cholineCl (low Na+); this medium still contained ∼32 mM Na (Na-HEPES, Na-lactate, Na-pyruvate and NaH2PO4). For sperm motility studies, +Na+ was supplemented with 3 mg/ml BSA and 25 mM Na-bicarbonate and this medium was also modified by substituting NaCl by cholineCl. The effect of zero-calcium medium was studied by removing calcium with EGTA. To do this, 3.5 mM EGTA (stock 0.5 M prepared in ∼2 M NaOH) was added to +Na+ medium. In this condition, the pH of the medium barely changes upon EGTA addition, and the external calcium decreases from 2.5 mM to 68 nM, according to the calcium concentration calculator program Maxchelator (V.2.1) written by Chris Patton from the Hopkins Marine Station (Stanford University).
Sperm purification and dye loading
Human semen was obtained from a panel of 12 healthy donors. The donors participated in this study after reading and signing a letter of informed consent, approved by the Ethics Committee of the Facultad de Medicine of UNAM, in agreement with the procedures used for human tissues expressed in the declaration of Helsinki. Sperm purification was performed using percoll gradients (Linares-Hernández et al., 1988) and purified sperm (0.6–1.5 × 108 cells) were resuspended in 2 ml +Na+ at 36°C and loaded with either 2 μM fura 2-AM for 40 min, 0.5 µM 2′,7′-bis-(carboxyethyl)-5(6′)-carboxyflorescein acetoxymethyl ester-AM for 20 min or 20 μM SBFI-AM + 0.6% pluronic acid for 90 min. Once washed, the cells were incubated in 25 ml +Na+ medium at 36°C and immediately used either for simultaneous detection of [Ca2+]i and membrane potential or [Na+]i.
Measurement of [Ca2+]i, [Na+]i and membrane potential
Membrane potential and [Ca2+]i were simultaneously detected with the fluorescent probes diSC3(5) and fura 2, respectively, with a PTI (Photon Technology International) fluorometer equipped with two photomultipliers as reported in Linares-Hernández et al. (1988). Briefly, fura 2-loaded sperm pellets (100 μl with ∼1 × 107 cells) were added to the fluorescence cuvette containing either 2.5 ml +Na+ or low Na+ + 500 nM diSC3(5), previously equilibrated at 36°C and under constant magnetic stirring. The fura 2 signal was detected with a 488 nm filter (Andover), exciting at 340/380 and calibrated at the end of the trace, after membrane potential calibration (see below), using a Kd = 260 nM as described (Linares-Hernández et al., 1988). [Na+]i was measured in a similar fashion using SBFI as described (Torres-Flores et al., 2008a). The diSC3(5) fluorescence was detected at 670 nm, exciting at 600 nm with an halide lamp, placed in front of the xenon source. Both excitation and emission wavelengths for diSC3(5) measurements were achieved with optical interference filters (Andover). Data was acquired and digitalized at 0.83 Hz with the PTI interface system. The diSC3(5) fluorescence was calibrated at the end of the experiment (before fura 2 calibration), using the K-ionophore valinomycin, that brings the plasma membrane potential to values close to Nernst potential for potassium distribution across the plasma membrane (Ek). After valinomicyn (Vm = −71 mV), three subsequent additions of KCl corresponding to −43, −30 and −15 mV, resulted in concomitant increases of diSC3(5) fluorescence. The fractional change of fluorescence with respect to the fluorescence value at −71 mV (Fo) as a function of Ek results in a linear relationship. The slope (m) and intercept (b) of this calibration curve were used to convert actual fluorescence data (F) to membrane potential (Vm), according to the following equation (González-Martínez, 2003):
Given that mitochondrial potential does not appreciably contribute to diSC3(5) signal in human sperm (Guzmán-Grenfell et al., 2000), simultaneous measurements of intracellular calcium and membrane potential were performed in the absence of mitochondrial inhibitors or uncouplers
Measurement of cAMP
cAMP was measured with the Cyclic AMP Competitive EIA kits from Cayman Chemical (Ann Harbor, MI) as described Torres-Flores et al. (2008b). Briefly, sperm pellets (∼1 × 107 cells) obtained from different experimental conditions were treated with 1 ml 0.05 M HCl. The sample was boiled for 3 min, cooled on ice and centrifuged for 10 min. The supernatant was used for cAMP determination. The sample and the appropriate cAMP standards were alkalinized and acetylated with acetic anhydride and used for the competition assay according to the manufacturer's instructions.
Measurement of ATP content
ATP content was measured in sperm extracts following the luciferin-luciferase reaction with the kit for ATP determination (Sigma). Sperm (∼1 × 107) assayed under different experimental conditions were pelleted and resuspended in 0.25 ml 0.05 M HCl, boiled for 3 min and the supernatant was used for determination of ATP content. Similarly, the ATP standard was diluted in 0.05 M HCl and boiled with the sperm extracts. The samples and standards were alkalinized to pH 7.8 with appropriate addition of Trizma base, and the luciferin-luciferase reaction was performed according to the manufacturer's instructions. Light emission was detected with a photomultiplier (PTI model 814).
Motility assays
Sperm motility (number of motile cells) as a function of time was characterized immediately after sperm purification. Analysis was based on the examination of 20 consecutive digitalized images obtained from a single field using an ×20 negative-phase contrast objective. The image capture speed was therefore one every 40 ms and images were taken throughout 1 s. For each sample, 2000–3000 sperm were analyzed with the Hamilton–Thorne HTM-IVOS-12 computer-assisted sperm motility analyzer (CASA). Twenty frames were acquired at a frame rate of 60 Hz, at a constant temperature of 37°C in +Na+ (+Na+ supplemented with 3 mg/ml BSA and 25 mM NaHCO3). The presence of BSA and bicarbonate in +Na+ medium, which also supports sperm capacitation when incubated for a few hours, helped preserve sperm motility and also avoid sperm adhesion when cells were incubated for long periods of time within the CASA slide chamber (Microcell; Conception Technologies, San Diego, CA, USA). CASA recordings were also performed in sperm loaded with either fura 2 or SBFI and in media that prevented the loss of motility when the NA+ media was depleted of Ca2+ with EGTA. The % motility was chosen as a general index since all other parameters such as VAP [smoothed path velocity (m/s)], VCL [track velocity (m/s)], VSL [straight line velocity (m/s)], ALH [amplitude of lateral head displacement (m)] and BCF [beat cross frequency (Hz)] also decreased upon Ca2+ removal in +Na+ medium.
Statistical analysis
Numeric results are expressed as mean ± standard error (SE); n means number of individuals tested and analyzed with Student's t-tests. Two-tailed P-values < 0.05 were considered statistically significant.
Results
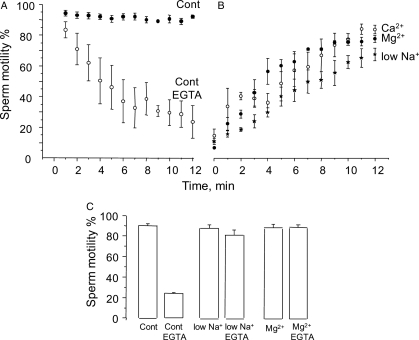
Figure 1A shows the effect of calcium removal from the medium on sperm motility. External free calcium was lowered from 2.5 mM to 68 nM by adding 3.5 mM EGTA. This procedure produced a reversible (see below) and gradual reduction in the percentage of motile sperm, which eventually brought almost the entire population to an immotile state. The exposure time to EGTA that brought 50% of the sperm population to the non-motile condition within the CASA chamber was t½ = 6.8 ± 1.8 min (n = 6, SE). The t½ values displayed a high degree of variability, ranging from 2.4 to 14 min. However, in control media containing calcium, sperm motility decreased on average only 2.5 ± 1.8% at t½, with a maximum and minimum range of 8% decrease and 3% increase.
Figure 1.
Effect of calcium removal from the medium on sperm motility. (A) Sperm isolated from percoll gradients were washed and incubated in +Na+ (20 × 106 cells/ml), the control medium (Cont, see Methods). Extracellular calcium was lowered from 2.5 mM to 68 nM by adding 3.5 mM EGTA, and sperm suspension was immediately transferred to a CASA plate kept at 37°C. Motility patterns were scored on the plate every minute until motility decreased below 10% (empty circles). The filled circles show the % of sperm motility on the plate of sperm incubated in +Na+ without EGTA, during the same time period. (B) Reversal of the calcium removal-induced sperm motility arrest by: restoring calcium to 2.5 mM (open circles; Ca2+), adding 3 mM MgCl2 (Mg2+, closed circles) or carrying out the experiment in low Na (low Na+) medium (stars). In the latter case, sperm in +Na+ (Cont) were treated with EGTA, microcentrifuged 10 s and then resuspended in low Na+. Motility was scored every minute until close to full recovery. (C) Addition of 3 mM MgCl2 or Ca2+ to +Na+, or suspending in low Na+, prevented the sperm motility decrease induced by external calcium removal with 3.5 mM EGTA. In 3.5 mM Mg2+ media, EGTA decreased free calcium to 133 nM. All media used for motility contained in addition 3 mg/ml BSA and 25 mM Na-bicarbonate, and the experiments were performed nine times [±standard error (SE)].
Since removal of external calcium causes a Na-dependent depolarization and sodium loading, we investigated whether the decrease in sperm motility was related to this phenomena. We tested two conditions that inhibit both Na-dependent depolarization and Na-loading; supplementing our control +Na+ media (Cont) with 3 mM MgCl2 (Mg2+) or by replacing NaCl from +Na+ with choline Cl (low Na+) (González-Martínez, 2003; Torres-Flores et al., 2008a) (see below text ). As shown in Fig. 1C, when sperm were incubated in low Na+, there was no effect on sperm motility, and under this condition calcium removal barely also decreased motility. Likewise, the presence of 3 mM MgCl2 in +Na+ did not affect normal sperm motility and it also prevented the inhibitory effect of calcium removal on sperm motility observed in normal medium. Figure 1B shows that either calcium restoration (Ca2+), the addition of 3 mM MgCl2 (Mg2+) or re-suspension in low Na+, fully restored motility in immotile sperm previously treated with EGTA. The times required to recover 50% of sperm motility were 4.4 ± 0.8, 3.9 ± 0.3 and 3.1 ± 0.3 min (n = 7 ± SE), respectively (see below text). As the time course of the decrease in sperm motility induced by calcium removal with EGTA is variable, in these experiments we waited until sperm motility decreased by <20% before implementing our indicated experimental test conditions (see Fig. 1B).
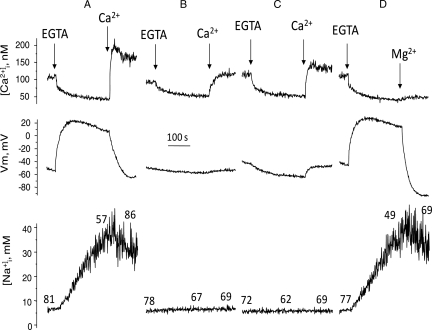
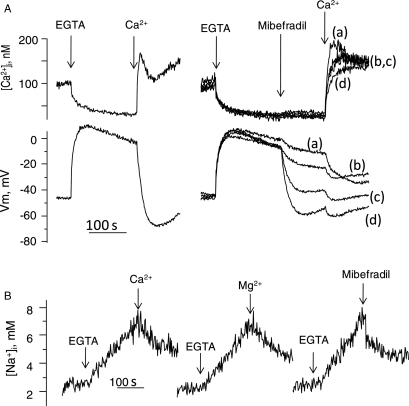
To better relate motility measurements with the ion transport mechanisms triggered by calcium removal, motility was determined in aliquots of spermatozoa taken from the simultaneous recordings of [Ca2+]i and Vm and from [Na+]i measurements. In human dye-loaded sperm incubated in +Na+ medium, EGTA addition induced Na-dependent depolarization, and a [Ca2+]i decrease and [Na+]i increase, as previously reported (González-Martínez, 2003; Torres-Flores et al., 2008a). As anticipated, after 4 min, the percentage of motile sperm fell from 95 to 45% (Fig. 1A). Motility recovered to 90% upon calcium re-addition. SBFI-loaded sperm revealed that motility recovers upon calcium re-addition even though [Na+]i was decreasing yet still from an elevated concentration level (Fig. 2A lower trace). In sperm incubated in low Na+ (Fig. 2B) or in Mg+ +Na+ (Fig. 2C), EGTA decreased [Ca2+]i but did not induce changes in membrane potential and intracellular sodium as previously observed (González-Martínez, 2003; Torres-Flores et al., 2008a), and the motility characteristics were preserved, as documented in the cells (Fig. 1C). As previously shown (González-Martínez, 2003), Mg2+ induced a ouabain-dependent hyperpolarization in EGTA treated sperm (Fig. 2D), halted the sodium influx and then decreased [Na+]i, without affecting the levels of [Ca2+]i (Torres-Flores et al., 2008a). Under this condition, the sperm motility inhibition induced by calcium removal was reversed, even in the presence of still elevated levels of intracellular sodium. Altogether, these results suggested that the decrease in sperm motility did not depend on [Ca2+]i but on: the Na-dependent depolarization, intracellular sodium loading or to the sum of both.
Figure 2.
Effect of calcium removal and subsequent calcium restoration on sperm motility obtained during simultaneous recordings of membrane potential and [Ca2+]i in fura 2-loaded sperm, or during [Na+]i detection in SBFI-loaded sperm incubated in different conditions. Dye-loaded sperm (∼15 × 106 cells) were added to the fluorescence cell containing 2.5 ml of the following media supplemented with 0.5 μM diSC3(5): (A) +Na+ our control medium, (B) low Na+, (C) Mg +Na+ and (D) +Na+; in this experiment, 3 mM MgCl2 was added after EGTA instead of calcium. The spectrofluorometer cell was previously thermostabilized at 36°C and under constant magnetic stirring. Aliquots (10 μl) were taken at three different timepoints during recordings: before EGTA addition, before calcium restoration or magnesium addition, and 2min after calcium restoration or magnesium addition. Sperm motility patterns were scored in CASA chambers at 36°C. Percentages of sperm motility are indicated on the traces.
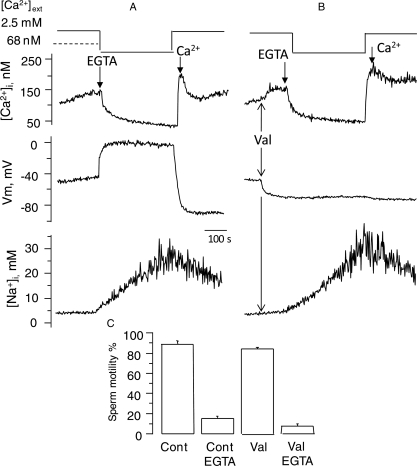
To discern the effects of plasma membrane potential and sodium loading on sperm motility, we induced sodium influx by calcium removal while clamping the membrane potential with the K+-ionophore valinomycin. Figure 3B shows that valinomycin prevented the depolarization induced by calcium removal in fura 2-loaded sperm, while barely affecting the decrease in [Ca2+]i, and the sodium influx, with levels close to the control (Fig. 3A). In experiments performed with unloaded cells, valinomycin slightly reduced sperm motility but calcium removal produced a marked decrease in sperm motility (Fig. 3C). These results suggested that intracellular sodium loading, but not membrane potential depolarization, is related to the decrease in sperm motility induced by external calcium removal.
Figure 3.
Clamping sperm membrane potential with valinomycin (Val) does not affect the sperm motility decrease or the sodium influx induced by external calcium removal in human sperm. (A) Representative control responses to calcium removal induced by 3.5 mM EGTA, and subsequent calcium restoration, while simultaneously detecting sperm [Ca2+]i and membrane potential, and separately [Na+]i. (B) Effect of valinomycin on these sperm responses. (C) Effect of calcium removal on sperm motility in sperm treated with valinomycin. Motility was determined in all samples at the time when it decreased to15% in sperm incubated in +Na+ + 3.5 mM EGTA. n = 6 individuals ± SE.
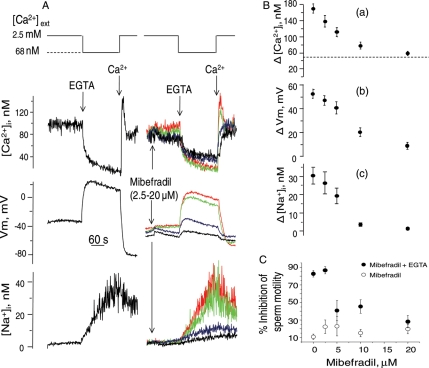
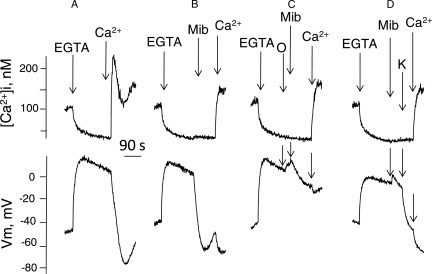
The effect of mibefradil, a calcium channel blocker, was then examined on the ion transport and motility patterns induced by external calcium removal in human sperm. As shown in Fig. 4A and B, the addition of this blocker inhibited the Na+-dependent depolarization (IC50= 7.1 ± 0.6 μM), decreased the calcium peak induced by calcium restoration (IC50 = 5.6 ± 1.2 μM) and reduced the [Na+]i increase induced by calcium removal (IC50= 5.7 ± 0.8 μM, n = 7 ± SE), for the three cases. It is worth emphasizing that Ca2+ restoration induces Ca2+ uptake through all possible paths. This is probably why mibefradil mainly inhibits the peak Ca2+ transient and not the return to the basal [Ca2+]i level [Fig. 4A top traces and B(a)]. Additionally, mibefradil, at > 10 μM, induced a slow and relatively minor Em hyperpolarization, a slight decrease in resting [Ca2+]i and a reduction in the calcium decrease induced by calcium removal. Mibefradil also produced a small decrease in the percentage of sperm motility and a concentration dependent protection of the sperm motility inhibition caused by calcium removal with EGTA (Fig. 4C). The addition of mibefradil after subjecting sperm to external calcium removal (Fig. 5A) induced a dose-dependent hyperpolarization (Fig. 5A central trace) and partially blocked the [Ca2+]i increase caused by calcium restoration. Similar to calcium restoration or Mg2+ addition, mibefradil arrested sodium influx followed by a decrease in [Na+]i triggered by external calcium removal (Fig. 5B). As expected, the mibefradil-induced hyperpolarization was inhibited by ouabain (Fig. 6C), and by incubating sperm in medium without external potassium, (Fig. 6D), but hyperpolarization ensued upon addition of 1 mM KCl. However, unlike Mg2+ and calcium, mibefradil was unable to reverse sperm motility arrest after calcium removal (not shown).
Figure 4.
Effect of mibefradil on: [Ca2+]i, [Na+]i, and membrane potential (Vm) changes and sperm motility induced by external calcium removal in human sperm. (A) Vm and [Ca2+]i were detected simultaneously in fura 2-loaded sperm; samples of the same donor were loaded with SBFI to detect [Na+]i. Mibefradil 2.5 μM (red traces), 5.0 μM (green traces), 10 μM (blue traces) and 20 μM (black traces) were added 2 min before EGTA. The leftmost traces show controls without mibefradil. (B) Dose response curves of the effect of mibefradil on either (a) the calcium influx induced by calcium restoration (the dotted line indicates the limit of a mibefradil sensitive fraction), (b) the extent of depolarization, measured as the EGTA-induced maximum depolarization minus resting membrane potential, and (c) the extent of sodium influx, measured as the [Na+]i increase 3 min after EGTA minus resting values. (C) Effect of mibefradil on sperm motility. In this series of experiments calcium removal was induced with 3.5 mM EGTA in +Na+ and the time required to decrease the percentage of motile sperm to ∼15% was determined. At this time (10–15 min), sperm motility was measured in sperm exposed to 2.5–20 μM mibefradil.
Figure 5.
Mibefradil hyperpolarizes sperm depolarized by calcium chelation and, similar to Ca2+ and Mg2+, it decreases [Na+]i. (A) The effect of differing concentrations of mibefradil on [Ca2+]i and membrane potential, detected simultaneously, in sperm depolarized by calcium chelation. Calcium removal was induced with 3.5 mM EGTA; after 3min mibefradil was added to the following final concentrations: (a) 2.5 μM, (b) 5.0 μM, (c) 10.0 μM and (d) 20 μM subsequently, calcium (Ca2+) was restored to 2.5 mM as indicated. The traces at the left are controls without mibefradil. (B) Addition of 2.5 mM (Ca2+), 3 mM MgCl2 or mibefradil (20 μM) decrease [Na+]i in SBFI-loaded sperm after Ca2+ chelation by EGTA.
Figure 6.
Induction of a Na+/K+ ATPase-dependent hyperpolarization by mibefradil in calcium removal-induced depolarized sperm. (A) Control trace, effect of EGTA and subsequent calcium restoration, (B) effect of EGTA and subsequent 20 μM mibefradil (Mib) addition; 1 min later external calcium (Ca2+) was restored, (C) ouabain (50 μM (O) was added 15 s before mibefradil, calcium was restored as indicated, (D) effect of EGTA and subsequent addition of mibefradil (20 μM) in +Na+ medium without potassium. To assure zero potassium, sperm were previously washed in +Na+ without potassium and the pellet added to the fluorescence cell containing 2.5 ml of this medium. One minute after mibefradil addition, 1 mM KCl was added and 1min later calcium was restored. These traces are representative of five experiments.
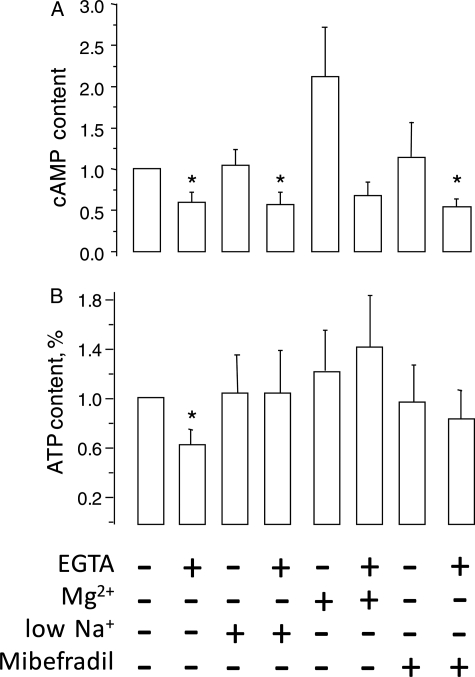
Since sperm motility is regulated by cAMP and depends on ATP, we measured these parameters in sperm subjected to calcium removal, and once their motility fell below 10%. Sperm cAMP content decreased in all media containing EGTA, even in low Na+ or in +Na+ Mg2+ which prevented the motility decrease induced by calcium removal. Therefore, it is unlikely that this second messenger is responsible for motility inhibition (Fig. 7A). In contrast, calcium removal generated a ∼40% decrease in the ATP content of sperm in normal +Na+, yet incubation in low Na+, +Na++Mg2+ or addition of mibefradil in +Na+ prevented this decrease in ATP (Fig. 7B).
Figure 7.
External calcium removal decreases human sperm cAMP and ATP content. Sperm were incubated either in +Na+, low Na+, +Na+ +Mg2+ or +Na+ + mibefradil (20 μM). Calcium was removed by adding 3.5 mM EGTA and the samples were incubated until their sperm motility fell to <10% of their value in +Na+. At this time the EGTA treated sperm in +Na++ Mg2+ displayed 92% motility. Sperm extracts were prepared (with 0.05 M HCl) for cAMP and ATP determinations after this time. (A) cAMP content, expressed as a fraction of control. The cAMP content was 0.98 ± 0.43 pmol/107 cells, n = 5 ± SE. (B) ATP content, expressed as a fraction of control. The ATP content was 292 ± 60 pmol/106 cells, n = 7 ± SE.
Discussion
Here we present evidence that calcium removal by chelation from the external medium progressively and indirectly decreases mammalian sperm motility (Feng et al., 1988; Aaberg et al., 1989; Jin et al., 2007) through sodium influx via calcium channels. In addition, the findings presented in this work indicate that these channels are blocked by mibefradil. Indeed, calcium removal did not induce motility arrest in conditions that inhibit the EGTA-induced sodium influx and the Na-dependent depolarization, that is, in low sodium medium (González-Martínez, 2003; Torres-Flores et al., 2008a), in normal medium supplemented with 3 mM MgCl2 (González-Martínez, 2003; Torres-Flores et al., 2008a), or in the presence of mibedradil (this work). This is true even when the experiments are prolonged for 12 min, the necessary time to observe ∼80% motility inhibition (not shown). Intracellular sodium loading, not the depolarization itself, was related to the motility arrest, as shown by the fact that valinomycin, which clamped membrane potential without affecting sodium influx, did not affect the decrease in motility induced by calcium removal. Therefore, human sperm undergo motility arrest when external calcium is removed not because [Ca2+]i decreases, but due to Na+ loading and its consequences. These findings are consistent with those made in demembranated cells (Feng et al., 1988), showing that initial motility does not require calcium and that the later arrest in sperm motility is due to an alternative membrane-associated process.
Interestingly, mibefradil, a T- and L-type calcium channel blocker that can also inhibit sodium (McNulty and Hanck, 2004) and potassium channels (Gomora et al., 1999), inhibited the calcium removal-induced Na+-dependent depolarization and sodium influx, and induced an ouabain-sensitive and external K+-dependent hyperpolarization. These effects are mechanistically identical to those produced by micromolar calcium or millimolar magnesium, and consequently are consistent with the interpretation that mibefradil blocks channels that, in the absence of external calcium allow sodium permeation in a high conductance mode and generate intracellular sodium loading. Thus, blocking these channels stops sodium influx, and the plasma membrane potential mainly depends on the electrogenic Na+/K+-ATPase, activated by the increase in [Na+]i that hyperpolarizes the cells. Our results also provide evidence that mibefradil blocks the same channels in the presence of external calcium, that is, in its presumptive calcium-conductance mode, since it blocked ∼70% of the [Ca2+]i increase induced by calcium addition in EGTA treated sperm, at IC50 values similar to those obtained with blockades of the Na+ influx and Na+-dependent depolarization. Likewise, mibefradil induced a gradual reduction in resting [Ca2+]i, suggesting that this channel contributes to regulate intracellular calcium levels by, allowing entry of this cation. In this regard, it is interesting to note that the calcium decrease induced by external calcium removal was progressively reduced as the mibefradil concentration increased, indicating that under appropriate conditions calcium can exit from the cell through this same Na+-conducting channel. The protective effect of mibefradil on the motility arrest induced by external calcium removal is also consistent with the protection afforded by calcium, magnesium and low sodium medium. However, unlike these later conditions, the mibefradil protection was partial and did not reverse the arrest of sperm motility caused by external calcium removal with EGTA. It is possible that this blocker has other effects which result in a decrease in sperm motility as it has been previously reported to decrease sperm motility by 10% in calcium containing medium (Treviño et al., 2004). In this regard, mibefradil has been shown to block, (i) T-type channels in spermatogenic cells (Arnoult, et al., 1998), (ii) calcium influx induced by external potassium (Blackmore and Eisoldt, 1999) and (iii) a potassium channel found in sperm flagella (Navarro et al., 2007).
Even though the sperm motility arrest induced by external calcium removal is related to intracellular sodium loading, the relationship is loose. Indeed, [Na+]i may reach values as high as 30–40 mM in approximately four minutes (Torres-Flores et al., 2008a), a condition that arrests motility in less than 50% of the sperm population in most samples. More prolonged incubations stabilize [Na+]i at values close to 40 mM (Torres-Flores et al., 2008a) but motility is further inhibited. It is evident that when sperm are incubated in the absence of external calcium, sodium entry elevates Na+/K+ ATPase activity, at the expense of energy consumption. This in turn would limit the supply of ATP to the dynein-ATPases required for sperm motility. In agreement with this proposal, external calcium removal results in a decrease in ATP levels, measured at the time when sperm motility is arrested below 10%. This ATP decrease is not observed when the media contains magnesium, mibefradil or is low in sodium, conditions which preserve sperm motility. However, it is interesting that a recovery in motility, induced either by calcium or magnesium, occurs when [Na+]i is still high (this work). It is worth noting that the hyperpolarization caused by these two divalent cations, calcium or magnesium, would decrease the Na+/K+ ATPase activity, thus increasing the availability of ATP required by the dynein-ATPases. Other processes, possibly involving glycolysis, may contribute to explain why this protection occurs while [Na+]i is elevated. In this respect, the alpha 4 ATPase isoform, found predominantly in the midpiece of the sperm flagellum, plays an important role in motility (Schuh et al., 2004). On the other hand, sAC knockout male mice are sterile and produce immotile sperm (Marquez and Suarez, 2008). In spite of this, the arrest of sperm motility observed here is not related to cAMP since its decrease upon external calcium removal occurred equally even under conditions that preserved sperm motility (with low sodium or in the presence of magnesium or mibefradil).
Our findings are consistent with those reported earlier (Carlson et al., 2009; Lishko et al., 2011) indicating that Catsper is the major transporter responsible for the sodium-dependent depolarization. This novel calcium channel present in mouse sperm flagella is required for hyperactivated motility (Ren et al., 2001; Jin et al., 2007); its absence results in infertility. Notably, sperm from Catsper(–,–) males retain their initial motility in the absence of external calcium, unlike those from wild-type mice (Jin et al., 2007). Furthermore, it is now known that mibefadril inhibits currents that are most likely due to Catsper in human sperm (Lishko et al., 2011; Strunker et al., 2011). According to the results presented here, external calcium removal would not depolarize nor increase [Na+]i in sperm from Catsper null mice, similar to that observed in human sperm incubated with mibefradil, or in media containing magnesium or low sodium.
Authors’ roles
V.T.-F. was responsible for experiments monitoring intracellular Ca2+, Na+ and membrane potential in human spermatozoa. G.P.-J. participated in the measurement of sperm motility. Y.H.-R. participated in the measurement of sperm motility and monitoring intracellular Ca2+. A.D. contributed with some ideas, carried out part of the writing and supported the project. M.T.G.-M. was the leader of this work.
Funding
This work was supported by grants IN213308-3 from PAPIIT-DGAPA/UNAM (Mexico) and 49517 from CONACYT (Mexico) and NIH R01 HD038082-07A1 (to A.D.).
Acknowledgements
We also acknowledge the Laboratorio Clínico y de Biogenética Eugenio Sue for their invaluable assistance in sperm motility recordings and for their generous donation of the Hamilton-Thorn CASA system, and Dr Christopher Wood for critical reading of the manuscript. V.T.-F. is a PhD student of the Postgraduate Program of Ciencias Biológicas, UNAM. During the course of this research the untimely and unfortunate death of M.G.-M. occurred.
References
- Aaberg RA, Sauer MV, Sikka S, Rajfer J. Effects of extracellular ionized calcium, diltiazem and cAMP on motility of human spermatozoa. J. Urol. 1989;141:1221–1224. doi: 10.1016/s0022-5347(17)41225-0. [DOI] [PubMed] [Google Scholar]
- Arnoult C, Villaz M, Florman HM. Pharmacological properties of the T-type Ca2+ current of mouse spermatogenic cell. Mol Pharmacol. 1998;53:1104–1111. [PubMed] [Google Scholar]
- Blackmore PF, Eisoldt S. The neoglycoprotein mannose–bovine serum albumin, but not progesterone, activates T-type calcium channels in human spermatozoa. Mol Hum Reprod. 1999;5:498–506. doi: 10.1093/molehr/5.6.498. [DOI] [PubMed] [Google Scholar]
- Carlson AE, Burnett LA, Del Camino D, Quill TA, Hille B, Chong JA, Moran MM, Babcock DF. Pharmacological targeting of native CatSper channels reveals a required role in maintenance of sperm hyperactivation. PLOS ONE. 2009;4:1–9. doi: 10.1371/journal.pone.0006844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darszon A, Treviño CL, Wood C, Galindo B, Rodríguez-Miranda E, Acevedo JJ, Hernandez-González EO, Beltrán C, Martínez-López P, Nishigaki T. Ion channels in sperm motility and capacitation. Soc Reprod Fertil Supp. 2007;65:229–244. [PubMed] [Google Scholar]
- Espinosa F, Darszon A. Mouse sperm membrane potential: changes induced by Ca2+ FEBS Lett. 1995;372:119–125. doi: 10.1016/0014-5793(95)00962-9. [DOI] [PubMed] [Google Scholar]
- Feng B, Bhattacharya A, Yanagimachi R. Ca2+ is essential for the motility of plasma membrane-intact, but not of demembranated, hamster spermatozoa. Andrologia. 1988;20:155–162. doi: 10.1111/j.1439-0272.1988.tb00680.x. [DOI] [PubMed] [Google Scholar]
- Gomora JC, Enyeart JA, Enyeart JJ. Mibefradil potently blocks ATP-activated K1 channels in adrenal cells. Mol Pharmacol. 1999;56:1192–1197. doi: 10.1124/mol.56.6.1192. [DOI] [PubMed] [Google Scholar]
- González-Martínez MT. Induction of a sodium-dependent depolarization by external calcium removal in human sperm. J Biol Chem. 2003;278:36304–36310. doi: 10.1074/jbc.M304479200. [DOI] [PubMed] [Google Scholar]
- Guzmán-Grenfell AM, Bonilla-Hernández MA, González-Martínez MT. Glucose induces a Na+,K+.-ATPase-dependent transient hyperpolarization in human sperm. I. Induction of changes in plasma membrane potential by the proton ionophore CCCP. Biochim Biophys Acta. 2000;1464:188–198. doi: 10.1016/s0005-2736(99)00247-3. [DOI] [PubMed] [Google Scholar]
- Jin J, Jin N, Zheng H, Ro S, Tafolla D, Sanders KM, Yan W. Catsper3 and Catsper4 Are Essential for Sperm Hyperactivated Motility and Male Fertility in the Mouse. Biol Reprod. 2007;77:37–44. doi: 10.1095/biolreprod.107.060186. [DOI] [PubMed] [Google Scholar]
- Linares-Hernández L, Guzmán-Grenfell AM, Hicks-Gómez JJ, González-Martínez MT. Voltage-dependent calcium influx in human sperm assessed by simultaneous optical detection of intracellular calcium and membrane potential. Biochim Biophys Acta. 1988;1372:1–12. doi: 10.1016/s0005-2736(98)00035-2. [DOI] [PubMed] [Google Scholar]
- Lishko PV, Kirichok Y. The role of Hv1 and CatSper channels in sperm activation. J Physiol. 2010;23:4667–4672. doi: 10.1113/jphysiol.2010.194142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lishko PV, Botchkina IL, Kirichok Y. Progesterona activates the principal Ca2+ channel of human sperm. Nature- Lett. 2011;471:387–391. doi: 10.1038/nature09767. [DOI] [PubMed] [Google Scholar]
- Marquez B, Suarez SS. Soluble adenylyl cyclase is required for activation of sperm but does not have a direct effect on hyperactivation. Reprod Fertil Dev. 2008;20:247–252. doi: 10.1071/rd07146. [DOI] [PubMed] [Google Scholar]
- McNulty MM, Hanck DA. State-dependent mibefradil block of Na+ channels. Mol Pharmacol. 2004;66:1652–1661. doi: 10.1124/mol.66.6.1652. [DOI] [PubMed] [Google Scholar]
- Navarro B, Kirichok Y, Clapham DE. KSper, a pH-sensitive K+ current that controls sperm membrane potential. Proc Natl Acad Sci USA. 2007;104:7688–7692. doi: 10.1073/pnas.0702018104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H, Moran MM, Navarro B, Chong JA, Krapivinsky G, Krapivinsky L, Kirichok Y, Ramsey IS, Quill TA, Clapham DE. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc Natl Acad Sci USA. 2007;104:1219–1223. doi: 10.1073/pnas.0610286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Navarro B, Perez G, Jackson AC, Hsu S, Shi Q, Tilly JL, Clapham DE. A sperm ion channel required for sperm motility and male fertility. Nature. 2001;413:603–609. doi: 10.1038/35098027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh K, Cartwright EJ, Jankevics E, Bundschu K, Liebermann J, Williams JC, Armesilla AL, Emerson M, Oceandy D, Knobeloch KP, et al. KSper, a pH-sensitive K+ current that controls sperm membrane potential. J Biol Chem. 2004;279:28220–28226. doi: 10.1074/jbc.M312599200. [DOI] [PubMed] [Google Scholar]
- Strunker T, Goodwin N, Breker C, Kashikar ND, Weyand I, Seifert R, Kaupp UB. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nat Lett. 2011;471:382–386. doi: 10.1038/nature09769. [DOI] [PubMed] [Google Scholar]
- Suarez SS, Ho HC. Hyperactivation of mammalian sperm. Cell Mol Biol. 2003;49:351–356. [PubMed] [Google Scholar]
- Suarez SS, Pacey AA. Sperm transport in the female reproductive tract. Hum Reprod Update. 2006;12:23–37. doi: 10.1093/humupd/dmi047. [DOI] [PubMed] [Google Scholar]
- Suarez SS, Wolf DP, Meizel S. Induction of the acrosome reaction in human spermatozoa by a fraction of human follicular fluid. Gamete Res. 1986;14:107–121. [Google Scholar]
- Torres-Flores V, García-Sánchez NL, González-Martínez MT. Intracellular sodium increase induced by external calcium removal in human sperm. J Androl. 2008a;29:63–69. doi: 10.2164/jandrol.107.003368. [DOI] [PubMed] [Google Scholar]
- Torres-Flores V, Hernández-Rueda YL, Neri-Vidaurri P, Jiménez-Trejo F, Calderón-Salinas V, Molina-Guarneros JA, González-Martínez MT. Activation of protein kinase A stimulates the progesterone-induced calcium influx in human sperm exposed to the phosphodiesterase inhibitor papaverine. J. Androl. 2008b;29:549–557. doi: 10.2164/jandrol.107.004614. [DOI] [PubMed] [Google Scholar]
- Treviño CL, Felix R, Castellano LE, Gutiérrez C, Rodríguez D, Pacheco J, López-González I, Gomora JC, Tsutsumi V, Hernández-Cruz A, et al. Expression and differential cell distribution of low-threshold Ca2+ channels in mammalian male germ cells and sperm. FEBS Lett. 2004;563:87–92. doi: 10.1016/S0014-5793(04)00257-1. [DOI] [PubMed] [Google Scholar]