Abstract
Ricin is known as a potent toxin against animals. It consists of two chains, Ricin Toxin A (RTA) and Ricin Toxin B (RTB). The toxic effect is known to be caused by RTA. Inhibitors for RTA with less efficiency have been reported. Hence, it is of interest to identify new inhibitors. Virtual screening methods (computer aided drug designing) to find similar molecules in drug database were used for screening new inhibitors against RTA. We used the structure of RTA in complex with Pteroic acid (PDB code: 1BR6) as target molecule. Ligand based virtual screening approach was used in which the known inhibitory molecule Pteroic acid (PTA) served as a template to identify similar ligands from the ZINC database. These ligands were docked inside the binding pocket of RTA by using the MVD (Molegro Virtual Docker). This approach successfully identified six novel compounds. These docked ligands interacted with Asn78, Ala79, Val81, Gly121 and Ser176 amino acids, which are key residues of the RTA active site. Three compounds in particular, ZINC05156321 (6, 7 diphenylpteridin-4-ol), ZINC05156324 (6, 7-bis (3-fluorophenyl) pteridin-4-ol) and ZINC08555900 (6, 7-bis (4-fluorophenyl)-1H-pteridin-4-one), showed higher binding affinity in comparison to PTA, with high interaction energy, better space fitting and electrostatic interactions. These molecules should be tested for in vitro and in vivo activities in future for consideration as effective inhibitors.
Keywords: Ricin Toxin A chain (RTA), Ricin Toxin B chain (RTB), Zinc Database, Virtual Screening, Molegro Virtual Docker, PTA (Pteroic Acid)
Background
Ricin is a powerful cytotoxic protein. The tertiary (30) structure of Ricin is a globular and glycosylated heterodimer, with an approximate molecular weight of 60-65 kDa (Figure 1) [1]. It consists of two chains namely Ricin Toxin A Chain (RTA) and Ricin Toxin B Chain (RTB). RTA and RTB are of approximately similar molecular weight i.e. 32 kDa and 34 kDa respectively. Ricin A Chain (Figure 1) is an N-glycoside hydrolase made up of 267 amino acids [2]. It has three structural domains in which approximately 50% of the polypeptides are arranged into α- helices and β-sheets [3]. The three domains form a prominent cavity that has formed an active site of RTA. Ricin B Chain (Figure 1a) is basically a lectin consisting of 262 amino acids which can bind to the terminal galactose residues on cell surfaces [4]. RTB forms a bilobal, barbell-like structure that lacks α-helices and β-sheets, although individual lobes consist of three subdomains. Ricin functions as a cytotoxin, in the process where RTA is reductively cleaved from RTB in order to remove a steric block, after the cleaving active site is exposed on RTA [5]. N-glycosidase activity of the ricin protein was studied [6]. In eukaryotic ribosomes RTA specifically and irreversibly cleaves the glycosidic bond of adenine at position 4324 within the 28S rRNA of the 60S subunit. However, the phosphodiester backbone of the RNA will remain intact [7]. RTA targets adenine at position 4324 (A4324) of the highly conserved sequence, which is about 12 nucleotides long and universally found in eukaryotic ribosomes. This highly conserved sequence, 5'-AGUACGAGAGGA-3', is known as the SRL (Sarcin-Ricin Loop) and is important for binding elongation factors during protein synthesis [8]. The depurination event caused by RTA rapidly and completely inactivates the ribosome, which results in toxicity, in turn leads to inhibition of protein synthesis. Approximately 1500 ribosomes are depurinated by a single RTA molecule in the cytosol within a minute [9]. There are several invariable amino acid residues in the active site of RTA that are involved in the depurination reaction of ribosomal RNA [10]. The exact mechanism of the depurination event is unknown but the key amino acid residues that are identified to be involved in depurination reaction includes Arginine (ARG) at position 180, Glutamic acid (GLU) at position 177 and Tyrosine (TYR) at positions 80 and 123. In particular, ARG180 and GLU177 are involved in the catalytic mechanism, but not in substrate binding [11]. This has been proved with enzyme kinetic studies involving RTA mutants [5]. PTA and its derivatives had been extensively studied, found proper binding with RTA and with good biological activity [12]. Hence, we have selected PTA as a seed molecule to perform ligand based virtual screening. Ligand based virtual screening method will give us number of molecules similar to the seed molecule [13]. Ligand-based techniques derive knowledge from given active molecule/molecules that are known to bind to the desired target molecule, to use this knowledge search the candidate molecules in large databases with similar properties [14]. This can be done by various methods, including similarity and substructure searching [15], 3D shape matching [16] or pharmacophore matching [17]. In this study, we have selected similarity and substructure based searching approach for screening the molecules with similar properties.
Figure 1.
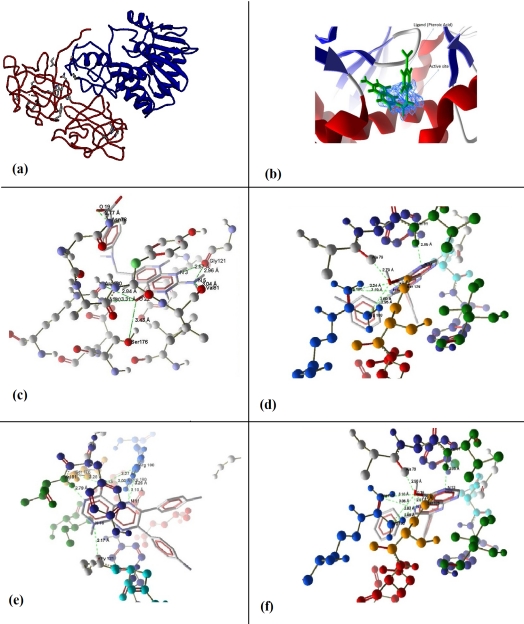
(a) Crystallographic structure of Ricin (Ricin-A chain is shown in blue color and Ricin-B chain is shown in red color), (b) Active site (Blue color) of Ricin Toxin A chain (RTA) predicted by Pocket-Finder, (c) Hydrogen bond interactions of Pteroic acid within the binding pocket of RTA, (d) Hydrogen bond interactions of ZINC05156324 (6, 7-bis (3-fluorophenyl) pteridin-4-ol) within the binding pocket of RTA, (e) Hydrogen bond interactions of ZINC08555900 (6, 7-bis (4-fluorophenyl)- 1H-pteridin-4-one) within the binding pocket of RTA, (f) Hydrogen bond interactions of ZINC05156321 (6, 7 diphenylpteridin-4-ol) within the binding pocket of RTA.
Methodology
X-ray crystallographic structure of target:
The 3-Dimensional crystal structure of Ricin Toxin-A Chain (RTA) in complex with Pteroic Acid (PTA) inhibitor (PDB code: 1BR6) was selected from the Protein DataBank (PDB) [18], as the receptor/target model in virtual screening process.
Ligand ZINC database (Version - Eight):
ZINC database [19] has been used screening. ZINC contains over 13 million compounds in 3-Dimensional structural format and ready-to-dock formats available for docking [20].
Pocket-Finder
Pocket-Finder [21] was used for active site (pocket) detection on RTA protein.
Molegro Virtual Docker (Version- 3.2):
In this work Molegro Virtual Docker (MVD) [22] has been used for the prediction of protein-ligand interactions study [23].
Results
Prediction of active site on RTA
Active site of RTA was predicted using the Pocket-Finder program (Figure 1b). Active site consists of following amino acid residues ASN78, TYR 80, VAL81, PHE93, GLY121, ASN122, TYR123, ARG134, ILE172, GLU177, ARG180, GLU208, ASN209 and TRP211 [10]. Results from earlier investigators had represented that active site of Ricin Toxin-A chain (RTA) has been complexed with ligand PTA (Figure 1b).
Virtual screening on ZINC database
Before screening ZINC database, the docking protocol was validated. PTA ligand was re-docked within the binding pocket of RTA (PDB ID: 1BR6) to obtain the docking based affinity information. The ZINC database was searched on the basis of structure of PTA in result of which 34 similar compounds were found. All these compounds were docked with Molegro Virtual Docker. Six high ranked compounds were then selected on the basis of their interaction energies, hydrogen bonding and internal energies etc., as described in Table 1 (see Table 1). Three molecules were then selected for structural docking with RTA protein, whose binding energies were more than pteroic acid binding energy.
Depiction of binding mode of Pteroic Acid (PTA) within the active site of RTA:
Interactions of Pteroic Acid (PTA) in the binding pocket of RTA are shown in Figure 1c. Pteroic Acid showed seven hydrogen bond interactions within the active site of RTA. ARG180 formed two hydrogen bonds with O22 of PTA (bond lengths are 2.57 Å and 2.96 Å), SER176 also forms a hydrogen bond with O22 of PTA (bond length is 3.43 Å), O19 of PTA formed a hydrogen bond with ASN78 (bond length is 2.77 Å), VAL81 formed a hydrogen bond with N5 of PTA (bond length is 3.04 Å) and GLY121 formed two hydrogen bonds with N3 and N5 of PTA (bond lengths are 2.57 Å and 2.96 Å respectively).
Binding studies of top three selected compounds after virtual screening:
Binding of 6, 7- Bis (3-fluorophenyl) pteridin-4-ol (ZINC05156324) and RTA is shown in Figure 1d. ZINC05156324 also formed seven hydrogen bonds within the active site of RTA. It formed two hydrogen bonds at O17 from ARG180 (bond lengths are 3.24 Å and 2.80 Å) and two hydrogen bonds at N12 with ARG180 (bond lengths are 3.19 Å and 2.95 Å) whereas, O17 formed one hydrogen bond with ALA79 (bond length is 2.70 Å), one hydrogen bond is formed by N14 with VAL81 (bond length is 2.85 Å). Further SER176 and O17 can act as both hydrogen bond donor and acceptor for each other (bond length is 3.08 Å).
Interaction between 6, 7-bis (4-fluorophenyl) - 1H- pteridin -4- one (ZINC08555900) and RTA is shown in Figure 1e. Seven hydrogen bond interactions are found between ZINC08555900 and RTA. It formed three hydrogen bonds with O13, one from SER176 and two from ARG180 (bond lengths are 3.28 Å, 3.27 Å and 3.00 Å respectively). It formed two hydrogen bonds at N11 from ARG180 (bond lengths are 3.10 Å and 3.26 Å) and formed one hydrogen bond at N14 from VAL81 and one at N16 from GLY121 (bond lengths are 2.79 Å and 3.17 Å respectively). Figure 1f illustrates the interaction between 6, 7 diphenyl pteridine − 4 - ol (ZINC05156321) and the active site of RTA. ZINC05156321 shows seven hydrogen bond interactions within the active site of RTA. ZINC05156321 formed a hydrogen bond at Ala 79 from O16 (bond length is 2.56 Å) and two hydrogen bonds with N11 from Arg180 (bond lengths are 3.05 Å and 2.89 Å). It has also formed two hydrogen bonds with O16 from Arg180 (bond lengths are 3.10 Å and 2.93 Å). Further one hydrogen bond is formed by N13 from Val81 (bond length is 2.85 Å), Ser176 and O16 act as both hydrogen bond donor and acceptor for each other (bond length is 3.29 Å).
Discussion
Novel RTA inhibitors help in the treatment of Racinin based toxicity effectively (Figure 1a& Figure 1b). Pteroic acid (PTA) has been proved to be one of such inhibitors. Interestingly, its docked structural information is also available. It is known that the Pterin ring of PTA inhibitor binds in the active site of RTA and forms hydrogen bonds with the active site residues [12]. Hence, virtual screenings were performed on RTA active site and 34 similar molecules have been found. Out of the 34 molecules, six molecules were selected based on binding energies, dock scores, interaction energies, HBond energy, VdW and internal energy of poses, number of hydrogen bonds etc., as shown in Table 1 Table 1. The six ligands were docked deep inside the binding pocket of RTA. Three molecules were then selected (Figure 1c, Figure 1d, Figure 1e & Figure 1f) based on their resemblance of similar orientation, as observed with Pteroic acid ligand [12] and having more binding energies than PTA. PTA formed seven hydrogen bonds with all surrounding residues of ASN78, VAL81, GLY121, SER176 and ARG180 with interaction energy of 138.4 Kcal/mole. It was spatially fit in to the active site with good electrostatic interaction. Whereas, best ranked ZINC05156324 (6, 7-bis (3- fluorophenyl) pteridin-4-ol) has been found to be potential inhibitor upon all the molecules by interacting with surrounding amino acids like ALA 79, VAL 81, SER 176 and ARG 180 by forming hydrogen bonds. It has formed seven hydrogen bonds with high interaction energy of 154.625, also with better electrostatic interaction and also spatially fit. Rest of the two molecules showed high interaction energies when compared to PTA with residues like ALA 79, VAL 81, GLY 121, SER 176 and ARG 180, both of the molecules showed seven hydrogen bonds approximately with 144.00 interaction energies. Hence, the above said three molecules can be considered as potential RTA inhibitors. The above said molecules may function as effective inhibiters because they are exactly binding with active site of RTA, which has a role in binding with ribosome and for RNA depurination function [12]. They may be tested on in vitro and in vivo conditions. As per earlier reports Miller et al., (2002) designed RTA inhibitory molecules based on structure of the RTA active site and also experimentally selected highly soluble molecules as Ricin inhibitors [24]. Robertus et al., (2008) has developed molecules based on RTA (Ricin Toxin A chain) active site structure based virtual screening. Whereas in our work ligand structure (PTA) based similarity [25] search approach (virtual screening) has been implemented on ZINC database.
Conclusion
Virtual screening methods are widely used for reducing the cost and time of drug discovery process. We show the identification of six new compounds as potent inhibitor of RTA using virtual screening. Nonetheless, these molecules should be tested for in vitro and in vivo activities in future for consideration as effective inhibitors.
Supplementary material
Acknowledgments
The authors are thankful to Dr. M.D. Tiwari, Director, IIIT Allahabad, for providing us infrastructure and continuous encouragement.
Footnotes
Citation:Mishra & Prasad, Bioinformation 7(2): 46-51 (2011)
References
- 1.PJ Aplin, T Eliseo. Med J Aust. 1997;167:260. doi: 10.5694/j.1326-5377.1997.tb125050.x. [DOI] [PubMed] [Google Scholar]
- 2.S Olnes, A Pihl. Biochemistry. 1973;12:3121. doi: 10.1021/bi00740a028. [DOI] [PubMed] [Google Scholar]
- 3.SA Weston, et al. J Mol Biol. 1994;244:410. doi: 10.1006/jmbi.1994.1739. [DOI] [PubMed] [Google Scholar]
- 4.R Wales, et al. J Biol Chem. 1991;266:19172. [PubMed] [Google Scholar]
- 5.MJ Lord, et al. Toxicol Rev. 2003;22:53. doi: 10.2165/00139709-200322010-00006. [DOI] [PubMed] [Google Scholar]
- 6.Y Endo, K Tsurugi. J Biol Chem. 1987;262:8128. [PubMed] [Google Scholar]
- 7.Y Endo, K Tsurugi. J Biol Chem. 1998;263:8735. [Google Scholar]
- 8.S Sperti, et al. Biochem J. 1973;136:813. doi: 10.1042/bj1360813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MB Sturm, VL Schramm. Anal Chem. 2009;81:2847. doi: 10.1021/ac8026433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.YS Kim, JD Robertus. Protein Eng. 1992;5:775. doi: 10.1093/protein/5.8.775. [DOI] [PubMed] [Google Scholar]
- 11.A Frankel, et al. Mol Cell Biol. 1990;10:6257. doi: 10.1128/mcb.10.12.6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.X Yan, et al. J Mol Biol. 1997;266:1043. doi: 10.1006/jmbi.1996.0865. [DOI] [PubMed] [Google Scholar]
- 13.WP Walters, et al. Drug Discov Today. 1998;3:160. [Google Scholar]
- 14.PD Lyne. Drug Discov Today. 2002;7:1047. doi: 10.1016/s1359-6446(02)02483-2. [DOI] [PubMed] [Google Scholar]
- 15.J Mestres, RMA Knegtel. Perspect Drug Des Discovery. 2000;20:191. [Google Scholar]
- 16.J Srinivasan, et al. J Med Chem. 2002;45:2494. doi: 10.1021/jm010494q. [DOI] [PubMed] [Google Scholar]
- 17.JS Mason, et al. Curr Pharm Des. 2001;7:567. [Google Scholar]
- 18. http://www.nature.com/nbt/journal/v25/n8/full/nbt0807-845.html.
- 19. http://zinc.docking.org.
- 20.JJ Irwin, BK Shoichet. J Chem Inf Model. 2005;45:177. doi: 10.1021/ci049714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. http://www.modelling.leeds.ac.uk/pocketfinder.
- 22. http://www.molegro.com/mvd-product.php.
- 23.R Thomsen, MH Christensen. J Med Chem. 2006;49:3315. doi: 10.1021/jm051197e. [DOI] [PubMed] [Google Scholar]
- 24.DJ Miller, et al. J Med Chem. 2002;45:90. [Google Scholar]
- 25. http://www.stormingmedia.us/37/3762/A376284.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.