Abstract
Anaemia and RBC (red blood cell) transfusion may be associated with worse clinical outcomes, especially with longer blood storage duration prior to transfusion. The mechanisms underlying these harmful effects are unknown. RBCs have been proposed to buffer plasma S1P (sphingosine 1-phosphate), a lysophospholipid essential for the maintenance of endothelial integrity and important in the regulation of haematopoietic cell trafficking. The present study examined the effect of anaemia, RBC transfusion and RBC storage duration on plasma S1P levels. Plasma S1P from 30 individuals demonstrated a linear correlation with Hct (haematocrit; R2=0.51, P<0.001) with no evidence for a plateau at Hct values as low as 19%. RBC transfusion in 23 anaemic patients with baseline mean Hct of 22.2±0.34% (value is the mean±S.D.) increased Hct to 28.3±0.6% at 72 h. Despite an Hct increase, RBC transfusion failed to elevate plasma S1P consistently. A trend towards an inverse correlation was observed between RBC storage duration and the post-transfusion increase in plasma S1P. After 30 days of storage, RBC S1P decreased to 19% of that observed in fresh (3–7-day-old) RBC segments. RBC membranes contain low levels of both S1P phosphatase and S1P lyase activities that may account for the decline in S1P levels with storage. Our results support a role for RBCs in buffering plasma S1P and identify a disturbance in the capacity after transfusion. Changes in S1P content may contribute to an RBC storage lesion. Further studies should investigate the clinical significance of alterations in circulating S1P levels and the potential value of enriching stored RBCs with S1P.
Keywords: anaemia, blood storage, lysophospholipid, sphingosine 1-phosphate, transfusion
Abbreviations: Apo, apolipoprotein; ESI, electrospray ionization; Hct, haematocrit; HDL, high-density lipoprotein; LPC, lysophosphatidylcholine; MRM, multiple reaction monitoring; MS/MS, tandem MS; RBC, red blood cell; S1P, sphingosine 1-phosphate; DH-S1P, dihydro-S1P
INTRODUCTION
Anaemia is associated with increased morbidity and mortality in a variety of clinical settings, including critically ill patients [1], those with acute coronary syndromes and surgical patients [2]. Traditionally, RBC (red blood cell) transfusions have been used to treat anaemia. Approx. 13.9 million transfusions are given to 4.8 million patients annually. However, growing evidence suggests that some of the risks associated with anaemia may actually arise from RBC transfusion. Studies have suggested that RBC transfusion may be associated with heightened systemic inflammation [3] and worse outcomes in certain settings [4–6]. For example, RBC transfusion correlates with an increased risk of adverse clinical outcomes in patients with myocardial ischaemia, who traditionally were viewed as a group that would benefit from restoration of RBC mass to improve oxygenation. In the CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the ACC/AHA Guidelines) registry, the ~10% of patients who received RBC transfusion were more likely to die (11.5% compared with 3.8% in-hospital mortality), suffer myocardial infarction or death (13.4% compared with 5.8%) and had longer lengths of hospital stay compared with their non-transfused counterparts [7]. This increased risk persisted after adjustment for potentially confounding clinical variables (estimated adjusted hazard ratio, 3.94) [7]. Likewise, an analysis of three randomized trials of patients with acute coronary syndromes revealed a higher 30 day mortality among those receiving transfusion [8]. The increased risk was especially evident among transfused patients with Hct (haematocrit) >25%.
Long duration of storage (age) of RBCs has also been associated with worse outcomes after transfusion [9,10]. For example, the transfusion of RBCs stored for more than 2 weeks has been associated with an increase in complications in patients undergoing cardiac surgery [10]. Those given older blood had higher in-hospital mortality, a need for intubation beyond 72 h, sepsis and worse 1 year outcomes as compared with patients who received newer blood. These and other observations have spawned the ‘RBC storage lesion’ hypothesis, which proposes that unfavourable cellular changes occur during the storage of RBCs [11]. However, the molecular alterations that might account for dysfunction associated with RBC storage are not known. Depletion of nitric oxide and biochemical changes in lipid content may occur in stored RBCs, with potential prothrombotic or immunomodulatory effects [12].
Modification of RBC phospholipid composition has been observed during storage. LPCs (lysophosphatidylcholines) accumulate in aged RBCs and have been proposed as a possible mediator of lung injury after transfusion [13]. RBCs also accumulate, store and release another lysophospholipid, S1P (sphingosine 1-phosphate) [14], which has been implicated as a critical regulator of endothelial barrier function [15] and lymphocyte egress from lymph tissue. The buffering capacity of RBCs for S1P may be a key determinant of plasma levels of this important bioactive mediator. Indeed, anaemia has been associated with lower levels of plasma S1P in humans [16]. Anaemia and blood transfusion may therefore alter plasma S1P homoeostasis, which in turn could affect vascular permeability and lymphocyte trafficking.
In the present study, we have examined the effect of anaemia, blood transfusion and RBC storage duration on S1P levels. Our purpose was to evaluate the role of RBCs in maintaining S1P homoeostasis, to determine how RBC transfusion affects S1P levels and to ascertain whether the relationship between RBC transfusion and S1P levels is itself influenced by RBC storage duration.
MATERIALS AND METHODS
Subjects
Normal donors and patients provided written consent, and blood was collected according to protocols approved by the Institutional Review Boards at the University of Kentucky. Normal donors were recruited from University staff and laboratory personnel. A total of 36 anaemic patients who had not received a blood transfusion in the last 30 days were recruited from the Chandler Medical Center; of these patients, 13 did not receive transfusions and 23 were scheduled to be transfused as deemed necessary by their treating physicians. Exclusion criteria included recent trauma, scheduled surgery, haemodynamic instability or pregnancy. Demographic characteristics, pertinent medical history and laboratory values (metabolic panel, lipid profile, and baseline and post-transfusion Hct) were abstracted from the medical record and are presented in Table 1. The number of blood units transfused and the duration of storage of each unit were recorded. For measurement of plasma levels of S1P, blood was collected into tubes containing sodium citrate (0.32% final concentration) before transfusion and at times up to 72 h post-transfusion. Plasma was rapidly separated by centrifugation, snap-frozen and stored at −80°C for further analysis.
Table 1. Demographic and clinical characteristics of anaemic patients.
Values are means±S.D. or percentages.
| Characteristic | Value |
|---|---|
| Age (years) | 62±16 |
| Female sex (%) | 65.2 |
| Patients with | |
| Diabetes (%) | 17.3 |
| Hypertension (%) | 39.1 |
| Body mass index (kg/m2) | 27.1±8.5 |
Quantification of S1P, LPC, sphingosine and hexadecenal by HPLC–ESI (electrospray ionization)–MS/MS (tandem MS)
Lipids were extracted from plasma, RBCs and in vitro enzyme assays using acidified organic solvents, as we have described previously [17]. Analysis of S1P, sphingosine and hexadecenal (as its semicarbazone derivative) were accomplished using a Shimadzu UFLC (ultra-fast liquid chromatography) coupled with an ABI 4000-Qtrap hybrid linear ion trap triple quadrupole mass spectrometer in MRM (multiple reaction monitoring) mode using C17-S1P, C17-sphingosine and d5-hexadecenal semicarbazone used as internal standards. S1P, DH-S1P (dihydro-S1P) and C17-S1P were separated using an Agilent Zorbax Eclipse XDB C8 column, 5 μm, 4.6×150 mm2. The mobile phase consisted of 3:1 methanol/water with formic acid (0.5%) and 5 mM ammonium formate (0.1%) as solvent A and 99:1 methanol/water with formic acid (0.5%) and 5 mM ammonium formate (0.1%) as solvent B. For the analysis of S1P and DH-S1P, separation was achieved using a gradient of 0% B for 1 min, 0–100% B in the next 1 min, maintained at 100% B for the next 10 min and equilibrated to the initial conditions in 3 min. The flow rate was 0.5 ml/min with a column temperature of 30°C. The sample injection volume was 10 μl. The mass spectrometer was operated in the positive ESI mode with optimal ion source settings determined using synthetic standards of S1P, DH-S1P and C17-S1P with a declustering potential of 61 V, entrance potential of 10 V, collision energy of 23 V, collision cell exit potential of 16 V, curtain gas of 20 lbf/in2 (1 lbf/in2=6.9 kPa), ion spray voltage of 5500 V, ion source gas1/gas2 of 40 lbf/in2 and temperature of 550°C. MRM transitions monitored were: 366.141/250 for C17-S1P, 382.133/284.2 for DH-S1P and 380.124/264.1 for S1P.
Sphingosine was analysed using a Waters XTerra C8, 3.5 μm, 3.0×100 mm2 column. The mobile phase consisted of 61:39:0.5 methanol/5 mM ammonium formate/formic acid as solvent A and 90:10:0.5:0.5 acetonitrile/chloroform/5 mM ammonium formate/formic acid as solvent B. Analysis of sphingosine was conducted by maintaining 0% B for the first 3 min, gradually increasing it from 0% B to 100% B in the next 5 min and maintaining it at 100% B for the last 2 min. Column equilibration time was 3 min. The flow rate was 0.5 ml/min with a column temperature of 30°C. The sample injection volume was 10 μl. The mass spectrometer was operated in the positive ESI mode with optimal ion source settings determined by a C17-sphingosine synthetic standard with a declustering potential of 76 V, entrance potential of 10 V, collision energy of 29 V, collision cell exit potential of 16 V, curtain gas of 30 lbf/in2, ion spray voltage of 5500 V, ion source gas1/gas2 of 40 lbf/in2 and temperature of 550°C.
Hexadecenal semicarbazone was analysed using a nucleodur C18 gravity, 5 μM, 125/2 column. The mobile phase consisted of 75:25 methanol/water with formic acid (0.5%) and 5 mM ammonium formate (0.1%) as solvent A and 80% [99:1 of methanol/water with formic acid (0.5%) and 5 mM ammonium formate (0.1%)]+20% chloroform as solvent B. Analysis of hexadecenal semicarbazone was conducted by maintaining 0% B for 1 min and gradually increasing it from 0% B to 100% B over the next 7 min and maintaining it at 100% B for the last 2 min. Column equilibration time was 3 min. The flow rate was 0.5 ml/min with a column temperature of 30°C. The sample injection volume was 10 μl. The mass spectrometer was operated in the positive ESI mode with optimal ion source settings determined by derivatizing synthetic standard of hexadecenal with semicarbazide hydrochloride, with the following settings: declustering potential of 101 V, entrance potential of 10 V, collision energy of 21 V, collision cell exit potential of 14 V, curtain gas of 20 lbf/in2, ion spray voltage of 5500 V, ion source gas1/gas2 of 40 lbf/in2 and temperature of 550°C. The MRM transitions monitored were as follows: 296.3/279.2, 296.3/253.2 and 296.3/97.3 for hexadecenal semicarbazone; and 301.3/258.2, 301.3/284.3 and 301.3/97 for d5-hexadecenal semicarbazone.
LPCs were quantified using methods that have been described previously [18].
Size-exclusion fractionation of plasma
Plasma was subjected to size-exclusion chromatography using a Superdex 75 HR 10/30 (1×30 cm2) column (Pharmacia). In brief, 100 μl of plasma was chromatographed at a flow rate of 0.5 ml/min using PBS as the mobile phase. A total of 20 fractions were collected, and the S1P content in each fraction was measured by HPLC–ESI–MS/MS as described above. Lipoprotein-containing fractions were identified by immunoblot analysis.
Measurement of S1P in stored blood
Storage segments containing samples of the RBC unit in integral tubing filled at the time the unit was prepared were obtained from 35 different 3–7-day-old RBC units from the Kentucky Blood Center on two separate occasions. Lipids were extracted from 50 μl of RBCs obtained from these segments. The segments were then stored at 4°C under blood bank conditions for an additional 35 days to produce samples that have ages of 38–43 days, at which time another sample was obtained for lipid extraction and analysed as described above.
Determination of S1P phosphatase and lyase activities
RBC membranes were prepared by hypo-osmotic lysis and repeated washing of freshly isolated RBCs. S1P lyase and phosphatase activities were determined using Triton X-100-solubilized substrates and assay conditions determined previously to be optimal for these enzymes [19]. S1P phosphatase activity was determined by monitoring the production of sphingosine using HPLC—ESI–MS/MS as described above with C17-sphingosine added as an internal standard to monitor recovery, and quantification of sphingosine was accomplished with reference to a calibration curve generated using an authentic standard. S1P lyase activity was determined by measuring the formation of hexadecenal detected as its semicarbazone derivative as described previously [20].
Statistical analysis
Results are expressed as means±S.D. Means were compared using paired Student's t tests (pre- compared with post-) or unpaired Student's t tests (one group compared with another). Associations between two quantitative variables were assessed by linear regression. To examine the relationship between duration of RBC storage and changes in S1P levels post-transfusion, data were analysed by weighted least-squares linear regression analysis with the weights inversely proportional to estimates of error variance. When fitting a regression model with S1P change as the dependent variable and days storage as the independent variable, one value appeared as a potential ‘outlier’ (change in S1P concentration ~−400 at ~2 days), with an externally studentized residual of −3.24, and was the only observation whose externally studentized residual exceeded 2.50 in absolute value. Therefore Spearman correlation was determined with the observation included and excluded. Statistical significance was determined by a P value less than 0.05 using SigmaSTAT version 3.5 (Systat Software).
RESULTS
Anaemia is associated with selective depletion of HDL (high-density lipoprotein)-associated S1P
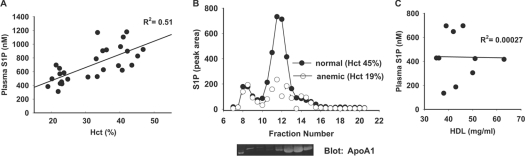
Previous studies have established that RBCs store and transport S1P and may be important in maintaining circulating S1P levels [14,21]. In keeping with these observations, plasma S1P is reportedly to be lower in anaemic individuals [22]. Our measurements of plasma S1P in 23 anaemic patients and four healthy donors confirm and extend this recent report [22]. Plasma S1P had a strong linear association with Hct (R2=0.51, P<0.001), with no suggestion of a plateau near the lowest Hct value of 19% (Figure 1A). S1P in plasma associates predominately with lipoprotein particles, particularly HDLs, and also with plasma proteins, most prominently albumin [21]. To determine whether anaemia alters the distribution of S1P between these pools, plasma from normal donors (n=3) and anaemic individuals (n=3) with HDL levels 40–50 mg/dl was fractionated by size-exclusion chromatography. Figure 1(B) displays the representative distribution using the values from one individual. In normal individuals, 77.4±0.6% of total plasma S1P was found in the HDL-rich fractions, identified by their Apo (apolipoprotein) A1 content. In plasma from anaemic patients, only 41.6±9% of total S1P was found in the HDL-rich fractions (P=0.002), suggesting that anaemia selectively depletes the HDL-bound pool of S1P. In keeping with these observations, S1P levels did not correlate with HDL levels in anaemic individuals with Hct <36% (Figure 1C; R2=0.00027, P=0.97). In this patient population, no significant correlation existed between Hct and HDL.
Figure 1. Effect of anaemia on plasma S1P levels and distribution.
(A) Total plasma S1P, measured as described in the Materials and methods section, from anaemic patients (n=23) and healthy volunteers (n=4) plotted against Hct. R2=0.51 (P<0.001) by linear regression analysis. (B) Representative S1P distribution in fractions of plasma separated by size-exclusion chromatography from normal and anaemic subjects. The peak of S1P correlated with the elution of HDL, as detected by immunoblotting the fractions for ApoA1. (C) Lack of correlation between plasma S1P and HDL in subjects (n=10) with anaemia (range, 21–36%). R2=0.00027 (P=0.97) by linear regression analysis.
Transfusion of RBCs variably restores S1P in anaemic patients
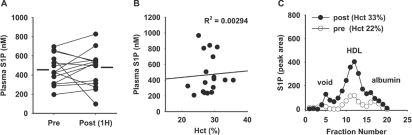
In mice engineered to lack S1P by conditional genetic inactivation of the two sphingosine kinase enzymes responsible for S1P production (‘S1P-less’ mice), RBC transfusion replenishes circulating S1P [23]. Therefore we sought to determine whether RBC transfusions given to improve Hct in humans increased circulating S1P levels in 23 patients with an average Hct of 22.2±1.1% prior to transfusion. On average, 1.8 units of leucoreduced RBCs were transfused per patient. At 1 h after transfusion, the average Hct increased by ~30% to 28.5±2.4% and remained stable up to 72 h, at which time the average Hct was 28.3±2.9%. Despite the elevation in average Hct, average plasma S1P increased by <5% at 1 h post-transfusion (Figure 2A). Unlike in pre-transfusion samples, plasma S1P at 12–24 h after transfusion did not correlate with Hct (Figure 2B), indicating that replacement of RBC alone may not restore plasma S1P levels in anaemic individuals. Indeed, there was substantial interindividual variability in the response to transfusion. At 12–24 h after transfusion, plasma S1P declined in four patients, despite increases in Hct. In 13 individuals, plasma S1P post-transfusion changed less than 200 nM from pre-transfusion; however, S1P increased by more than 200 nM in six patients. Plasma from individuals whose S1P concentrations increased after transfusion was fractionated by size exclusion. The increase in plasma S1P paralleled a restoration in HDL-associated S1P (Figure 2C).
Figure 2. Effect of blood transfusion on plasma S1P levels and distribution.
(A) Plasma S1P levels in individuals pre- and 1 h post-transfusion. The mean levels (458±161 nM and 465±180 nM; P=0.80, as determined using a paired Student's t test) are indicated by horizontal bars. (B) Plasma S1P as a function of Hct at 12–24 h after transfusion. Data were analysed by linear regression. (C) S1P distribution in fractions of plasma separated by size-exclusion chromatography pre- and post-transfusion from an anaemic patient who displayed an increase in total plasma S1P after transfusion.
RBC S1P declines with storage
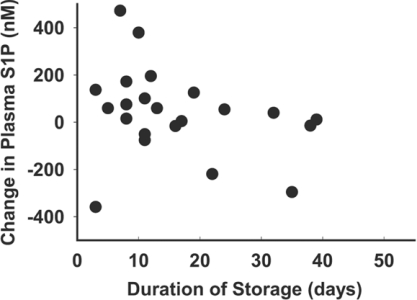
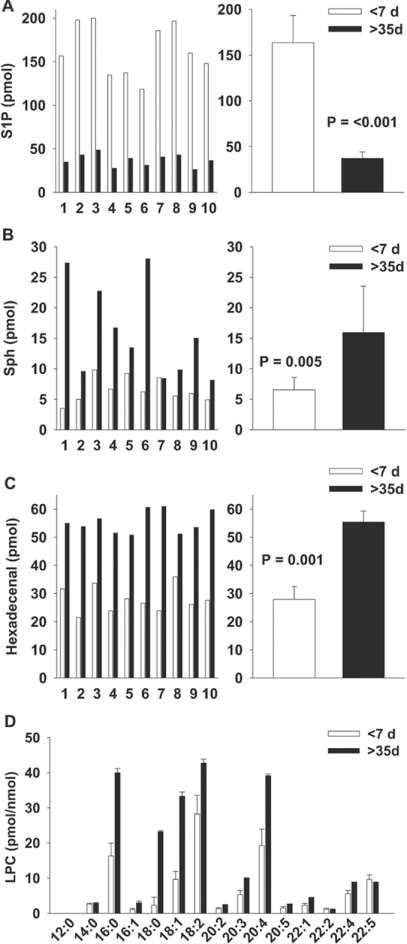
To understand why RBC transfusion did not consistently restore plasma S1P, we examined the relationship between the age of the RBC unit transfused and the post-transfusion change in plasma S1P in 22 patients (Figure 3). When analysed by weighted least-squares linear regression analysis, there was a negative, but not statistically significant, correlation (P=0.058) between the age of the RBC unit and the post-transfusion change in plasma S1P. However, one observation appeared as a potential ‘outlier’. The Spearman correlation between S1P change and days storage was −0.547 (P=0.01) with the observation excluded and −0.354 (P=0.1) with the observation included. To explore further the relationship between RBC age and S1P delivery, we measured the S1P content in RBC storage segments from 3–7-day-old RBC transfusion units (Figure 4A). In 35 segments, there was considerable variability between S1P across different segments from these ‘young’ RBC units, with up to a 3-fold difference in S1P levels. After storage of the 35 segments for an additional 35 days, the S1P content consistently and markedly declined by 81±9%. We next sought to determine the fate of S1P during storage. S1P can be degraded by lyase and phosphatase activities. Dephosphorylation of S1P by lipid phosphate phosphatases yields sphingosine, which accumulates in RBC segments with storage (Figure 4B). S1P lyase cleaves S1P at the C2–C3 bond to generate ethanolamine phosphate and hexadecenal. With storage, RBC segments also accumulate hexadecenal (Figure 4C). In the same samples, aged-associated increases occurred in LPC, specifically those of chain lengths 16:0, 18:0, 18:1 and 20:4 (Figure 4D), as reported previously by other investigators [13].
Figure 3. Effect of RBC storage duration on post-transfusion plasma S1P levels.
Change in S1P levels from baseline to 12–24 h post-transfusion compared with the age of the RBC unit transfused. For patients receiving >1 unit, the average storage duration of transfused units was plotted. R2=0.085 (P=0.06) by linear regression analysis with weighted least squares.
Figure 4. Effect of RBC storage duration on levels of S1P, its degradation products and LPC.
(A) Effect of RBC storage duration on S1P levels in 50 μl obtained from 10 RBC unit ‘segments’ (numbered 1–10) stored for the indicated times. Results are given as means±S.D. P<0.001, as determined using a paired Student's t test. Results are representative of results obtained in different experiments from 35 segments. (B) Accumulation of sphingosine (Sph) in RBC unit ‘segments’ (numbered 1–10). Results are given as means±S.D. (n=10). P=0.005, as determined using a Student's t test. (C) Accumulation of hexadecenal in RBC unit ‘segments’ stored for the indicated times. Results are given as means±S.D. (n=10). P=0.001, as determined using a paired Student's t test. S1P, Sph and hexadecenal levels are reported as the amount measured in 50 μl. (D) LPC content as means±S.D. (n=23) in pmol/nmol of lipid phosphate for individual LPC species denoted by chain length.
Degradation of S1P by RBC membraneassociated lyase and phosphatase activities
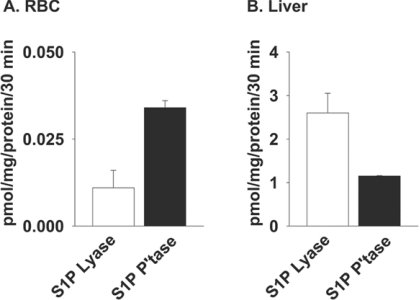
The results obtained with RBC segments suggest the presence of S1P lyase and phosphatase activities in stored RBC. However, RBC S1P-degrading activities have been reported to be undetectable in intact RBCs or membrane preparations based on measurements made using radiochemical- or fluorescence-based assays [24]. We used more sensitive MS-based assays to monitor metabolism of exogenously provided S1P by RBCs. RBC S1P lyase activity was evident when the formation of hexadecenal, detected as its semicarbazone derivative, was measured under assay conditions established previously to be optimal for the enzyme (Figure 5A). Likewise, the presence of S1P phosphatase activity was detected by the appearance of C17-sphingosine after incubation of RBC membranes with C17-S1P (Figure 5A). S1P lyase and phosphatase activities in RBC membranes were much lower than reported in other mammalian tissues and cell lines, and were only 0.4 and 3% respectively of those in a mouse liver homogenate assayed in parallel as a positive control (Figure 5B). Such low levels may explain previous reports that S1P lyase and phosphatase activities were not detectable in RBCs when less sensitive assays were employed [24]. The low levels of activity may be sufficient to degrade RBC S1P during prolonged storage and account for elevations in both sphingosine and hexadecenal. RBC release S1P [22], and it is possible that released S1P is degraded by RBC-surface-associated lipid phosphate phosphatases.
Figure 5. Determination of S1P phosphatase and lyase activity in liver homogenates and RBC membranes.
Membrane fractions were prepared from washed RBCs (A) or liver tissue (B). S1P phosphatase (P'tase) and S1P lyase activities were quantified by measuring the rates of formation of sphingosine and hexadecenal (detected as its semicarbazone) as described in the Materials and methods section.
DISCUSSION
In the vasculature, S1P plays an essential role in maintaining endothelial barrier function and regulating immune cell function [15,21,23]. HDL is a major carrier of plasma S1P, and evidence suggests that some of the pleotropic beneficial effects of HDL in the cardiovascular system may involve HDL-mediated delivery of S1P to vascular cells. Thus the biological activity of S1P may be regulated by both its total circulating level and its distribution between RBCs and plasma protein compartments. Understanding the mechanism(s) that regulate plasma S1P levels and distribution of plasma S1P between protein-bound pools may therefore be important for evaluating the functional consequences of alterations in circulating S1P levels. RBCs buffer plasma S1P by sequestering and releasing this bioactive lipid. Previous work indicates that anaemia in humans is associated with a reduction in plasma S1P levels without a change in RBC-associated S1P, suggesting that total RBC S1P ‘capacity’ influences plasma S1P levels in humans, although the same may not be true in mice [14,22,25]. Both HDL and albumin elicit the release of S1P from RBCs [22]. These observations, coupled with the fact that isolated RBCs can take up S1P from media, have led to a proposed model of circulating S1P homoeostasis, in which RBCs buffer this lipid and the binding capacity of plasma components, such as HDL and albumin, regulate the release of the lipid from RBCs. The present study identifies an effect of anaemia on S1P distribution between plasma protein pools and extends these observations by describing the effects of RBC transfusion on S1P homoeostasis. The findings may have broad implications for understanding the mechanisms responsible for the deleterious effects of RBC transfusions in anaemic patients. Our results suggest that one potential source for disruption of S1P homoeostasis in transfusion recipients could be the use of RBC units with altered S1P content, particularly aged RBCs in which S1P levels have become depleted.
Our findings confirm a correlation between RBC indices and plasma S1P concentration and indicate that the relationship remains linear at the lowest tested Hct (19%). Anaemia also predicts a change in plasma distribution of S1P, with less of the total pool of S1P associating with HDL in plasma from anaemic individuals. A recent study establishes that ApoM content of HDL correlates with human HDL-S1P levels [26]. Mice with absent or heightened ApoM expression have corresponding decreases and increases in plasma S1P respectively. Importantly, genetic deficiency of ApoM in mice, which reduces plasma S1P levels by approx. 50%, disrupts endothelial barrier function and promotes permeability [26]. Thus apoM appears essential for the S1P-protective effects of HDL. The IC50 for ApoM binding to S1P is approx. 0.9 μM [26], which is close to the plasma concentration of S1P. Our results suggest equilibrium between ApoM and RBC-pools of S1P that may reflect the affinity of ApoM for S1P. Loss of ApoM-associated S1P could diminish endothelial-protective effects exerted by HDL and could contribute to a systemic inflammatory response and endothelial dysfunction described in anaemia [12], which has been cited as a cause of increased morbidity and mortality that cannot solely be explained by a decrease in circulating RBC mass.
Despite lower S1P levels in plasma from anaemic individuals, RBC transfusion did not consistently increase plasma S1P, despite consistent improvements in Hct. One possible explanation for the lack of an increase in S1P post-transfusion is a decline in RBC S1P content during storage due to S1P-degrading activities in RBCs. In support of this possibility, a trend towards an inverse correlation between the age of the transfused unit and a change in plasma S1P was observed. Unfortunately, in the present study, we did not measure S1P content in the transfused RBC units, an important value necessary to correlate post-transfusion changes in plasma S1P with RBC S1P content. Given the consistent and striking decline in RBC S1P content during storage, it is reasonable to assume that the older transfusion units contained less S1P. However, the RBC S1P content of the younger units (3–7 days) is variable and may also contribute to inconsistent increases in post-transfusion S1P.
In summary, previous studies have identified a correlation between Hct and circulating S1P levels that is probably due to the capacity of RBCs to buffer, that is take up and release S1P. The present work indicates that RBC transfusion disrupts normal S1P homoeostasis. It may be possible that a disturbance in S1P levels or distribution between plasma-protein-associated pools after RBC transfusion may alter its physiological effects. Two firmly established effects of circulating S1P are to maintain endothelial barrier function and to regulate immune cell trafficking. ‘S1P-less’ mice develop lung vascular leakage and display impairment of the S1P blood–lymph gradient that mediates egress of lymphocytes from lymphoid tissue [15,23]. Thus attenuation of S1P signalling may promote lung permeability and pulmonary oedema, impair immune cell function and affect infection rates, all of which are adverse clinical events that occur after transfusion. Indeed, the recognition of the risks associated with transfusion has led to the widespread adoption of strict indications for transfusion. RBC storage may contribute to the risk associated with transfusion and therefore understanding the changes in the properties of stored RBCs becomes important for modifying practices to improve the quality of transfused blood and clinical outcomes. The present work supports the concept that changes in the lysopholipid content of RBCs during storage could have biological and clinical ramifications. One logical extension of our findings is to investigate ways of enriching stored blood with S1P as a means to investigate the effect that restoration of circulating S1P has on clinical outcomes.
AUTHOR CONTRIBUTION
Samy Selim performed the experiments, interpreted the data and wrote/revised the paper. Manjula Sunkura, Abdelghaffar Salous and Steve Leung performed the experiments, interpreted the data and revised the paper. Evgeny Berdyshev developed the methods and revised the paper. Alison Bailey designed the study, interpreted the data and revised the paper. Charles Campbell designed the study, interpreted the data and revised the paper. Richard Charnigo performed the statistical analysis and revised the paper. Andrew Morris designed the study, developed the methods, interpreted the data and wrote/revised the paper. Susan Smyth designed the study, interpreted the data and wrote/revised the paper.
ACKNOWLEDGEMENTS
This material is the result of work supported with the resources and/or use of the facilities at the Lexington VA Medical Center.
FUNDING
This work was supported by the National Institutes of Health [grant numbers HL078663, GM050388, P20RR021954, T32HL091812], and fellowships from the Society for Cardiac Angiography and Interventions and the American Heart Association.
References
- 1.Caro J. J., Salas M., Ward A., Goss G. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer. 2001;91:2214–2221. [PubMed] [Google Scholar]
- 2.Carson J. L., Duff A., Poses R. M., Berlin J. A., Spence R. K., Trout R., Noveck H., Strom B. L. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;348:1055–1060. doi: 10.1016/S0140-6736(96)04330-9. [DOI] [PubMed] [Google Scholar]
- 3.Campbell C. L., Steinhubl S. R., Hooper W. C., Jozic J., Smyth S. S., Bernstein D., De Staercke C., Syros G., Negus B. H., Stuckey T., et al. Bleeding events are associated with an increase in markers of inflammation in acute coronary syndromes: an ACUITY trial substudy. J. Thromb. Thrombolysis. 2011;31:139–145. doi: 10.1007/s11239-010-0513-1. [DOI] [PubMed] [Google Scholar]
- 4.Charles A., Shaikh A. A., Walters M., Huehl S., Pomerantz R. Blood transfusion is an independent predictor of mortality after blunt trauma. Am. Surg. 2007;73:1–5. doi: 10.1177/000313480707300101. [DOI] [PubMed] [Google Scholar]
- 5.Corwin H. L., Gettinger A., Pearl R. G., Fink M. P., Levy M. M., Abraham E., MacIntyre N. R., Shabot M. M., Duh M. S., Shapiro M. J. The CRIT study: anemia and blood transfusion in the critically ill – current clinical practice in the United States. Crit. Care Med. 2004;32:39–52. doi: 10.1097/01.CCM.0000104112.34142.79. [DOI] [PubMed] [Google Scholar]
- 6.Doyle B. J., Rihal C. S., Gastineau D. A., Holmes D. R., Jr Bleeding, blood transfusion, and increased mortality after percutaneous coronary intervention: implications for contemporary practice. J. Am. Coll. Cardiol. 2009;53:2019–2027. doi: 10.1016/j.jacc.2008.12.073. [DOI] [PubMed] [Google Scholar]
- 7.Yang X., Alexander K. P., Chen A. Y., Roe M. T., Brindis R. G., Rao S. V., Gibler W. B., Ohman E. M., Peterson E. D. The implications of blood transfusions for patients with non-ST-segment elevation acute coronary syndromes: results from the CRUSADE National Quality Improvement Initiative. J. Am. Coll. Cardiol. 2005;46:1490–1495. doi: 10.1016/j.jacc.2005.06.072. [DOI] [PubMed] [Google Scholar]
- 8.Rao S. V., Jollis J. G., Harrington R. A., Granger C. B., Newby L. K., Armstrong P. W., Moliterno D. J., Lindblad L., Pieper K., Topol E. J., et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA, J. Am. Med. Assoc. 2004;292:1555–1562. doi: 10.1001/jama.292.13.1555. [DOI] [PubMed] [Google Scholar]
- 9.Purdy F. R., Tweeddale M. G., Merrick P. M. Association of mortality with age of blood transfused in septic ICU patients. Can. J. Anaesth. 1997;44:1256–1261. doi: 10.1007/BF03012772. [DOI] [PubMed] [Google Scholar]
- 10.Koch C. G., Li L., Sessler D. I., Figueroa P., Hoeltge G. A., Mihaljevic T., Blackstone E. H. Duration of red-cell storage and complications after cardiac surgery. N. Engl. J. Med. 2008;358:1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 11.Rao S. V., Califf R. M. Is old blood bad blood? Am. Heart J. 2010;159:710–712. doi: 10.1016/j.ahj.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 12.Twomley K. M., Rao S. V., Becker R. C. Proinflammatory, immunomodulating, and prothrombotic properties of anemia and red blood cell transfusions. J. Thromb. Thrombolysis. 2006;21:167–174. doi: 10.1007/s11239-006-5206-4. [DOI] [PubMed] [Google Scholar]
- 13.Vlaar A. P., Hofstra J. J., Kulik W., van Lenthe H., Nieuwland R., Schultz M. J., Levi M. M., Roelofs J. J., Tool A. T., de Korte D., Juffermans N. P. Supernatant of stored platelets causes lung inflammation and coagulopathy in a novel in vivo transfusion model. Blood. 2010;116:1360–1368. doi: 10.1182/blood-2009-10-248732. [DOI] [PubMed] [Google Scholar]
- 14.Hanel P., Andreani P., Graler M. H. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 2007;21:1202–1209. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- 15.Camerer E., Regard J. B., Cornelissen I., Srinivasan Y., Duong D. N., Palmer D., Pham T. H., Wong J. S., Pappu R., Coughlin S. R. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammationinduced vascular leak in mice. J. Clin. Invest. 2009;119:1871–1879. doi: 10.1172/JCI38575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohkawa R., Nakamura K., Okubo S., Hosogaya S., Ozaki Y., Tozuka M., Osima N., Yokota H., Ikeda H., Yatomi Y. Plasma sphingosine-1-phosphate measurement in healthy subjects: close correlation with red blood cell parameters. Ann. Clin. Biochem. 2008;45:356–363. doi: 10.1258/acb.2007.007189. [DOI] [PubMed] [Google Scholar]
- 17.Mathews T. P., Kennedy A. J., Kharel Y., Kennedy P. C., Nicoara O., Sunkara M., Morris A. J., Wamhoff B. R., Lynch K. R., Macdonald T. L. Discovery, biological evaluation, and structure–activity relationship of amidine based sphingosine kinase inhibitors. J. Med. Chem. 2010;53:2766–2778. doi: 10.1021/jm901860h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su W., Yeku O., Olepu S., Genna A., Park J. S., Ren H., Du G., Gelb M. H., Morris A. J., Frohman M. A. 5-Fluoro-2-indolyl des-chlorohalopemide (FIPI), a phospholipase D pharmacological inhibitor that alters cell spreading and inhibits chemotaxis. Mol. Pharmacol. 2009;75:437–446. doi: 10.1124/mol.108.053298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berdyshev E. V., Goya J., Gorshkova I., Prestwich G. D., Byun H. S., Bittman R., Natarajan V. Characterization of sphingosine-1-phosphate lyase activity by electrospray ionization-liquid chromatography/tandem mass spectrometry quantitation of (2E)-hexadecenal. Anal. Biochem. 2011;408:12–18. doi: 10.1016/j.ab.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berdyshev E. V., Goya J., Gorshkova I., Prestwich G. D., Byun H. S., Bittman R., Natarajan V. Characterization of sphingosine-1-phosphate lyase activity by electrospray ionization-liquid chromatography/tandem mass spectrometry quantitation of (2E)-hexadecenal. Anal. Biochem. 2011;408:12–18. doi: 10.1016/j.ab.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yatomi Y. Plasma sphingosine 1-phosphate metabolism and analysis. Biochim. Biophys. Acta. 2008;1780:606–611. doi: 10.1016/j.bbagen.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Bode C., Sensken S. C., Peest U., Beutel G., Thol F., Levkau B., Li Z., Bittman R., Huang T., Tolle M., et al. Erythrocytes serve as a reservoir for cellular and extracellular sphingosine 1-phosphate. J. Cell. Biochem. 2010;109:1232–1243. doi: 10.1002/jcb.22507. [DOI] [PubMed] [Google Scholar]
- 23.Pappu R., Schwab S. R., Cornelissen I., Pereira J. P., Regard J. B., Xu Y., Camerer E., Zheng Y. W., Huang Y., Cyster J. G., Coughlin S. R. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 24.Ito K., Anada Y., Tani M., Ikeda M., Sano T., Kihara A., Igarashi Y. Lack of sphingosine 1-phosphatedegrading enzymes in erythrocytes. Biochem. Biophys. Res. Commun. 2007;357:212–217. doi: 10.1016/j.bbrc.2007.03.123. [DOI] [PubMed] [Google Scholar]
- 25.Venkataraman K., Lee Y. M., Michaud J., Thangada S., Ai Y., Bonkovsky H. L., Parikh N. S., Habrukowich C., Hla T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ. Res. 2008;102:669–676. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christoffersen C., Obinata H., Kumaraswamy S. B., Galvani S., Ahnstrom J., Sevvana M., Egerer-Sieber C., Muller Y. A., Hla T., Nielsen L. B., Dahlback B. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc. Natl. Acad. Sci. U.S.A. 2011;108:9613–9618. doi: 10.1073/pnas.1103187108. [DOI] [PMC free article] [PubMed] [Google Scholar]