Abstract
Objectives:
To quantify the association between systemic levels of the chemokine regulated on activation normal T-cell expressed and secreted (RANTES/CCL5), interferon-γ-inducible protein-10 (IP-10/CXCL10), monocyte chemoattractant protein-1 (MCP-1/CCL2), and eotaxin-1 (CCL11) with future coronary heart disease (CHD) and ischemic stroke events and to assess their usefulness for CHD and ischemic stroke risk prediction in the PRIME Study.
Methods:
After 10 years of follow-up of 9,771 men, 2 nested case-control studies were built including 621 first CHD events and 1,242 matched controls and 95 first ischemic stroke events and 190 matched controls. Standardized hazard ratios (HRs) for each log-transformed chemokine were estimated by conditional logistic regression.
Results:
None of the 4 chemokines were independent predictors of CHD, either with respect to stable angina or to acute coronary syndrome. Conversely, RANTES (HR = 1.70; 95% confidence interval [CI] 1.05–2.74), IP-10 (HR = 1.53; 95% CI 1.06–2.20), and eotaxin-1 (HR = 1.59; 95% CI 1.02–2.46), but not MCP-1 (HR = 0.99; 95% CI 0.68–1.46), were associated with ischemic stroke independently of traditional cardiovascular risk factors, hs-CRP, and fibrinogen. When the first 3 chemokines were included in the same multivariate model, RANTES and IP-10 remained predictive of ischemic stroke. Their addition to a traditional risk factor model predicting ischemic stroke substantially improved the C-statistic from 0.6756 to 0.7425 (p = 0.004).
Conclusions:
In asymptomatic men, higher systemic levels of RANTES and IP-10 are independent predictors of ischemic stroke but not of CHD events. RANTES and IP-10 may improve the accuracy of ischemic stroke risk prediction over traditional risk factors.
Chemokines participate in the inflammatory process of atherosclerosis1–4 by attracting T-cells and macrophages into atherosclerotic lesions, and in the mobilization of inflammatory and progenitor cells from the bone marrow to the circulating blood.5–7 Experimental studies have found raised levels of chemokines in human atherosclerotic lesions.8,9 Clinical studies have shown that circulating chemokines and especially monocyte chemoattractant protein-1 (MCP-1/CCL2) were independent predictors of recurrent coronary heart disease (CHD) and cardiovascular death.10–13 However, whether plasma levels of chemokines are independent predictors of incident cardiovascular disease in asymptomatic subjects remains unclear.14–17 Previous studies have mostly investigated a single chemokine, although several chemokines are involved in the progression of atherosclerosis. Moreover, to our knowledge, none of these previous studies have examined the association between systemic chemokines and the risk of ischemic stroke.
We therefore examined the association between systemic levels of the chemokines regulated on activation normal T-cell expressed and secreted (RANTES/CCL5), interferon-γ-inducible protein-10 (IP-10/CXCL10), MCP-1 (CCL2), and eotaxin-1 (CCL11), and future CHD and ischemic stroke events in middle-aged European male participants of the PRIME Study (étude Prospective sur l'Infarctus du Myocarde).
METHODS
Study population.
Overall, 10,602 men aged 50 to 59 years were recruited between 1991 and 1993 by 4 collaborating WHO MONICA centers in Belfast (Northern Ireland), Lille, Strasbourg, and Toulouse (France).18 Among these, 823 subjects with coronary disease, 77 with a history of stroke at baseline examination, were excluded from the present analysis, leaving a study population of 9,711 men.
Baseline examination.
General characteristics.
Briefly, a self-administered health questionnaire was completed by subjects in their homes and was subsequently checked by trained interviewers at the clinic. It covered a broad range of clinical information, smoking habits, and use of medication. Diabetes was defined by current oral hypoglycemic treatment or use of insulin. Blood pressure was measured in the sitting position using identical automatic devices (Spengler SP9, Spengler, Cachan, France). Hypertension was defined as a blood pressure higher than 140/90 mm Hg or the use of antihypertensive medication. A 12-lead EKG was also recorded.18
Biological measurements.
Blood was drawn after overnight fasting. A subset of biological measurements was carried out using fresh plasma for the entire cohort. Plasma lipid analyses were centralized (SERLIA INSERM U325, The Lille Pasteur Institute, France). Total cholesterol and high-density lipoprotein cholesterol (HDL cholesterol) were measured by enzymatic methods using commercial kits in an automated analyzer (Boehringer, Mannheim, Germany). Fibrinogen was assessed by the Laboratory of Hemostasis at La Timone Hospital in Marseilles, France, using commercially available ELISA kits from Diagnostica Stago (Asnières-sur-Seine, France). Aliquots of serum and plasma were also frozen in liquid nitrogen for the analysis of biomarkers in nested case-control studies (see below).
Follow-up and verification of cases.
During the 10-year follow-up period, subjects were contacted annually by letter and asked to complete a clinical event questionnaire. For all subjects reporting a possible event, clinical information was sought directly from hospital or general practitioner records. All details concerning electrocardiograms, hospital admissions, enzyme levels, surgical intervention, angioplasty, and medical treatment were collected. Where applicable, the circumstances of death were obtained from the practitioner or the patient's family. In the few cases where these were not available from the practitioner or the family, death certificates were checked for supporting clinical or postmortem information on the cause of death.
CHD and ischemic stroke events were adjudicated by 2 independent medical committees. CHD events (stable and unstable angina, myocardial infarction, and coronary death) were defined as previously described using clinical, biological, stress test, scintigraphic, or angiographic criteria.18 Stroke was defined as previously reported19 according to WHO MONICA criteria as an acute focal cerebral function deficit including acute hemiparesis or hemiplegia, hemianopia, diplopia, dysarthria or aphasia, ataxia, acute loss of balance or coordination or an acute global cerebral function deficit including coma or the 4 limbs and cranial nerve palsy, of a vascular origin and persisting for more than 24 hours (except if the symptoms were interrupted by a surgical intervention or death). It included 1) ischemic stroke due to occlusion of small arteries, so-called lacunar infarct, occlusion of large arteries, i.e., atherothrombotic stroke, and stroke due to cardiac embolism; 2) intracerebral hemorrhage; and 3) subarachnoid hemorrhage. Transient or permanent cerebral focal deficit caused by a blood disease, a cerebral tumor or metastasis, or secondary to a trauma, were not considered by the stroke medical committee. Clinical information, computerized tomodensitometry scans (compatible signs), and angiographic and autopsy data were used to distinguish between ischemic and hemorrhagic stroke events. After 10 years of follow-up, the CHD and ischemic stroke event status was available for 95.1% of the cohort.
Standard protocol approvals, registrations, and patient consents.
The protocol was approved by the institutional review board of Broussais Hospital, Paris, France. Written informed consent was obtained for each subject who agreed to participate in the PRIME Study.
Nested case-control studies.
At the end of the 10 years of follow-up, 635 men developed a first coronary event and 98 a first ischemic stroke but baseline plasma samples were available, respectively, for 621 and 95 men (cases). Moreover, 1,242 and 190 matched controls (2 controls per case), respectively, were randomly selected from the initial cohort, and used for analysis (nested case-control study design). Matched controls were study participants recruited by the same center on the same day (±3 days), and of the same age (±3 years) as the corresponding case, and who were free from CHD and ischemic stroke at the time of the index date (CHD or ischemic stroke event).20
Measurements of biomarkers were performed blind with respect to the case-control status. Circulating levels of high sensitivity C-reactive protein (hs-CRP), RANTES, IP-10, MCP-1, and eotaxin-1 were determined by a multiplex bioassay using commercially available kits. Commercial kits for hs-CRP, IP-10, and eotaxin-1 were obtained from R&D Systems (Minneapolis, MN) and for MCP-1 and RANTES from Bio-Rad (Hercules, CA). Each multiplex assay was performed according to the manufacturer's specifications. The plates were read on a Luminex® 200− instrument system.
Statistical analysis.
The baseline characteristics of cases and matched controls were compared using conditional logistic regression. In the CHD control group, correlation coefficients were estimated by the Spearman rank test, while the median values of the chemokines were compared according to risk factor categories using the Wilcoxon-Mann-Whitney or Kruskal-Wallis tests. Hazard ratios (HR) and 95% confidence intervals (CIs) of each chemokine were estimated for CHD and for ischemic stroke using separate conditional logistic regression analyses, and were given for 1 SD of the log-transformed chemokine levels calculated for each control group. Analysis was adjusted for smoking status, hypertension, diabetes, body mass index, HDL and total cholesterol, triglycerides, and thereafter for hs-CRP and fibrinogen. Calibration of our final model was estimated by the Hosmer Lemeshow statistics in which a p value greater than 0.20 indicates adequate fit. Discrimination of our model was assessed by the C-statistic which was calculated from unconditional logistic regression adjusted for matched variables and the C-statistic of the model with and without chemokines was compared using a nonparametric test developed by Delong et al.21 appropriate for nonindependent data. Discrimination was also quantified by calculating the Net Reclassification Improvement index (NRI).22 To correct for the overestimation of the individual risk estimation due to the case-control design of the study, a correction on the intercept was made as proposed by Janes et al.23 Thereafter the tertiles of these corrected individual probabilities were used to define risk categories. Due to their skewed distribution, chemokines, triglycerides, hs-CRP, and fibrinogen were log-transformed for statistical analyses. All comparisons were 2-sided and a p value of less than 0.05 indicated a statistically significant difference. Analyses were performed using STATA− software version 9.1 (StataCorp, College Station, TX).
RESULTS
Of the 9,771 men who had no past history of stroke and CHD at baseline and after 10 years of follow-up, 635 had a first CHD event (408 in France and 227 in Northern Ireland) and 122 a first stroke including 98 of ischemic origin (64 in France and 34 in Northern Ireland) yielding incidence rates of 7.02 per 1,000 person-years for CHD and of 1.08 per 1,000 person-years for ischemic stroke events.
Baseline clinical characteristics.
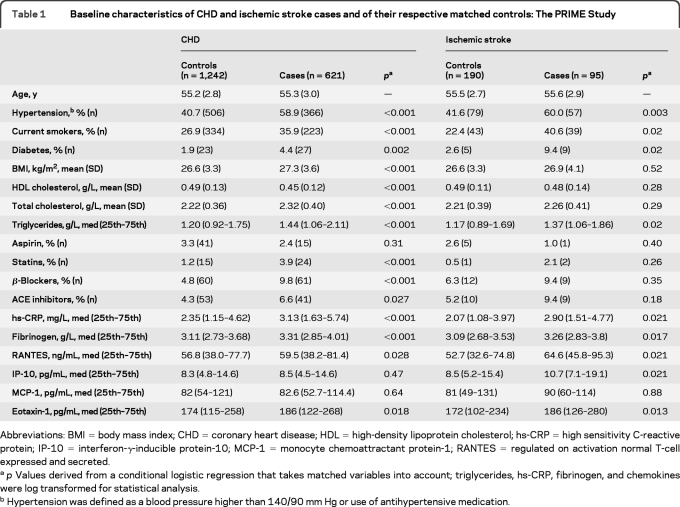
As shown in table 1, CHD cases had significantly higher levels of traditional cardiovascular risk factors (but a lower level of HDL cholesterol) as well as of hs-CRP, fibrinogen, RANTES, and eotaxin-1, and used more statins, β-blockers, and ACE inhibitors compared to controls. Ischemic stroke cases were more likely to be diabetic, hypertensive, and current smokers, and had higher triglyceride, hs-CRP, fibrinogen, RANTES, IP-10, and eotaxin-1 levels compared to controls.
Table 1.
Baseline characteristics of CHD and ischemic stroke cases and of their respective matched controls: The PRIME Study
Abbreviations: BMI = body mass index; CHD = coronary heart disease; HDL = high-density lipoprotein cholesterol; hs-CRP = high sensitivity C-reactive protein; IP-10 = interferon-γ-inducible protein-10; MCP-1 = monocyte chemoattractant protein-1; RANTES = regulated on activation normal T-cell expressed and secreted.
p Values derived from a conditional logistic regression that takes matched variables into account; triglycerides, hs-CRP, fibrinogen, and chemokines were log transformed for statistical analysis.
Hypertension was defined as a blood pressure higher than 140/90 mm Hg or use of antihypertensive medication.
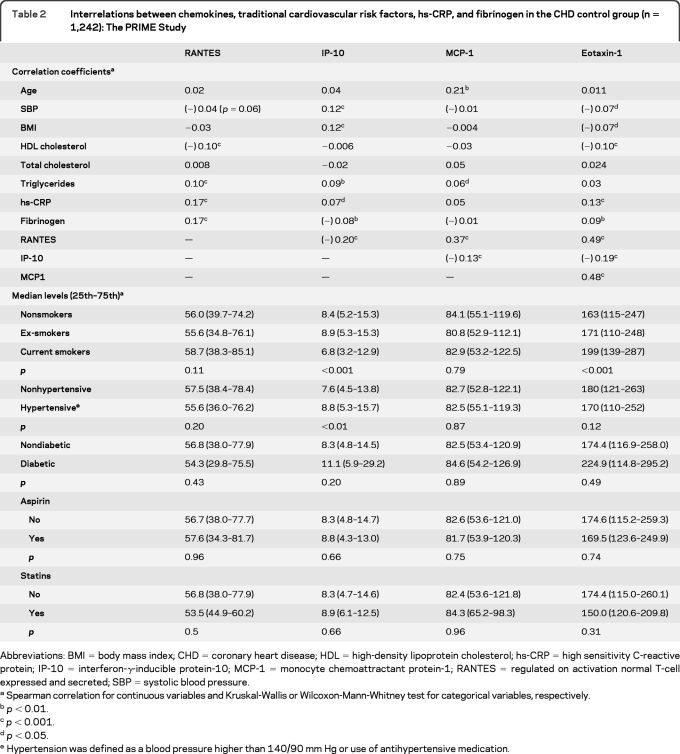
Correlates of chemokines in CHD controls.
As shown in table 2, chemokines were modestly correlated with traditional cardiovascular risk factors, hs-CRP and fibrinogen, with significant correlation coefficients ranging from −0.07 to +0.21. Correlations between chemokines ranged from −0.20 to +0.49. The circulating level of IP-10 decreased with current smoking status but increased with hypertension. The level of eotaxin-1 increased with current smoking status. The median levels of the chemokines did not differ according to statin or aspirin use.
Table 2.
Interrelations between chemokines, traditional cardiovascular risk factors, hs-CRP, and fibrinogen in the CHD control group (n = 1,242): The PRIME Study
Abbreviations: BMI = body mass index; CHD = coronary heart disease; HDL = high-density lipoprotein cholesterol; hs-CRP = high sensitivity C-reactive protein; IP-10 = interferon-γ-inducible protein-10; MCP-1 = monocyte chemoattractant protein-1; RANTES = regulated on activation normal T-cell expressed and secreted; SBP = systolic blood pressure.
Spearman correlation for continuous variables and Kruskal-Wallis or Wilcoxon-Mann-Whitney test for categorical variables, respectively.
p < 0.01.
p < 0.001.
p < 0.05.
Hypertension was defined as a blood pressure higher than 140/90 mm Hg or use of antihypertensive medication.
Circulating chemokines and future CHD events.
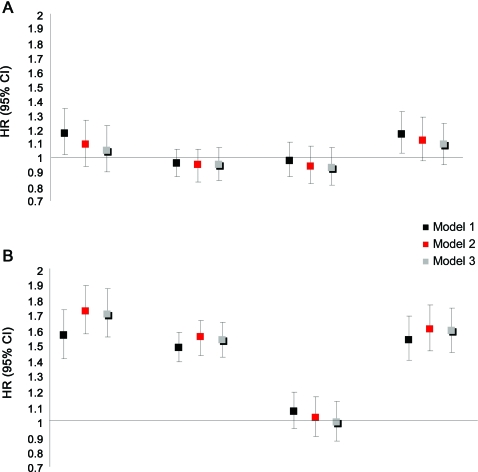
The figure, A, depicts the standardized HRs for each chemokine for the development of CHD. In unadjusted analysis, higher RANTES (HR = 1.17; 95% CI 1.02–1.34; p = 0.03) and eotaxin-1 levels (HR = 1.16; 95% CI 1.03–1.32; p = 0.014) were associated with CHD whereas IP-10 (HR = 0.96; 95% CI 0.87–1.06; p = 0.44) and MCP-1 (HR = 0.98; 95% CI 0.77–1.46; p = 0.73) were not. However, after adjustment for traditional risk factors and medications, hs-CRP and fibrinogen, RANTES (HR = 1.10; 95% CI 0.95–1.29; p = 0.22) and eotaxin-1 (HR = 1.09; 95% CI 0.95–1.24; p = 0.20) were no longer associated with CHD. Furthermore, RANTES (HR = 1.21; 95% CI 1.03–1.43; p = 0.021), eotaxin-1 (HR = 1.14; 95% CI 0.99–1.32; p = 0.06), and possibly MCP-1 (HR = 1.09; 95% CI 0.98–1.21; p = 0.11) were associated with acute coronary syndrome (n = 409) but not with stable angina (n = 212) in unadjusted analyses, but not after further adjustment for traditional cardiovascular risk factors, hs-CRP and fibrinogen (not shown). Moreover, none of the 4 chemokines were associated with the 29 fatal CHD events (not shown).
Figure. HRs and 95% CI of chemokines for CHD (A) and ischemic stroke (B): The PRIME Study.
(A) Standardized adjusted hazard ratios (HRs) and 95% confidence interval (CI) for regulated on activation normal T-cell expressed and secreted (RANTES), interferon-γ-inducible protein-10 (IP-10), monocyte chemoattractant protein-1 (MCP-1), and eotaxin-1 for coronary heart disease (CHD). The PRIME Study. HRs were estimated by conditional logistic regression and expressed for a 1-SD increase in the log-transformed chemokine values in controls. Model 1 = unadjusted analysis. Model 2 = model 1 + hypertension, smoking, diabetes, body mass index (BMI), high-density lipoprotein (HDL) and total cholesterol, triglycerides. Model 3 = model 2 + high sensitivity C-reactive protein (hs-CRP) and fibrinogen. (B) Standardized adjusted HRs and 95% CI for RANTES, IP-10, MCP-1, and eotaxin-1 for ischemic stroke. The PRIME Study. HRs were estimated by conditional logistic regression and expressed for a 1-SD increase in the log-transformed chemokine values in controls. Model 1 = unadjusted analysis. Model 2 = model 1 + hypertension, smoking, diabetes, BMI, HDL and total cholesterol, triglycerides. Model 3 = model 2 + hs-CRP and fibrinogen.
Circulating chemokines and future ischemic stroke events.
The standardized HRs for each chemokine for the development of ischemic stroke are shown in the figure, B. In unadjusted analyses, higher RANTES (HR = 1.56; 95% CI 1.04–2.34; p = 0.032), IP-10 (HR = 1.48; 95% CI 1.06–2.08; p = 0.022), and eotaxin-1 (HR = 1.53; 95% CI 1.05–2.24; p = 0.026) were associated with ischemic stroke. These associations were unaffected by further adjustment for traditional cardiovascular risk factors, medications, hs-CRP, and fibrinogen. MCP-1, however, was not associated with ischemic stroke even in unadjusted analysis (HR = 1.06; 95% CI 0.77–1.46; p = 0.73).
When RANTES, IP-10, and eotaxin-1 were considered in the same model adjusted for cardiovascular risk factors, medications, hs-CRP and fibrinogen, RANTES (HR = 1.83; 95% CI 1.21–2.75; p = 0.004), and IP-10 (HR = 1.95; 95% CI 1.33–2.87; p = 0.001) but not eotaxin-1 (HR = 1.14; 95% CI 0.76–1.71; p = 0.54) remained associated with ischemic stroke (backward analysis). In the same model, hypertension (HR = 2.57; 95% CI 1.42–4.65; p = 0.002) and current smoking status (HR = 3.32; 95% CI 1.62–6.80; p = 0.001) were the remaining independent predictors. In sensitivity analysis, the exclusion of ischemic stroke of cardioembolic origin (n = 27) yielded standardized unadjusted HRs for RANTES and IP-10 of 1.61 (0.94–2.77, p = 0.08) and 1.37 (0.90–2.07, p = 0.14) respectively. Fatal ischemic strokes were too few (n = 7) to assess reliably their association with chemokines.
Contribution of systemic RANTES and IP-10 levels to ischemic stroke risk prediction.
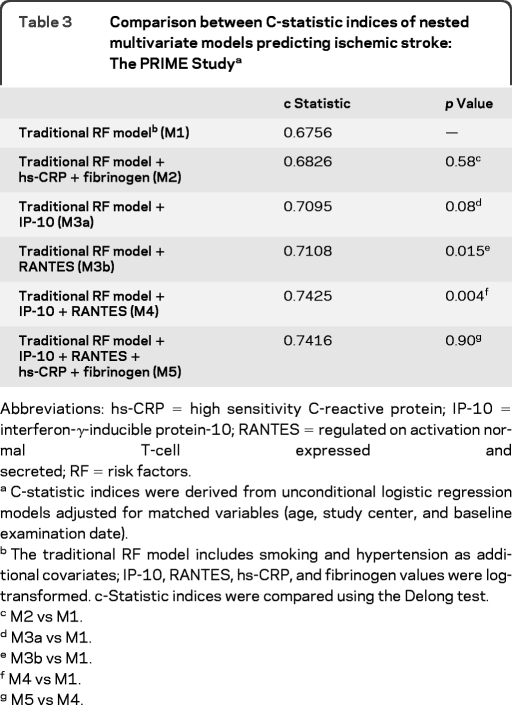
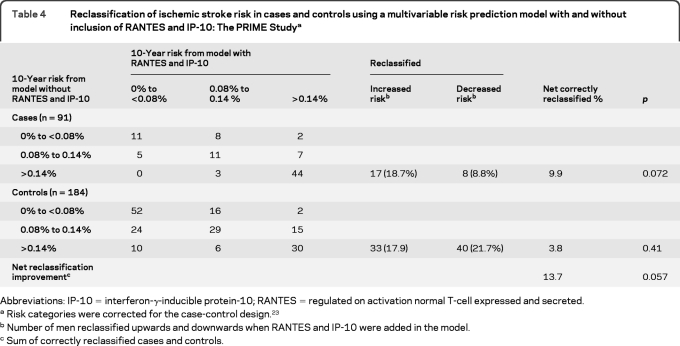
The goodness of fit of the final prediction model that included hypertension and smoking status in addition to age and study center was adequate (p value of the Hosmer Lemeshow statistic = 0.31). As shown in table 3, the C-statistic of the traditional risk factors–based model was improved by the addition of IP-10 (borderline significant) or RANTES (statistically significant) and improvement was even higher when both chemokines were added simultaneously. As shown in table 4, the addition of both chemokines reclassified 13.7% of the men.
Table 3.
Comparison between C-statistic indices of nested multivariate models predicting ischemic stroke: The PRIME Studya
Abbreviations: hs-CRP = high sensitivity C-reactive protein; IP-10 = interferon-γ-inducible protein-10; RANTES = regulated on activation normal T-cell expressed and secreted; RF = risk factors.
C-statistic indices were derived from unconditional logistic regression models adjusted for matched variables (age, study center, and baseline examination date).
The traditional RF model includes smoking and hypertension as additional covariates; IP-10, RANTES, hs-CRP, and fibrinogen values were log-transformed. c-Statistic indices were compared using the Delong test.
M2 vs M1.
M3a vs M1.
M3b vs M1.
M4 vs M1.
M5 vs M4.
Table 4.
Reclassification of ischemic stroke risk in cases and controls using a multivariable risk prediction model with and without inclusion of RANTES and IP-10: The PRIME Studya
Abbreviations: IP-10 = interferon-γ-inducible protein-10; RANTES = regulated on activation normal T-cell expressed and secreted.
Risk categories were corrected for the case-control design.23
Number of men reclassified upwards and downwards when RANTES and IP-10 were added in the model.
Sum of correctly reclassified cases and controls.
DISCUSSION
Incidence rate of first IS in the French and the Northern Irish component of the PRIME Study was consistent with that published in the French Dijon stroke registry and in the OXVASC study in the United Kingdom.24,25 The higher incidence rate of IS in Northern Irish than in French men in PRIME is also consistent with the north to south gradient of IS in Europe. The age range of 50 to 59 years may also explain the apparent disproportional ratio between CHD and ischemic stroke rates in PRIME.26,27
In asymptomatic subjects, evidence for an association between systemic chemokines and subclinical atherosclerosis or future CHD events is lacking. In 3 cross-sectional studies, the association between MCP-1 and subclinical atherosclerosis was not independent of traditional cardiovascular risk factors, especially age.28–30 In prospective analyses, no firm conclusion was drawn from the ARIC study regarding the association of MCP1 with CHD.15 In the EPIC-Norfolk study, higher IL-8 was independently associated with CHD over 6 years.16 The MONICA/KORA Augsburg Study is the only population-based study to evaluate the association of several chemokines with future CHD events.14 In this study, which included 2,358 healthy participants who developed 381 first CHD events over 11 years, elevated MCP-1, IL-8, and IP-10 levels at baseline were associated with a higher risk of CHD in univariate analysis but not after adjustment for traditional cardiovascular risk factors.
In the PRIME Study, with twice the number of CHD events as compared to the MONICA/KORA Augsburg Study,14 we not only confirm the lack of an independent association between MCP-1 and IP-10 and the risk of CHD, but we also extend these results by showing that neither RANTES nor eotaxin-1 are independent predictors of future CHD. Taken together, data from the current and previous studies suggest that systemic chemokines may not be useful for the estimation of CHD risk in asymptomatic subjects.
In the current study, 3 of the 4 chemokines tested were strong and independent predictors of future ischemic stroke, arguing against the possibility that these results were due solely to chance. Moreover, that RANTES and IP-10 remained predictive of stroke when included in the same model suggests that they were involved through different but potentially complementary mechanistic pathways, a view supported by their negative statistical correlation.
The apparently selective association of systemic chemokines with ischemic stroke as opposed to CHD requires confirmation. From a mechanistic point of view, the absence of an independent association between chemokines involved in monocyte/macrophage trafficking (MCP-1 or IL-8) and incident symptomatic CHD or stroke is, a posteriori, not completely unexpected. Besides its role in the promotion of inflammatory atherosclerotic lesion growth, monocyte/macrophage recruitment into the ischemic brain or myocardium is also required to ensure adaptive collateral vessel growth, neovascularization, and tissue repair,31,32 all of which might significantly limit disease severity and reduce the occurrence/incidence of sustained symptomatic disease. In addition, resident microglia greatly contributes to the response of the brain to injury,33 possibly substituting for and substantially limiting the need for monocyte recruitment. In fact, this might also explain why T-cell recruitment and activation in the injured brain parenchyma could be the critical step in the progression and severity of brain injury,34,35 and why T cell-directed chemokines such as RANTES and IP-10 are particularly important in stroke prediction. Circulating T cells have been shown to closely interact with meningeal vascular structures, crawling along and monitoring the luminal vascular surface, and crossing the vessel wall in response to brain injury.36 They then interact with resident perivascular and brain phagocytes for sustained activation.35,36 Consistent with this, several recent experimental studies have highlighted the critical role of T cells in the progression of brain injury in response to ischemia.34,35 It is worth noting that RANTES/CCR5 signaling and IP-10 are known promoters of T-cell recruitment and activation.37,38 In addition, RANTES/ CCR5 restrains IL-10 production37 while IP-10 inhibits regulatory T-cell recruitment,38 2 pathways recently shown to play critical roles in the control of the inflammatory response to brain injury and in brain protection after an ischemic insult.34,35 All these putative mechanisms arise from experimental models in which chemokine levels were elevated at the time or after brain injury. Whether the same mechanisms already operate but at much lower degrees several years prior to the events should be assessed. The residual confounding effect of a silent carotid artery stenosis or a silent cerebral injury might also explain that subjects who would have an ischemic stroke had higher levels of IP-10 and RANTES several years prior to the event in the present study.
Our results may have important clinical implications, as we show that the consideration of RANTES and IP-10 may improve the ability of a model to discriminate between those men who are likely to develop a first ischemic stroke from those who are not. Moreover, recent experimental evidence suggests that the combined inhibition of CCL2, CX3CR1, and CCR5,5 the inhibition of the functional interaction of RANTES with CXCL4,39 or the use of a RANTES antagonist40 reduces the progression of atherosclerosis in mice models, suggesting that chemokines may represent novel therapeutic targets for atherosclerotic disorders in the near future. It should be remembered, however, that at present, assays for systemic chemokines lack standardization. In addition, chemokine levels have short half-life and their stability over time (intraindividual variation) is not known yet. Whether repeated assessments over time would yield more precise estimates of their predictive power for vascular risk should be assessed in the future.
Analyses by ischemic stroke phenotype could not be conducted given the reduced numbers of stroke events. The incremental value of IP-10 and RANTES for ischemic stroke risk prediction should be evaluated in an independent dataset. Statin therapy could impact systemic chemokine levels but such therapy was used sparingly when the PRIME Study began in the early 1990s. This study needs to be confirmed in other ethnicities, in women, and in the elderly.
ACKNOWLEDGMENT
The authors thank the following organizations which allowed the recruitment of the PRIME subjects: the health screening centers organized by the Social Security of Lille (Institut Pasteur), Strasbourg, Toulouse, and Tourcoing; Occupational Medicine Services of Haute-Garonne, of the Urban Community of Strasbourg; the Association Interentreprises des Services Médicaux du Travail de Lille et environs; the Comité pour le Développement de la Médecine du Travail; the Mutuelle Générale des PTT du Bas-Rhin; the Laboratoire d'Analyses de l'Institut de Chimie Biologique de la Faculté de Médecine de Strasbourg; the Department of Health and Social Services and Personal Safety (NI), its Research and Development Office, and the Northern Ireland Chest Heart and Stroke Association.
APPENDIX
The PRIME Study was organized under an agreement between INSERM and the Merck, Sharp and Dohme-Chibret Laboratory, with the following participating laboratories: The Strasbourg MONICA Project, Laboratory of Epidemiology and Public Health, EA 3430, Strasbourg; University of Strasbourg, Strasbourg, F-67085, France (D. Arveiler, B. Haas); The Toulouse MONICA Project, INSERM, U558; Department of Epidemiology, Paul Sabatier University-Toulouse Purpan, Toulouse, France (J. Ferrieres, J.B. Ruidavets); The Lille MONICA project, INSERM, U744; University of Lille Nord de France; UDSL, Lille, France; The UKCRC Centre of Excellence for Public Health (NI), the Queen's University, Belfast, Northern Ireland (A. Evans, J. Yarnell, F. Kee); INSERM, U545; Department of Atherosclerosis, Lille; The Lille Pasteur Institute, Lille; the Faculty of Medicine, and University of Lille Nord de France, Lille, France (G. Luc, J.M. Bard); INSERM, U626, Laboratory of Hematology, Marseille; La Timone Hospital, Marseille, France (I. Juhan-Vague, P. Morange); The Laboratory of Endocrinology, INSERM, U563, Toulouse, France (B. Perret); The Vitamin Research Unit, The University of Bern, Bern, Switzerland (F. Gey); The Nutrition and Metabolism Group, Centre for Public Health, Queen's University Belfast, Northern Ireland (J. Woodside, I. Young); The DNA Bank, INSERM/UPMC, Paris University UMRS 937, Paris (F. Cambien); The Coordinating Centre, University of Paris-Sud XI, Villejuif, France (P. Ducimetiere); INSERM, Unit 970, Villejuif, France; and University of Paris V, The Paris Cardiovascular Research Centre (PAARC), Paris, France (A. Bingham).
Editorial, page 1116
Disclosure: Author disclosures are provided at the end of the article.
- CHD
- coronary heart disease
- CI
- confidence interval
- HDL
- high-density lipoprotein cholesterol
- HR
- hazard ratio
- hs-CRP
- high sensitivity C-reactive protein
- IP-10
- interferon-γ-inducible protein-10
- MCP-1
- monocyte chemoattractant protein-1
- NRI
- Net Reclassification Improvement
- RANTES
- regulated on activation normal T-cell expressed and secreted
CONTRIBUTORS
Pr. L. Guize, MD (APHP, France, PRIME Validation Event Committee, deceased), C. Morrison, MD (Scottish MONICA Cardiovascular Epidemiology Unit; Dundee University; Ninewells Hospital Dundee Scotland, PRIME Validation Event Committee), M.-T. Guillanneuf, MD (INSERM, France, PRIME Validation Event Committee), Pr. M. Giroud, MD (Dijon Stroke Registry, France, PRIME Validation Event Committee), Aurelien Belot, MSc (Hospices Civils de Lyon, Biostatistics Department, France, Statistical Support).
AUTHOR CONTRIBUTIONS
F. Canouï-Poitrine, P. Ducimetiere, J. Ferrieres, F. Kee, D. Arveiler, P. Amouyel, and J.P. Empana take responsibility for the integrity of the work as a whole, from inception to published article. The statistical analysis was conducted by F. Canouï-Poitrine, INSERM U970. F. Canouï-Poitrine conducted the statistical analysis and drafted the paper. G. Luc, E. Machez, J.B. Ruidavets, M. Montaye, B. Haas: substantial contributions to acquisition of data and critical revision of the manuscript for important intellectual content. P.E. Morange, J. Ferrieres, F. Kee, J. Yarnell, P. Amouyel, P. Ducimetiere, D. Arveiler: substantial contributions to conception and design and critical revision of the manuscript for important intellectual content. Z. Mallat: substantial contributions to critical revision of the manuscript for important intellectual content. P. Ducimetiere and A. Bingham: general coordination and critical revision of the manuscript for important intellectual content. J.P. Empana: supervision of statistical analysis, interpretation of the data, and writing of the manuscript.
DISCLOSURE
Dr. Canouï-Poitrine and Dr. Luc report no disclosures. Dr. Mallat serves as an Associate Editor for Arteriosclerosis Thrombosis and Vascular and Atherosclerosis and on the editorial boards of the Journal of Molecular Medicine and Circulation Research; has received research support from INSERM, France, Agence Nationale de la Recherche, France, and the British Heart Foundation; and has contractual rights for receipt of future royalty payments from Aterovax SA for patents re: cardiovascular biomarkers. Dr. Machez, A. Bingham, Dr. Ferrieres, Dr. Ruidavets, and Dr. Montaye report no disclosures. Dr. Yarnell serves on the editorial board of Health Education and receives publishing royalties for Epidemiology and Prevention: A System-based Approach (Oxford University Press, 2006). Dr. Haas, Dr. Arveiler, Dr. Morange, and Dr. Kee report no disclosures. Dr. Evans receives research support from the EU Framework Programme. Dr. Amouyel serves on the scientific advisory board for sanofi-aventis, Pfizer Inc, the Leducq Foudation, and Ipsen; serves on the editorial board of the European Journal of Prevention and Cardiac Rehabilitation; receives research support from Pfizer Inc and sanofi-aventis; and holds stock in Genoscreen. Dr. Ducimetiere serves as an Associate Editor of the European Journal of Epidemiology and Revue d'Epidémiologie et de Santé Publique and the editorial advisory board of the European Journal of Cardiovascular Prevention and Rehabilitation. Dr. Empana reports no disclosures.
REFERENCES
- 1. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685–1695 [DOI] [PubMed] [Google Scholar]
- 2. Packard RR, Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clin Chem 2008;54:24–38 [DOI] [PubMed] [Google Scholar]
- 3. Aukrust P, Halvorsen B, Yndestad A, et al. Chemokines and cardiovascular risk. Arterioscler Thromb Vasc Biol 2008;28:1909–1919 [DOI] [PubMed] [Google Scholar]
- 4. Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med 2006;354:610–621 [DOI] [PubMed] [Google Scholar]
- 5. Combadiere C, Potteaux S, Rodero M, et al. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice Circulation 2008;117:1649–1657 [DOI] [PubMed] [Google Scholar]
- 6. Si Y, Tsou CL, Croft K, Charo IF. CCR2 mediates hematopoietic stem and progenitor cell trafficking to sites of inflammation in mice. J Clin Invest 2010;120:1192–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weber C. Platelets and chemokines in atherosclerosis: partners in crime. Circ Res 2005;96:612–616 [DOI] [PubMed] [Google Scholar]
- 8. Damas JK, Smith C, Oie E, et al. Enhanced expression of the homeostatic chemokines CCL19 and CCL21 in clinical and experimental atherosclerosis: possible pathogenic role in plaque destabilization. Arterioscler Thromb Vasc Biol 2007;27:614–620 [DOI] [PubMed] [Google Scholar]
- 9. Wang N, Tabas I, Winchester R, Ravalli S, Rabbani LE, Tall A. Interleukin 8 is induced by cholesterol loading of macrophages and expressed by macrophage foam cells in human atheroma. J Biol Chem 1996;271:8837–8842 [DOI] [PubMed] [Google Scholar]
- 10. de Lemos JA, Morrow DA, Blazing MA, et al. Serial measurement of monocyte chemoattractant protein-1 after acute coronary syndromes: results from the A to Z trial. J Am Coll Cardiol 2007;50:2117–2124 [DOI] [PubMed] [Google Scholar]
- 11. Kervinen H, Manttari M, Kaartinen M, et al. Prognostic usefulness of plasma monocyte/macrophage and T-lymphocyte activation markers in patients with acute coronary syndromes. Am J Cardiol 2004;94:993–996 [DOI] [PubMed] [Google Scholar]
- 12. Kraaijeveld AO, de Jager SC, de Jager WJ, et al. CC chemokine ligand-5 (CCL5/RANTES) and CC chemokine ligand-18 (CCL18/PARC) are specific markers of refractory unstable angina pectoris and are transiently raised during severe ischemic symptoms. Circulation 2007;116:1931–1941 [DOI] [PubMed] [Google Scholar]
- 13. Rothenbacher D, Muller-Scholze S, Herder C, Koenig W, Kolb H. Differential expression of chemokines, risk of stable coronary heart disease, and correlation with established cardiovascular risk markers. Arterioscler Thromb Vasc Biol 2006;26:194–199 [DOI] [PubMed] [Google Scholar]
- 14. Herder C, Baumert J, Thorand B, et al. Chemokines and incident coronary heart disease: results from the MONICA/KORA Augsburg case-cohort study, 1984–2002. Arterioscler Thromb Vasc Biol 2006;26:2147–2152 [DOI] [PubMed] [Google Scholar]
- 15. Hoogeveen RC, Morrison A, Boerwinkle E, et al. Plasma MCP-1 level and risk for peripheral arterial disease and incident coronary heart disease: Atherosclerosis Risk in Communities study. Atherosclerosis 2005;183:301–307 [DOI] [PubMed] [Google Scholar]
- 16. Boekholdt SM, Peters RJ, Hack CE, et al. IL8 plasma concentrations and the risk of future coronary artery disease in apparently healthy men and women: the EPIC-Norfolk prospective population study. Arterioscler Thromb Vasc Biol 2004;24:1503–1508 [DOI] [PubMed] [Google Scholar]
- 17. van Wijk DF, van Leuven SI, Sandhu MS, et al. Chemokine ligand 2 genetic variants, serum monocyte chemoattractant protein-1 levels, and the risk of coronary artery disease. Arterioscler Thromb Vasc Biol 2010l;30:1460–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ducimetiere P, Ruidavets JB, Montaye M, Haas B, Yarnell J. Five-year incidence of angina pectoris and other forms of coronary heart disease in healthy men aged 50–59 in France and Northern Ireland: the Prospective Epidemiological Study of Myocardial Infarction (PRIME) Study. Int J Epidemiol 2001;30:1057–1062 [DOI] [PubMed] [Google Scholar]
- 19. Canouï-Poitrine F, Luc G, Bard JM, et al. , PRIME Study Group Relative contribution of lipids and apolipoproteins to incident coronary heart disease and ischemic stroke: the PRIME Study. Cerebrovasc Dis 2010;30:252–259 [DOI] [PubMed] [Google Scholar]
- 20. Empana JP, Canoui-Poitrine F, Luc G, et al. Contribution of novel biomarkers to incident stable angina and acute coronary syndrome: the PRIME Study. Eur Heart J 2008;29:1966–1974 [DOI] [PubMed] [Google Scholar]
- 21. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–845 [PubMed] [Google Scholar]
- 22. Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–172 [DOI] [PubMed] [Google Scholar]
- 23. Janes H, Pepe MS, Gu W. Assessing the value of risk predictions by using risk stratification tables. Ann Intern Med 2008;149:751–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Benatru I, Rouaud O, Durier J, et al. Stable stroke incidence rates but improved case-fatality in Dijon, France, from 1985 to 2004 Stroke 2006;37:1674–1679 [DOI] [PubMed] [Google Scholar]
- 25. Rothwell PM, Coull AJ, Silver LE, et al. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study). Lancet 2005;366:173–183 [DOI] [PubMed] [Google Scholar]
- 26. Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke 2001;32:2735–2740 [DOI] [PubMed] [Google Scholar]
- 27. Gentil A, Béjot Y, Lorgis L, et al. Comparative epidemiology of stroke and acute myocardial infarction: the Dijon Vascular project (Diva). J Neurol Neurosurg Psychiatry. 2009;80:1006–1011 [DOI] [PubMed] [Google Scholar]
- 28. Deo R, Khera A, McGuire DK, et al. Association among plasma levels of monocyte chemoattractant protein-1, traditional cardiovascular risk factors, and subclinical atherosclerosis. J Am Coll Cardiol 2004;44:1812–1818 [DOI] [PubMed] [Google Scholar]
- 29. Tang W, Pankow JS, Carr JJ, et al. Association of sICAM-1 and MCP-1 with coronary artery calcification in families enriched for coronary heart disease or hypertension: the NHLBI Family Heart Study. BMC Cardiovasc Disord 2007;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thakore AH, Guo CY, Larson MG, et al. Association of multiple inflammatory markers with carotid intimal medial thickness and stenosis (from the Framingham Heart Study). Am J Cardiol 2007;99:1598–1602 [DOI] [PubMed] [Google Scholar]
- 31. Glod J, Kobiler D, Noel M, et al. Monocytes form a vascular barrier and participate in vessel repair after brain injury. Blood 2006;107:940–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ito WD, Arras M, Winkler B, Scholz D, Schaper J, Schaper W. Monocyte chemotactic protein-1 increases collateral and peripheral conductance after femoral artery occlusion. Circ Res 1997;80:829–837 [DOI] [PubMed] [Google Scholar]
- 33. Schilling M, Strecker JK, Schabitz WR, Ringelstein EB, Kiefer R. Effects of monocyte chemoattractant protein 1 on blood-borne cell recruitment after transient focal cerebral ischemia in mice. Neuroscience 2009;161:806–812 [DOI] [PubMed] [Google Scholar]
- 34. Liesz A, Suri-Payer E, Veltkamp C, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med 2009;15:192–199 [DOI] [PubMed] [Google Scholar]
- 35. Shichita T, Sugiyama Y, Ooboshi H, et al. Pivotal role of cerebral interleukin-17-producing gamma delta T cells in the delayed phase of ischemic brain injury. Nat Med 2009;15:946–950 [DOI] [PubMed] [Google Scholar]
- 36. Bartholomaus I, Kawakami N, Odoardi F, et al. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature 2009;462:94–98 [DOI] [PubMed] [Google Scholar]
- 37. Potteaux S, Combadiere C, Esposito B, et al. Role of bone marrow-derived CC-chemokine receptor 5 in the development of atherosclerosis of low-density lipoprotein receptor knockout mice. Arterioscler Thromb Vasc Biol 2006;26:1858–1863 [DOI] [PubMed] [Google Scholar]
- 38. Heller EA, Liu E, Tager AM, et al. Chemokine CXCL10 promotes atherogenesis by modulating the local balance of effector and regulatory T cells. Circulation 2006;113:2301–2312 [DOI] [PubMed] [Google Scholar]
- 39. Koenen RR, von Hundelshausen P, Nesmelova IV, et al. Disrupting functional interactions between platelet chemokines inhibits atherosclerosis in hyperlipidemic mice. Nat Med 2009;15:97–103 [DOI] [PubMed] [Google Scholar]
- 40. Braunersreuther V, Steffens S, Arnaud C, et al. A novel RANTES antagonist prevents progression of established atherosclerotic lesions in mice. Arterioscler Thromb Vasc Biol 2008;28:1090–1096 [DOI] [PubMed] [Google Scholar]