Abstract
Objective:
We conducted a study of minocycline to assess its safety, tolerability, and efficacy for the treatment of HIV-associated cognitive impairment.
Methods:
HIV-1-infected individuals with progressive neurocognitive decline were enrolled in a double-blind, placebo-controlled study of minocycline. Participants were randomized to receive minocycline 100 mg or matching placebo orally every 12 hours. The primary efficacy measure was change in a neuropsychological test composite z score (NPZ-8) from baseline to week 24. Measures of safety included the frequency of adverse events and changes over time in laboratory tests. After 50% of participants completed the double-blind phase, an interim analysis of futility for the primary outcome measure was performed, and our Data and Safety Monitoring Board recommended early study termination.
Results:
A total of 107 HIV-1-infected individuals with cognitive impairment were enrolled. The minocycline group did not show improvement in the primary outcome measure (NPZ-8) (mean 24-week change = 0.12) compared to placebo (mean 24-week change = 0.17) (95% confidence interval = [−0.26, 0.39], p = 0.70). There were few severe adverse events or laboratory abnormalities in either treatment group.
Conclusion:
Minocycline was safe and well-tolerated in individuals with HIV-associated cognitive impairment, but cognitive improvement was not observed.
Classification of evidence.
This interventional study provides Class II evidence for the safety, tolerability, and efficacy of minocycline for the treatment of HIV-associated cognitive impairment.
Cognitive impairment continues to be an important manifestation of HIV-1 infection in 40%–50% of HIV-1 seropositive (HIV+) individuals in the era of highly active antiretroviral therapy (HAART).1 The use of HAART may improve cognitive performance2–4 but the treatment response is frequently incomplete, or the antiretroviral drugs are unable to penetrate into the CNS. An adjunctive therapy that interferes with the cascade of inflammatory events triggered by the HIV virus within the CNS is likely to play an important role in the future treatment of HIV-associated neurocognitive disorders (HAND).5
Minocycline has both anti-inflammatory and neuroprotective effects in in vitro models6 and has been shown to affect cytokine production by monocytes and T lymphocytes,7 inhibit microglia activation,8 reduce excessive matrix metalloproteinase activity,9 inhibit nitric oxide synthesis,10 and inhibit apoptotic cell death.11–13 Minocycline offers a unique therapeutic strategy for HAND because it may also have a direct effect in inhibiting HIV replication.14 In SIV encephalitis, an animal model for HAND, minocycline has been linked to suppressed activation of p38 MAP kinase. In this model, minocycline-treated animals demonstrated a reduction in the severity of SIV encephalitis and lower levels of viral RNA in both CSF and brain homogenates compared with SIV-infected, untreated controls. Minocycline-treated macaques also had lower brain levels of activated p38 MAP kinase, a proapoptotic neurodegenerative MAP kinase. In addition, minocycline may also inhibit both SIV and HIV replication in cultured primary macrophages14,15 and lymphocytes, the predominant target cells productively infected by the viruses. Viral replication (quantitation of SIV measured by p27 expression, and HIV measured by p24 expression) was suppressed in a dose-dependent fashion. Based upon these preliminary data, we conducted a phase II, randomized, double-blind, placebo-controlled study of minocycline to assess the safety, tolerability, and efficacy of minocycline for the treatment of HIV-associated cognitive impairment.
METHODS
Recruitment/enrollment.
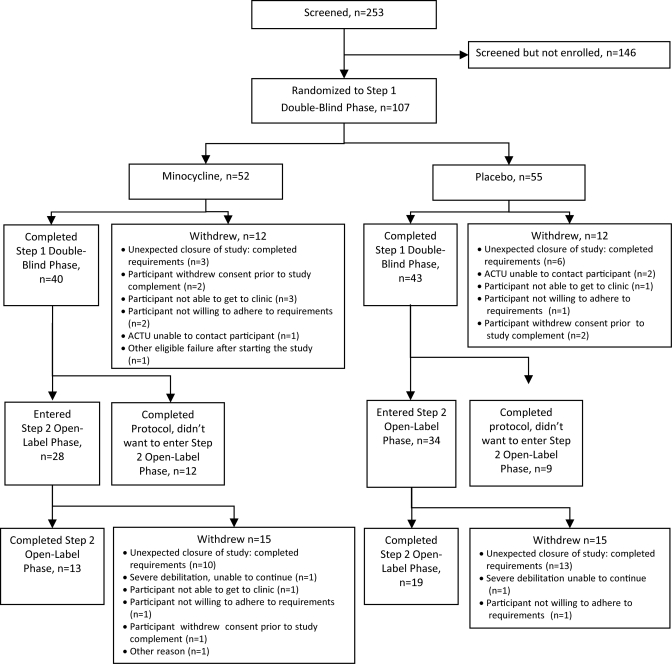
From March 2007 to September 2009, 107 HIV+ individuals with cognitive impairment and on a stable antiretroviral regimen for at least 16 weeks prior to study entry were enrolled across 16 US sites. The number of individuals completing each study phase is shown in the figure. Cognitive impairment was defined as performance compared to age-, education-, and, where available, gender- and race-matched controls at least 1.0 SD below on 3 or more independent neuropsychological tests, or at least 2.0 SD on one test and at least 1.0 SD on a second test at the screening visit. Normative values were obtained from HIV-negative neurologically asymptomatic individuals from the Multicenter AIDS Cohort Study.16 In addition to impaired performance at screening, progressive neurocognitive decline established by either objective or subjective criteria was also a requirement for enrollment. Objective criteria were defined as a decline of at least 1.0 SD below on 2 or more independent tests or 2.0 SD below age-matched and education-matched controls on one test between 2 neuropsychological test batteries performed in clinical practice or a research study within 12 months prior to entry. Subjective criteria were defined as cognitive decline noted by the participant, or a participant's family member, caregiver, or primary provider, and a Center for Epidemiologic Studies Depression Scale (CES-D) score <16 within 45 days prior to study entry. Participants were excluded if they were <18 or >65 years of age, had an estimated premorbid IQ <70 (as determined by the vocabulary section of the Wechsler Adult Intelligence Scale–Revised [WAIS-R]), or had a Karnofsky Functional Performance score <60. Participants also were excluded if they were pregnant or breast-feeding, or had concurrent conditions including an active symptomatic AIDS-defining opportunistic infection within 45 days prior to entry, a current neoplasm, severe premorbid psychiatric illness, confounding neurologic disorder, CNS infection, active drug or alcohol use or dependence, or serious illness requiring systemic treatment that in the opinion of the investigator would interfere with the study requirements.
Figure. Consort flow diagram of trial participation.
Standard protocol approvals, registrations, and patient consents.
The protocol was reviewed and approved by all appropriate committees of the AIDS Clinical Trials Group (ACTG) and the Institutional Review Boards at all participating sites. The study is registered in clinicaltrials.gov (NCT 00361257). Informed consent was obtained from all participants or their authorized representatives.
Randomization.
At entry, stratified randomization was utilized to assign treatment: minocycline 100 mg orally every 12 hours or matching placebo orally every 12 hours using permuted blocks with a block size of 4. The randomization sequence for each stratification combination was generated by the statistical and data management center at the Harvard School of Public Health and Frontier Science Technology Research Foundation. Investigators at sites enrolled participants, and interventions were assigned by site pharmacists. Two factors were used in the stratification: detectable (≥30 RNA copies/mL) vs undetectable (<30 RNA copies/mL) CSF HIV viral load vs no lumbar puncture, and progressive neurocognitive decline defined by objective vs subjective criteria. HIV-1 RNA in CSF and plasma was measured using a reverse transcriptase PCR assay performed at the University of Washington.17
Therapy and follow-up.
Participants received the study drug daily for 24 weeks during the double-blind phase. Participants were re-evaluated at 2, 4, 8, 12, 18, and 24 weeks after randomization. At each visit, participants were assessed for adverse clinical events. A neurologic examination and safety laboratory tests including serum chemistry profiles, hematology, CD4+ T-lymphocyte counts, and plasma HIV RNA levels were performed at screening and weeks 4, 12, and 24. A neuropsychological test battery was performed at screening, pre-entry, entry, and at 12 and 24 weeks. The battery included the Hopkins Verbal Learning Test Revised (HVLT-R), Trail Making Test parts A and B,18 Grooved Pegboard Test with the dominant and nondominant hand,19 Symbol Digit Test,20 Timed Gait Test, California Computerized Assessment Package (CALCAP), Choice and Sequential Reaction Time Test,21 Stroop Color Interference Task,22 and the International HIV Dementia Scale.23 Estimated premorbid intellectual function was assessed with the WAIS-R vocabulary subtest at screening. The presence of depression symptomatology was assessed with the CES-D24 at screening, entry, and weeks 12 and 24. Functional performance was measured with the Karnofsky Performance Score, the Instrumental Activities of Daily Living, and the performance-based Modified Medication Management Test25,26 at screening, entry, and weeks 12 and 24.
Outcome measures.
The primary research question was to evaluate the efficacy and safety for the treatment of HAND. The primary measure of efficacy in this study was the change in the composite neuropsychological z score (NPZ-8) from baseline to week 24. The NPZ-8 was defined as the average of z scores for the Trail Making Test parts A and B, Grooved Pegboard Test with the dominant and nondominant hand, CALCAP Choice and Sequential Reaction Time Test, Timed Gait Test, and Symbol Digit Test. These specific tests were chosen because they are sensitive measures of HAND and have been used in previous clinical trials for HAND.26 Secondary outcome measures of efficacy included the Global Deficit Score,27,28 performance on individual neuropsychological tests, changes in neuropsychological test z scores grouped by cognitive domains, changes in individual subjective and functional performance assessments, and changes in the Investigator and Subject Clinical Global Impressions.26 Primary measures of safety included the frequency of adverse events and abnormal results on laboratory tests, and changes over time in vital signs and laboratory tests.
Statistical methods.
For the primary analysis, an intention-to-treat analysis was conducted by first adopting a multiple imputation method to impute missing 24-week outcomes, and a general linear model was fit to estimate the minocycline effect on the 24-week change of NPZ-8. As a part of exploratory analyses, we conducted an observed data analysis using a general linear model, and also a longitudinal data analysis with a mixed model utilizing the repeatedly measured NPZ-8 at weeks 12 and 24. For the secondary analyses, no data were imputed and only the observed data analyses were conducted. For continuous outcomes, general linear models were used to estimate the minocycline effect. For categorical outcomes, new dichotomous variables were created (i.e., better vs no change/worse at 24 weeks compared to baseline), and logistic regressions were used to estimate the effect.
Including both primary and secondary endpoints, 30 statistical tests were conducted to examine the effect of minocycline compared to placebo. Since Type I error of 0.10 was used for each test, it was expected to observe 3 positive test results by chance.
Sample size was calculated to be 100, which provided 85% power (α = 0.10) to detect a 0.5 difference of NPZ-8 changes between groups assuming an SD of changes = 0.7 adjusting for 17% loss to follow-up, 10% noncompliance, and potential need for nonparametric testing (Mann-Whitney).
After 50% of the participants completed the double-blind phase, an interim analysis of primary and secondary outcome measures was performed. The results were reviewed by the Neurologic AIDS Research Consortium Data Safety and Monitoring Board on November 6, 2009, and they recommended early termination of the study, and participants returned for the final study visits.
RESULTS
Baseline characteristics.
Among the 107 HIV-infected individuals with cognitive impairment enrolled, 55 were randomized to placebo, and 52 were randomized to minocycline 100 mg every 12 hours. Eleven percent of the participants entered the study with objective neuropsychological test decline, and the remaining 89% entered the study with subjective criteria for neurocognitive decline. The 2 treatment groups were similar with respect to demographics (table 1). The mean age for the participants enrolled was 51 years, 83% were male, and 50% were white. Previous IV drug use was reported by 23% of the participants. The mean CD4 lymphocyte count was 543 cells/mm3. The participants, all on a stable antiretroviral regimen for at least 16 weeks prior to study entry, predominantly exhibited well-controlled viral replication with 86% of study participants having an undetectable plasma HIV RNA <30 copies/mL, and 93% (among those participants with available CSF HIV RNA data) having an undetectable CSF HIV RNA <30 copies/mL. Most participants remained on the same antiretroviral regimen with the exception of 17 participants in the placebo group and 14 participants in the minocycline group who changed antiretroviral therapy during the study period.
Table 1.
Baseline demographic characteristics
Abbreviations: ADC = AIDS Dementia Complex; CES-D = Center for Epidemiologic Studies Depression Scale; IHDS = International HIV Dementia Scale.
Among those participants with available CSF HIV RNA data (n = 41).
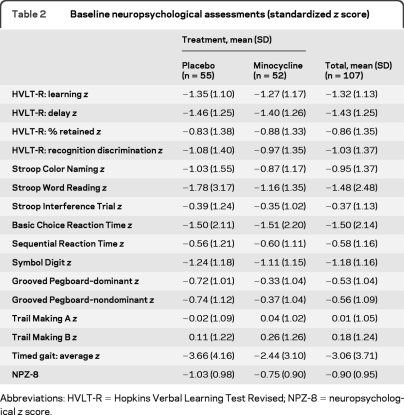
At enrollment, 51% of the participants were judged to have asymptomatic/subclinical cognitive impairment (AIDS Dementia Complex [ADC]) stage 0.5, whereas 38% had mild dementia (ADC stage 1), and 7% had moderate dementia (ADC stage 2).29 The baseline neuropsychological test z scores (table 2) were similar across the 2 groups except for Grooved Pegboard Test (dominant and nondominant hand tests), where participants in the placebo group had a trend for a more impaired z score compared to the minocycline group for both the dominant hand test (placebo mean z score = −0.72, minocycline mean z score = −0.33) and the nondominant hand test (placebo mean z score = −0.74, minocycline mean z score = −0.37).
Table 2.
Baseline neuropsychological assessments (standardized z score)
Abbreviations: HVLT-R = Hopkins Verbal Learning Test Revised; NPZ-8 = neuropsychological z score.
Efficacy.
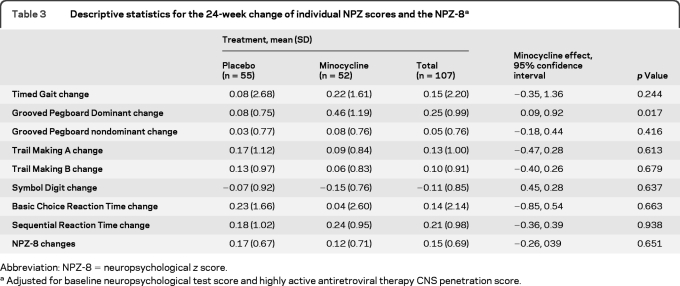
The changes from baseline to week 24 for the primary outcome measure (NPZ-8) and individual neuropsychological test secondary outcome measures are reported in table 3. The minocycline treatment group did not show improvement in the primary outcome measure (NPZ-8) (mean 24-week change in NPZ-8 score = 0.12) compared with the placebo group (mean 24-week change in NPZ-8 score = 0.17) (95% confidence interval −0.26, 0.39, p = 0.70). None of the secondary cognitive outcome measures showed a difference between the 2 treatment groups with one exception. The 24-week change in the Grooved Pegboard dominant hand z score in a general linear model adjusting for baseline neuropsychological test score and HAART CNS penetration effectiveness score30 showed a benefit for the minocycline group (mean 24-week z score change = 0.46) compared to the placebo group (mean 24-week z score change = 0.08); the minocycline effect was estimated to be 0.505, 95% confidence interval 0.094, 0.915, p = 0.017). However, after adjusting for multiple comparisons, the result was no longer significant. Also, no benefit for the minocycline group was seen with the Grooved Pegboard nondominant hand 24-week z score change (data not shown). Differences were not detected in the Global Deficit Score between the 2 treatment groups. The results from the observed data and longitudinal data analyses also showed that the minocycline effect was not different from the placebo effect.
Table 3.
Descriptive statistics for the 24-week change of individual NPZ scores and the NPZ-8a
Abbreviation: NPZ-8 = neuropsychological z score.
Adjusted for baseline neuropsychological test score and highly active antiretroviral therapy CNS penetration score.
No differences between the minocycline and placebo groups were seen on the subject and investigator clinical global impressions, subjective and performance-based functional measures, assessments of mood (CES-D score), CD4 count, and viral load assessments. There was no correlation between either the CES-D score or CD4 count and individual neuropsychological tests. Of note, 2 participants in the minocycline group changed from an undetectable plasma HIV RNA at baseline to a detectable HIV RNA at 24 weeks. In addition, 2 participants in the placebo group changed from a detectable HIV RNA at baseline to an undetectable HIV RNA at 24 weeks.
Safety.
There were no deaths reported in the study. In the placebo group, severe adverse events included general body pain (n = 2), fever (n = 1), headache (n = 1), cough (n = 1), vomiting (n = 1), and depression (n = 1). In the minocycline group, severe adverse events included tooth discoloration (n = 1) and mental status changes with suicidal ideation (n = 1). Twenty-five participants in the placebo group and 20 participants in the minocycline group had laboratory abnormalities. Life-threatening (grade 4) laboratory abnormalities were seen in no participants in the placebo group and one participant in the minocycline group (increased amylase). Severe (grade 3) laboratory abnormalities observed in the placebo group included changes in phosphorus (n = 6), fasting glucose (n = 1), absolute neutrophil count (n = 1), and lipase (n = 1). Severe laboratory abnormalities observed in the minocycline group included changes in phosphorus (n = 2), albumin (n = 1), nonfasting glucose (n = 1), total bilirubin (n = 1), and creatinine (n = 1). Mild to moderate hyperpigmentation, a known side effect from any tetracycline, was noted in 10 patients, with severe hyperpigmentation (tooth discoloration) seen in one patient on minocycline.
DISCUSSION
In this study, minocycline was safe and well-tolerated in individuals with HIV-associated cognitive impairment. Overall, there were few severe adverse events or laboratory abnormalities in either treatment group, and there were no deaths reported. No differences were detected in the primary outcome measure, the NPZ-8, among participants receiving minocycline compared to placebo. Similarly, no differences were detected among the 2 groups in the change scores for almost all of secondary outcome measures of cognitive performance with one exception, the Grooved Pegboard dominant hand. Differences in change scores in functional performance were not detected among the minocycline or placebo groups.
HIV+ individuals with progressive cognitive impairment defined by either subjective or objective criteria were recruited for this study. It was hypothesized that individuals with HAND who had progressive cognitive impairment were most likely to have active CNS inflammation due to HIV and thus most likely to obtain a therapeutic benefit from minocycline.
We required individuals to be on a stable antiretroviral regimen at entry so as not to confound any effects from minocycline with those that might result from changes in antiretroviral therapy. The proportion of study participants on antiretroviral therapy with active viral replication was low, with 86% of participants having an undetectable plasma HIV RNA and 93% of participants having an undetectable CSF HIV RNA. Thus, there are insufficient data to determine whether minocycline treatment might have a direct effect on inhibiting HIV replication as suggested by in vitro data in cultured macrophages and lymphocytes.14,15
There are a number of potential explanations for why this study failed to demonstrate a clinical benefit for minocycline. The treatment period of 24 weeks may have been too short to produce a neuroprotective effect. In part, the cognitive impairment in our study participants may have been due to non-HIV comorbid conditions such as hepatitis C coinfection or other comorbid conditions such as remote effects from illicit drug or alcohol use, depression, or side effects from medications. Minocycline at the dose used may have had inadequate penetration within the CNS, although among antibiotics, minocycline has relatively good brain penetration.31,32 HIV enters the CNS shortly after infection and may cause neuronal injury or dysfunction that after treatment with antiretroviral therapy is unresponsive to potential anti-inflammatory or neuroprotective effects from minocycline. Surrogate markers such as neuroimaging (e.g., magnetic resonance spectroscopy) or CSF biomarkers may be needed to evaluate early evidence of neuroprotection within the CNS rather than the clinical neuropsychological tests and functional outcomes used in this evaluation.
Minocycline has been shown to have a neuroprotective effect in animal models of other neurologic conditions such as multiple sclerosis (MS),33,34 Parkinson disease,11 Huntington disease,35,36 amyotrophic lateral sclerosis (ALS),36,37 and traumatic brain disease.10,38 In some of these conditions, minocycline has had a therapeutic benefit in clinical trials. In a small open-label study of 10 individuals with relapsing-remitting MS, minocycline decreased MRI activity as measured by the number of gadolinium-enhancing MRI lesions.39 Also, in an acute ischemic stroke study of 152 patients, participants receiving minocycline treatment had better outcomes on the NIH Stroke Scale, a modified Rankin Scale, and the Barthel Index over a 90-day period compared to participants receiving placebo.6 However, in a study of 412 individuals with ALS, participants on minocycline had faster deterioration on an ALS functional rating scale measuring gross and fine motor tasks, bulbar function, and respiratory function compared to those receiving placebo.40
The reason for the discrepancy between the minocycline effects in the SIV encephalitis model and those in HIV+ individuals is unclear. Further studies are needed to define the precise anti-inflammatory and neuroprotective properties of minocycline in the SIV encephalitis model. Although this study failed to demonstrate a beneficial impact of minocycline on HAND, additional studies using more sensitive markers of neurologic change may offer better opportunities to further assess minocycline as a potential anti-inflammatory and neuroprotectant treatment for CNS conditions associated with inflammation such as HAND, MS, and acute stroke.
GLOSSARY
- ACTG
AIDS Clinical Trials Group
- ADC
AIDS Dementia Complex
- ALS
amyotrophic lateral sclerosis
- CALCAP
California Computerized Assessment Package
- CES-D
Center for Epidemiologic Studies Depression Scale
- HAART
highly active antiretroviral therapy
- HAND
HIV-associated neurocognitive disorder
- HVLT-R
Hopkins Verbal Learning Test Revised
- MS
multiple sclerosis
- NPZ-8
neuropsychological z score
- WAIS-R
Wechsler Adult Intelligence Scale–Revised.
AUTHOR CONTRIBUTIONS
All authors were involved in revising the manuscript for content, including medical writing for content. Drs. Miyahara, Evans, and Sacktor and L. Deng were involved in the analysis or interpretation of the data. Drs. Sacktor, Evans, Schifitto, Cohen, and Clifford were involved in the study concept or design.
STUDY FUNDING
Supported in part by the AIDS Clinical Trials Group (ACTG) (full protocol available from the ACTG upon request) funded by: NIAID, AI38858, AI38855, AI27670, AI27668, AI27658, AI34853, AI127660, AI27664, AI27659, AI25903, AI25915, AI046376, AI46370, AI46381, AI50410, AI25868, AI46386, CFAR AI 127757, the Neurologic AIDS Research Consortium, NS32228, the National Institute of Mental Health, MH71150, MH64409, AI 068634, and GCRC Units funded by the National Center for Research Resources (NCRR), RR00052, RR00044, RR00046. The NCT number for this study is NCT 00361257. This study is registered in Clinical Trials.gov. Participating site ACTG Clinical Trials Unit (CTU) grant numbers include the following: University of California, San Diego Antiviral Research CTU Grant AI069432, Johns Hopkins University CTU Grant AI069465, CTSA Grant UL1 RR025005, UCLA School of Medicine CTU Grant AI069424, Washington University (St. Louis) CTU Grant AI069495, The Research & Education Group-Portland CRS CTU Grant AI069503, Henry Ford Hospital CRS CTU Grant AI069503, Massachusetts General Hospital CTU Grant AI069472, NYU/NYC HHC at Bellevue CTU Grant AI069532, University of Colorado Hospital CTU Grant AI069450, Northwestern University CTU Grant AI069471, Virginia Commonwealth University Medical Center CRS CTU Grant AI069503, University of Washington (Seattle) CTU Grant AI069434, University of North Carolina CTU Grant AI069423, University of Rochester Medical Center CTU Grant AI069511, Emory University, The Ponce de Leon Center CTU Grant AI069452, University of Pennsylvania, Philadelphia CTU Grant AI069467–04. Manufacture of the minocycline and matching placebo was funded with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services under contract no. N01-AI-05414. The project described was supported by Award AI068636 from the National Institute of Allergy and Infectious Diseases, National Institute of Mental Health (NIMH), and National Institute of Dental and Craniofacial Research (NIDCR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
DISCLOSURE
Dr. Sacktor receives support from the NIH (NIMH, NINDS, NIA, NIDA, Fogerty Institute, NIAID). Dr. Miyahara receives support from the NIH (NINDS, NIAID). L. Deng is supported by grants for the Neurologic AIDS Research Consortium (NINDS, NIH), the Statistical and Data Management Center of the Adult AIDS Clinical Trials Group (NIAID, NIH), and the Oral HIV/AIDS Research Alliance (NIDCR, NIH). Dr. Evans serves on data monitoring committees/scientific advisory boards for the FDA, NIH (NIAID and NICHD), Massachusetts General Hospital, Boston University, HIV Neurobehavioral Research Center (University of California at San Diego), Pfizer Inc, Genentech, Inc., Roche, CIS Biotech, Inc., InterMune, FzioMed, Inc., Alcon, Millennium Pharmaceuticals, Inc., and Averion International Corp.; and receives research support from the NIH (NINDS, NIAID, NIAID, NIMH, and NIDCR). Dr. Schifitto served as a consultant for Pfizer, Novartis, and Tibotec Therapeutics; received funding for travel from Biogen Idec; and receives research support from NIH. Dr. Cohen served as a consultant for Acorda Therapeutics Inc., Genzyme Corporation, Novartis, Pfizer Inc, and Astellas Pharma Inc.; served on speakers' bureaus for Bayer Schering Pharma, EMD Serono, Inc., and Teva Pharmaceutical Industries Ltd.; and received research support from Biogen Idec, Bristol-Myers Squibb, EMD Serono, Inc., Novartis, sanofi-aventis, and the NIH (NINDS, NIAID). Dr. Paul receives research support from the NIH. Dr. Robertson serves on scientific advisory boards for and has received funding for travel or speaker honoraria from GlaxoSmithKline, Abbott, and Tibotec Therapeutics; and receives research support from the NIH (NIMH, NIAID, and NINDS). B. Jarocki reports no disclosures. Dr. Scarsi receives research and salary support from the NIH (NIAID and Fogarty International Center) and the Department of Health and Human Services; and receives research support from the Ralph and Marion Falk and the Society of Infectious Diseases Pharmacists. Dr. Coombs received an honorarium for serving as an adjudicator for a Merck-sponsored study; served on a scientific advisory board for Abbott; serves as a consultant for NexBio, Inc.; and receives research support from the NIH (NIAID/DAIDS). Dr. Zink serves on the editorial board of the Journal of Neurovirology; serves as a consultant for AkaRx, Inc.; and receives research support from the NIH. Dr. Nath serves on scientific advisory boards for Biogen Idec and DioGenix, Inc.; serves as an Associate Editor for the Journal of Neurovirology; may accrue revenue on patents re: Tat as an immunogen; Diosgenin for treatment of neurodegenerative diseases; Role of Kv channels in neuroregeneration and protection; Role of limonoid compounds as neuroprotective agents; and Tat ELISA; has served as a consultant for N DioGenix, Inc. and Elan Corporation; receives research support from the NIH (NINDS, NIMH); and has provided expert advice in medico-legal cases. Dr. Smith is employed by NIAID, NIH, and DHHS and has no other disclosure. Dr. Ellis has served on the speakers' bureaus for and received speaker honoraria from Abbott and GlaxoSmithKline; served on the editorial board of the Journal of Neuroimmune Pharmacology; has received research support from the NIH; and his spouse holds stock in Abbott. Dr. Singer has received funding for travel from Biogen Idec and Pfizer Inc; and receives research support from Pfizer Inc and the NIH (NIMH, NCI, NINDS, NIDA). Dr. Weihe, S. McCarthy, and L. Hosey report no disclosures. Dr. Clifford serves/has served on scientific advisory boards for Biogen Idec, Elan Corporation, Roche, Forest Laboratories, Inc., Genentech, Inc., GlaxoSmithKline, Millennium Pharmaceuticals, Inc., Schering-Plough Corp., Bristol-Meyers Squibb, and Genzyme Corporation; received speaker honoraria and funding for travel from GlaxoSmithKline, Millennium Pharmaceuticals, Inc., and Genentech Inc.; has received research support from Pfizer Inc, Schering-Plough Corp., Bavarian Nordic, NeurogesX, GlaxoSmithKline, Tibotec Therapeutics, Boehringer Ingelheim, and Gilead Sciences, Inc.; and receives research support from the NIH (NIMH, NINDS, NIAID, and Fogarty Institutes).
REFERENCES
- 1. McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: mind the gap. Ann Neurol 2010;67:699–714 [DOI] [PubMed] [Google Scholar]
- 2. Gendelman HE, Zheng J, Coulter CL, et al. Suppression of inflammatory neurotoxins by highly active antiretroviral therapy in human immunodeficiency virus-associated dementia. J Infect Dis 1998;178:1000–1007 [DOI] [PubMed] [Google Scholar]
- 3. Ferrando S, van Gorp W, McElhiney M, et al. Highly active antiretroviral treatment in HIV infection: benefits for neuropsychological function. AIDS 1998;12:F65–F70 [DOI] [PubMed] [Google Scholar]
- 4. Sacktor N, Lyles RH, Skolasky RL, et al. Combination antiretroviral therapy improves psychomotor speed performance in HIV+ homosexual men. Neurology 1999;52:1640–1647 [DOI] [PubMed] [Google Scholar]
- 5. Epstein LG, Gendelman HE. Human immunodeficiency virus type 1 infection of the nervous system: pathogenetic mechanisms. Ann Neurol 1993;33:429–436 [DOI] [PubMed] [Google Scholar]
- 6. Lampl Y, Boaz M, Gilad R, et al. Minocycline treatment in acute stroke: an open-label, evaluator-blinded study. Neurology 2007;69:1404–1410 [DOI] [PubMed] [Google Scholar]
- 7. Kloppenburg M, Brinkman BM, de Rooij-Dijk HH, et al. The tetracycline derivative minocycline differentially affects cytokine production by monocytes and T lymphocytes. Antimicrob Agents Chemother 1996;40:934–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yrjanheikki J, Keinanen R, Pellikka M, et al. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci USA 1998;95:15769–15774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ryan LA, Zheng J, Brestser M, et al. Plasma soluble CD14 and TNF-a receptor two levels correlate with cognitive dysfunction during HIV-1 infection. J Infect Dis 2001;184:699–706 [DOI] [PubMed] [Google Scholar]
- 10. Amin AR, Attur MG, Thakker GD, et al. A novel mechanism of action of tetracyclines: effects on nitric oxide synthases. Proc Natl Acad Sci USA 1996;93:14014–14019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Du Y, Ma Z, Lin S, et al. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. Proc Natl Acad Sci USA 2001;98:14669–14664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee PL, Yiannoutsos CT, Ernst T, et al. A multi-center 1H MRS study of the AIDS dementia complex: validation and preliminary analysis. J Magn Reson Imaging 2003;17:625–633 [DOI] [PubMed] [Google Scholar]
- 13. Teng YD, Choi H, Onario RC, et al. Minocycline inhibits contusion-triggered mitochondrial cytochrome c release and mitigates functional deficits after spinal cord injury. Proc Natl Acad Sci USA 2004;101:3071–3076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zink MC, Uhrlaub J, DeWitt J, et al. Neuroprotective and anti-human immunodeficiency virus activity of minocycline. JAMA 2005;293:2003–2011 [DOI] [PubMed] [Google Scholar]
- 15. Si Q, Cosenza M, Zhao M, et al. A novel action of minocycline: inhibition of HIV-1 infection in microglia and macrophages (presentation number 395.9, 2002 Neuroscience Meeting Planner) Presented at the Society for Neuroscience, Orlando, Florida, 2002 [Google Scholar]
- 16. Miller EN, Selnes OA, McArthur JC, et al. Neuropsychological performance in HIV-1 infected homosexual men: The Multicenter AIDS Cohort Study (MACS). Neurology 1990;40:197–203 [DOI] [PubMed] [Google Scholar]
- 17. Zuckerman RA, Lucchetti A, Whittington WL, et al. Herpes simplex virus (HSV) suppression with valacyclovir reduces rectal and blood plasma HIV-1 levels in HIV-1/HSV-2-seropositive men: a randomized, double-blind, placebo-controlled crossover trial. J Infect Dis 2007;196:1500–1508 [DOI] [PubMed] [Google Scholar]
- 18. Reitan R. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills 1958;8:271–276 [Google Scholar]
- 19. Klove H. Clinical neuropsychology. In: Forster FM. The Medical Clinics of North America. New York: WB Saunders; 1963 [PubMed] [Google Scholar]
- 20. Smith A. Symbol digit modalities test manual. Los Angeles: Western Psychological Services; 1973 [Google Scholar]
- 21. California Computerized Assessment Package (CALCAP). Los Angeles, CA: Norland Software; 1990 [Google Scholar]
- 22. Comalli PE, Wapner S, Werner H. Interference effects of Stroop color-word test in childhood, adulthood, and aging. J Gen Psychol 1962;100:47–53 [DOI] [PubMed] [Google Scholar]
- 23. Sacktor NC, Wong M, Nakasujja N, et al. The International HIV Dementia Scale: a new rapid screening test for HIV dementia. AIDS 2005;19:1367–1374 [PubMed] [Google Scholar]
- 24. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401 [Google Scholar]
- 25. Albert SM, Flater SR, Clouse R, et al. Medication management skill in HIV: I: evidence for adaptation of medication management strategies in people with cognitive impairment: II: evidence for a pervasive lay model of medication efficacy. AIDS Behav 2003;7:329–338 [DOI] [PubMed] [Google Scholar]
- 26. Schifitto G, Zhang J, Evans SR, et al. A multicenter trial of selegiline transdermal system for HIV-associated cognitive impairment. Neurology 2007;69:1314–1321 [DOI] [PubMed] [Google Scholar]
- 27. Carey CL, Woods SP, Gonzalez R, et al. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol 2004;26:307–319 [DOI] [PubMed] [Google Scholar]
- 28. Heseltine PNR, Goodkin K, Atkinson JH, et al. Randomized double-blind placebo-controlled trial of peptide T for HIV-associated cognitive impairment. Arch Neurol 1998;55:41–51 [DOI] [PubMed] [Google Scholar]
- 29. Price RW, Brew BJ. The AIDS dementia complex. J Infect Dis 1988;158:1079–1083 [DOI] [PubMed] [Google Scholar]
- 30. Letendre S, Marquie-Beck J, Capparelli E, et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol 2008;65:65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brogden RN, Avery GS. New antibiotics: epicillin, minocycline and spectinomycin a summary of their antibacterial activity, pharmacokinetic properties and therapeutic efficacy. Drugs 1972;3:314–330 [DOI] [PubMed] [Google Scholar]
- 32. Brogden RN, Speight TM, Avery GS. Minocycline: a review of its antibacterial and pharmacokinetic properties and therapeutic use. Drugs 1975;9:251–291 [DOI] [PubMed] [Google Scholar]
- 33. Brundula V, Rewcastle NB, Metz LM, et al. Targeting leukocyte MMPs and transmigration: minocycline as a potential therapy for multiple sclerosis. Brain 2002;125:1297–1308 [DOI] [PubMed] [Google Scholar]
- 34. Popovic N, Schubart A, Goetz BD, et al. Inhibition of autoimmune encephalomyelitis by a tetracycline. Ann Neurol 2002;51:215–223 [DOI] [PubMed] [Google Scholar]
- 35. Chen M, Ona VO, Li M, et al. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med 2000;6:797–801 [DOI] [PubMed] [Google Scholar]
- 36. Wang X, Zhu S, Drozda M, et al. Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of Huntington's disease. Proc Natl Acad Sci USA 2003;100:10483–10487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tikka TM, Vartiainen NE, Goldsteins G, et al. Minocycline prevents neurotoxicity induced by cerebrospinal fluid from patients with motor neurone disease. Brain 2002;125:722–731 [DOI] [PubMed] [Google Scholar]
- 38. Arvin KL, Han BH, Du Y, et al. Minocycline markedly protects the neonatal brain against hypoxic-ischemic injury. Ann Neurol 2002;52:54–61 [DOI] [PubMed] [Google Scholar]
- 39. Zhang Y, Metz LM, Yong VW, et al. Pilot study of minocycline in relapsing-remitting multiple sclerosis. Can J Neurol Sci 2008;35:185–191 [DOI] [PubMed] [Google Scholar]
- 40. Gordon PH, Moore DH, Miller RG, et al. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol 2007;6:1045–1053 [DOI] [PubMed] [Google Scholar]