Abstract
OBJECTIVE:
Recent studies have reported that duodenal heterotopic gastric mucosa (HGM) has been observed in 8.9% of patients who undergo esophagogastroduodenoscopy. However, there are few reports concerning the endoscopic and endoscopic ultrasound characteristics of submucosal tumour-like HGM in the duodenum.
METHODS:
Endoscopic, endoscopic ultrasound (EUS) and histological findings were analyzed in six patients with submucosal tumour-like HGM, which were confirmed by pathological examination of biopsy or endoscopic polypectomy specimens.
RESULTS:
Endoscopically, the lesions appeared as a solitary, sessile submucosal tumour-like mass with a depression at the top. In four of six patients, small granular structures were found in the depressed area of the mass. On EUS, all masses demonstrated a heterogeneous pattern, among which four patients presented anechoic areas while two patients showed no anechoic areas. All lesions were localized within the mucosa and submucosa on EUS. Histologically, they consisted of gastric glands and some dilated glands, and were covered with normal duodenal epithelium. In four of six lesions, the tumours were composed of gastric-type foveolar epithelium showing papillary growth, fundic glands and pyloric glands, while the others consisted of gastric-type foveolar epithelium and pyloric glands.
CONCLUSION:
A heterogeneous pattern on EUS and small granular structures on esophagogastroduodenoscopy represent valuable diagnostic features of submucosal tumour-like HGM.
Keywords: Duodenal submucosal tumour, Endoscopic ultrasound, Heterotopic gastric mucosa
Abstract
OBJECTIF :
Selon des études récentes, on observe une muqueuse gastrique hétérotopique (MGH) du duodénum chez 8,9 % des patients qui ont subi une œsophagogastroduodénoscopie. Cependant, peu de rapports portent sur les caractéristiques des endoscopies et des échographies endoscopiques de la sous-muqueuse duodénale de la MGH d’aspect tumoral.
MÉTHODOLOGIE :
Les auteurs ont analysé les observations de l’endoscopie, de l’échographie endoscopique (ÉES) et de l’histologie chez six patients ayant une sous-muqueuse de la MGH d’aspect tumoral, confirmée par examen pathologique des prélèvements de biopsie ou de polypectomie endoscopique.
RÉSULTATS :
Sur le plan endoscopique, les lésions prenaient la forme d’une masse sous-muqueuse solitaire et sessile d’aspect tumoral surmontée d’une dépression. Chez quatre des six patients, on observait de petites structures granulaires dans la zone déprimée de la masse. À l’ÉES, toutes les masses avaient un aspect hétérogène, caractérisé par des zones anéchoïques chez quatre patients et par l’absence de zones anéchoïques chez deux patients. D’après l’ÉES, toutes les lésions étaient situées dans la muqueuse et la sous-muqueuse. Sur le plan histologique, elles se composaient de glandes gastriques et de quelques glandes dilatées et étaient couvertes d’épithélium duodénal normal. Dans quatre des six lésions, les tumeurs se composaient d’épithélium fovéolaire de type gastrique comportant une croissance papillaire, des glandes fondiques et des glandes pyloriques, tandis que les deux autres se composaient d’épithélium fovéolaire de type gastrique et de glandes pyloriques.
CONCLUSION :
Un modèle hétérogène d’après l’ÉES et de petites structures granulaires d’après l’œsophagogastroduodénoscopie représentent des caractéristiques diagnostiques précieuses de la MGH d’aspect tumoral de la sous-muqueuse.
The presence of heterotopic gastric mucosa (HGM) is well known in all organs of the gastrointestinal tract, particularly in the esophagus, duodenum and ileum (Meckel’s diverticulum) (1–4). A recent study (5) observed duodenal HGM in 8.9% of 28,210 patients who underwent esophagogastroduodenoscopy (EGD), and is endoscopically recognized as solitary or multiple small nodules (4,6,7). Morphologically, submucosal tumour-like masses are frequently of the solitary type (2,7–9). Solitary large HGMs may be difficult to differentiate from malignant neoplasms such as carcinoma, carcinoid tumours or lymphoma (1); however, a differential diagnosis for it has yet to be established.
Herein, we describe the endoscopic and endoscopic ultrasound (EUS) features of six cases of duodenal HGM with submucosal tumour-like appearance in the bulb and descending duodenum.
METHODS
Patients
Six patients with histologically proven duodenal HGM were evaluated by EGD and EUS. HGM was defined as a lesion consisting of gastric-type foveolar epithelium and/or fundic glands containing parietal cells and chief cells. The patients included six men, ranging in age from 49 to 71 years, all of whom presented with unremarkable symptoms. Two patients were referred for endoscopy due to undiagnosed gastric tumours, one for follow-up examination of gastric ulcer, one for further examination of gastric cancer, one for papillary cancer and one for colon cancer. All duodenal HGMs were incidental findings during endoscopy.
All of the patients underwent routine EGD (GIF-Q240, GIF-Q260, Olympus Optical Co, Japan) followed by EUS using a miniature probe system (12 MHz to 20 MHz; UM2R, UM3R, Olympus, Japan) after a fast of 12 h or longer. All patients received a single 20 mg intramuscular bolus of scopolamine butylbromide (Buscopan [Boehringer, Japan]) before endoscopy.
EUS images were obtained after filling the spaces around the lesions with deaerated (boiled) water. At each examination, 10 to 15 photographs were taken for analysis of size, echo level, borders of the lesions and layer of origin. Because it was difficult to exclude carcinoid tumour or carcinoma with submucosal growth patterns, three lesions (from patients 1, 2 and 3) were removed by endoscopic polypectomy and subsequently diagnosed histologically as HGM. Biopsy specimens of three lesions removed endoscopically from patients 4, 5 and 6 were diagnosed as HGM.
RESULTS
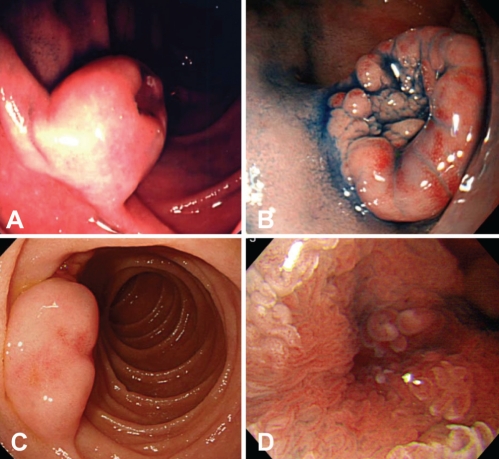
The clinical, endoscopic and EUS results are summarized in Table 1. The affected sites were the descending duodenum in four patients and the bulb in two. Endoscopically, the lesions appeared as solitary, sessile, submucosal tumour-like masses with a wide base and a depression at the top of the lesion (Figures 1A, 1B and 1C). The remaining surface of the masses was covered mainly with normal duodenal mucosa. In four of six lesions analyzed, small granular structures were found in the depressed area (Figure 1D).
Table 1.
Endoscopic, endoscopic ultrasound and histological findings in six men with duodenal heterotopic gastric mucosa
| Patient: age (years) |
Endoscopic ultrasound |
Histology | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
Endoscopy |
Location (layer) | Size*, mm | Internal echo | Anechoic lesion | |||||
| Portion | Configuration | Surface | Top | ||||||
| 1: 71 | 2nd | SMT-like mass | Normal mucosa | Depressed granular | II–III | 11×8 | Heterogenous | + | Foveolar epithelium, fundic glands, pyloric gland |
| 2: 49 | Bulb | SMT-like mass | Normal mucosa | Depressed granular | II–III | 12×10 | Heterogenous | + | Foveolar epithelium, pyloric gland |
| 3: 78 | 2nd | SMT-like mass | Normal mucosa | Depressed flat | II–III | 16×8 | Heterogenous | + | Foveolar epithelium, pyloric gland |
| 4: 68 | 2nd | SMT-like mass | Normal mucosa | Depressed flat | II–III | 7×6 | Heterogenous | + | Foveolar epithelium, fundic glands, pyloric gland |
| 5: 54 | 2nd | SMT-like mass | Normal mucosa | Depressed granular | II–III | 11×6 | Heterogenous | + | Foveolar epithelium, fundic glands, pyloric gland |
| 6: 64 | Bulb | SMT-like mass | Normal mucosa | Depressed granular | II–III | 13×6 | Heterogenous | N/A | Foveolar epithelium, fundic glands, pyloric gland |
Width × height. + Positive; N/A Not available; SMT Submucosal tumour
Figure 1).
A and B: Patient 1 and 6. Endoscopy showing a wide-based sessile submucosal tumour-like mass with depression at the top of the lesion. The lesions are covered mainly by normal duodenal mucosa. C Patient 3. Endoscopy showing a wide-based sessile submucosal tumour-like mass without granular structures at the top of the lesion. D Patient 4. Small granular structures are found in the depressed area
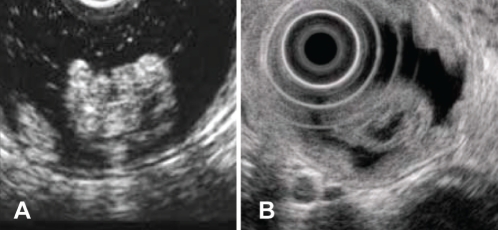
On EUS, the tumours were demonstrated to be heterogeneously hypoechoic masses with small anechoic areas (Figures 2A and 2B). The tumours ranged from 6 mm to 16 mm in diameter, and were localized within the second to third layers, which appeared to correspond to the mucosa and submucosa, respectively.
Figure 2).
A Patient 1. Endoscopic ultrasound images showing a heterogeneously hyperechoic mass with several anechoic lesions located predominantly in the submucosa. B Patient 3. Endoscopic ultrasound image showing a heterogeneously hyperechoic mass with anechoic lesions located predominantly in the submucosa
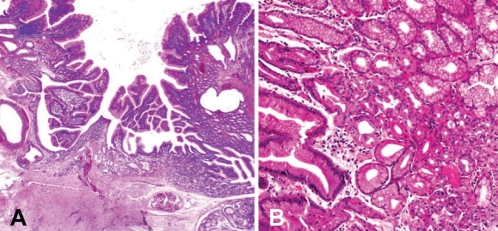
Histologically, all lesions consisted of gastric glands with or without some dilated glands, which were visualized as small anechoic areas on EUS (Figures 2A and 2B) and covered with normal duodenal epithelium without metaplastic change (Figure 3A). In four cases, tumours were composed of gastric-type foveolar epithelium showing papillary growth involving the fundic glands and pyloric glands (Figure 3B). The others consisted of gastric-type foveolar epithelium and pyloric glands. No atypical cells were observed in any cases. No tumour recurrence developed in the three patients who underwent polypectomy.
Figure 3).
A Patient 1. Histological examination of a polypectomy specimen showing the lesion covered by normal duodenal mucosa consisting of gastric-type foveolar epithelium, gastric fundic glands and pyloric glands with some dilated glands. B Patient 4. Biopsy specimen showing a lesion consisting of gastric fundic glands and pyloric glands
DISCUSSION
Jepsen et al (10) reported that duodenal polyps were found in 4.6% of patients referred for upper endoscopy, and that 1.4% of these duodenal polyps were HGM. A more recent study (5) reported a prevalence of duodenal HGM of 8.9% in patients undergoing EGD.
Histologically, previous reports (4,6,7) described HGM as comprised of solitary or multiple nodules. The proportion of the latter exceed 70%, whereas that of solitary lesions was small and ranged from 0% to 30%. Uraoka et al (7) described solitary lesions as hemispherically shaped and approximately 10 mm in diameter. Conversely, multiple-type lesions were demonstrated to be small nodules of various sizes. Each nodule exhibited an ovoid shape and plate-like elevation, with a diameter of 2 mm to 5 mm. In the present report, we described six cases of hemispherical HGM with a submucosal tumour-like appearance. Similar endoscopic findings of HGM have been reported by other investigators (2,7–9). Possible differential diagnoses include Brunner’s gland hyperplasia, ectopic pancreatic tissue, benign lymphoid hyperplasia, lymphoma, leiomyoma, leiomyosarcoma, adenoma, carcinoma and carcinoid tumours (1,8).
In the present series, a unique endoscopic finding was identified in 66% of patients, specifically small granular or lobular structures within the depressed portion of the lesion. Some investigators reported that large solitary duodenal HGMs tended to exhibit central depression (7,9,11). However, these features can also be seen in Brunner’s gland hyperplasia, carcinoid tumour, metastatic neoplasm or malignant lymphoma (1,8,9). Thus, it may be difficult to distinguish duodenal HGMs from other submucosal tumours using endoscopy.
EUS provides valuable information for the diagnosis of submucosal tumours of the gastrointestinal tract (12) because it is able to safely and precisely visualize size, extent, the layer of origin of the lesions and obtain internal images precisely (13). However, specific EUS features of duodenal HGMs are scarce. Hizawa et al (14) described that duodenal HGMs reveal simple anechoic masses within the submucosal layer on EUS. The EUS findings in our cases included hypoechoic and several small anechoic areas predominantly within the submucosal layer. We believe that the internal echo pattern of HGMs is variable and in accordance with the size of dilated gastric-type glands, which is characteristic of HGM.
An alternative approach for the differential diagnosis of HGM is Tc-99m pertechnetate scintigraphy. Tc-99m pertechnetate abdominal scintigraphy is a useful method for diagnosing and locating ectopic gastric mucosa in Meckel’s diverticulum, enteric or gastric duplications and cysts, Barrett’s esophagus and islands of HGM in an otherwise normal bowel. The method has an overall accuracy of 90% and a sensitivity of 85% in surgically proven cases (15).
Excessive surgery should be avoided because HGM is essentially a benign entity. However, if the HGM is large, it may still require laparoscopic or endoscopic resection due to the risk of ulceration, bleeding, adenoma or adenocarcinoma in the HGM, as previously reported (8,9,16–18). Endoscopic resection may be applied in the case of duodenal HGMs when EUS can verify that the tumour is localized in the third layer, which corresponds to the submucosa of the duodenal wall. EUS demonstrated that three lesions (patients 1, 2 and 3) were localized in the mucosa and submucosa. We carefully performed endoscopic polypectomy with electrocautery snare for removal, and obtained precise histological diagnosis of the tumours. These three patients developed no complications from the resection.
CONCLUSION
We showed that heterogeneously hypoechoic masses with several small anechoic lesions on EUS, and small granular structures on the depressed portions of the lesion, may be a characteristic feature of HGMs. Finally, EUS may not only provide characteristic findings, but also indicate the appropriateness of endoscopic resection.
REFERENCES
- 1.Vizcarrondo FJ, Wang TY, Brady PG. Heterotopic gastric mucosa: Presentation as a rugose duodenal mass. Gastrointest Endosc. 1983;29:107–11. doi: 10.1016/s0016-5107(83)72542-3. [DOI] [PubMed] [Google Scholar]
- 2.Kundrotas LW, Camara DS, Meenaghan MA, et al. Heterotopic gastric mucosa: A case report. Am J Gastroenterol. 1985;80:253–6. [PubMed] [Google Scholar]
- 3.Bernheim J, Novis BH. Heterotopic and metaplastic gastric mucosa in the duodenum. Isr J Med Sci. 1989;25:321–3. [PubMed] [Google Scholar]
- 4.Langkemper R, Hoek AC, Dekker W, et al. Elevated lesions in the duodenal bulb caused by heterotopic gastric mucosa. Radiology. 1980;137:621–4. doi: 10.1148/radiology.137.3.7444047. [DOI] [PubMed] [Google Scholar]
- 5.Genta RM, Kinsey RS, Singhal A, et al. Gastric foveolar metaplasia and gastric heterotopia in the duodenum: No evidence of an etiologic role for Helicobacter pylori. Human Pathol. 2010;41:1593–600. doi: 10.1016/j.humpath.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Lessells AM, Martin DF. Heterotopic gastric mucosa in the duodenum. J Clin Pathol. 1982;35:591–5. doi: 10.1136/jcp.35.6.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uraoka M, Fujitani T, Iida M, et al. Clinico-pathological investigation of heterotopic gastric mucosa of the duodenum. Gastroenterol Endosc. 1986;28:3078–85. (in Japanese with English abstract). [Google Scholar]
- 8.Shiraishi S, Yasuda K, Bandoh T, et al. Laparoscopic resection for ectopic gastric mucosa of the duodenum: Report of a case. Surg Today. 1999;29:351–3. doi: 10.1007/BF02483061. [DOI] [PubMed] [Google Scholar]
- 9.Kawamoto K, Ushio K, Ino S, et al. Diagnosis of neoplastic-like duodenal small lesions, adenoma and cancer (in Japanese) Stom Intest. 2001;36:1507–27. [Google Scholar]
- 10.Jepsen JM, Perssen M, Jakobsen NO, et al. Prospective study of prevalence and endscopic and histopathologic characteristics of duodenal polyps is patients submitted to upper endoscopy. Scand J Gastroenterol. 1994;29:483–7. doi: 10.3109/00365529409092458. [DOI] [PubMed] [Google Scholar]
- 11.Yoshimitsu K, Yoshida M, Motooka M, et al. Heterotopic gastric mucosa of the duodenum mimicking a duodenal cancer. Gastrointest Radiol. 1989;14:115–7. doi: 10.1007/BF01889173. [DOI] [PubMed] [Google Scholar]
- 12.Kawamoto K, Ueyama T, Iwashita I, et al. Colonic submucosal tumors, comparison of endoscopic US and target air-enema CT with barium enema study and colonoscopy. Radiology. 1994;192:697–702. doi: 10.1148/radiology.192.3.8058936. [DOI] [PubMed] [Google Scholar]
- 13.Rösch T, Lorenz R, Dancygier H, et al. Endosonographic diagnosis of submucosal upper gastrointestinal tract tumors. Scan J Gastroenterol. 1992;27:1–8. doi: 10.3109/00365529209011157. [DOI] [PubMed] [Google Scholar]
- 14.Hizawa K, Matsumoto T, Kouzuki T, et al. Cystic submucosal tumors in the gastrointestinal tract: Endosonographic findings and endoscopic removal. Endoscopy. 2000;32:712–4. doi: 10.1055/s-2000-9025. [DOI] [PubMed] [Google Scholar]
- 15.Sfakianakis GN, Conway JJ. Detection of ectopic gastric mucosa in Meckel’s diverticulum and in other aberrations by scintigraphy. II. Indications and methods-a 10 year experience. J Nucl Med. 1981;22:732. [PubMed] [Google Scholar]
- 16.Russin V, Krevsky B, Caroline DF, et al. Mixed hyperplastic and adenomatous polyp arising from ectopic gastric mucosa of the duodenum. Arch Pathol Lab Med. 1986;110:556–8. [PubMed] [Google Scholar]
- 17.Pasic F, Kesic V, Jokic A, et al. Duodenal polyps of gastric origin. Med Arch. 1993;47:95–6. [PubMed] [Google Scholar]
- 18.Kushima R, Ruthlein HJ, Stolte M, et al. Pyloric gland-type adenoma arising in heterotopic gastric mucosa of the duodenum, with dysplastic progression of the gastric type. Virchows Arch. 1999;435:452–7. doi: 10.1007/s004280050425. [DOI] [PubMed] [Google Scholar]