Abstract
Statins have been suggested to protect against Alzheimer’s disease (AD). Recently, however, we reported that aged dogs that underwent chronic statin treatment exhibited cognitive deficits compared with age matched controls. In human studies, blood levels of Coenzyme Q10 (CoQ10) decrease with statin use. CoQ10 is important for proper mitochondrial function and is a powerful antioxidant, two important factors for cognitive health in aging. Thus, the current study tested the hypothesis that CoQ10 levels in the serum and/or parietal cortex are decreased in statin treated dogs and are associated with poorer cognition. Six aged beagles (>8 years) were administered 80 mg/day of atorvastatin for 14.5 months and compared with placebo-treated animals. As predicted, serum CoQ10 was significantly lower in statin-treated dogs. Parietal cortex CoQ10 was not different between the two groups. However, poorer cognition was correlated with lower parietal cortex CoQ10. This study in dogs suggests that serum CoQ10 is reduced with atorvastatin treatment. CoQ10 levels in brain may linked to impaired cognition in response to atorvastatin, in agreement with previous reports that statins may have a negative impact on cognition in the elderly.
Keywords: Alzheimer’s disease, Canine, Dog, Lipitor, Statins
Introduction
Alzheimer’s disease (AD) is the most common cause of dementia in the elderly, affecting an estimated 5.5 million people in the United States. This number is expected to rise as the average age of the population increases. AD is pathologically characterized by senile plaques formed from beta-amyloid (Aβ) peptides and neurofibrillary tangles (NFTs) formed from hyper-phosphorylated tau [1, 14]. Inhibiting Aβ production is one possible means of preventing the development of AD. HMG-CoA reductase inhibitors (statins) prescribed to lower plasma cholesterol can modify Aβ levels. High intracellular cholesterol levels may influence the amyloid precursor protein (APP) to increase the production of Aβ. Therefore, the reduction of cholesterol through statins may reduce Aβ production [42]. Previous cell culture and rodent studies have found that statins improve cognitive function and reduce the production of Aβ [5, 16, 35].
Statin use has been associated with reduced risk of AD in human epidemiological studies [4, 13]. Li and colleagues reported an association between statin treatment and decreased AD-related neuropathological changes [18]. However, recent randomized controlled trials to determine if statin use prevented AD found that statins have no benefit in preventing AD when compared with controls, although statin therapy as a treatment for AD showed promise [20]. It is clear that the discussion of whether statin use is a possible treatment for AD remains controversial.
Aged dogs are a useful model for exploring chronic statin treatment to prevent AD. Dogs naturally develop human sequence Aβ deposits, vascular pathology and cognitive impairment [9]. Additionally, dogs were used to establish the efficacy and safety of statins, due to their similarity with humans with respect to responsiveness, drug tolerance and metabolism [26]. The efficacy and safety of most statins on the market were established in dogs given chronic, physiologically-relevant doses for over 2 years [7]. A recent pilot study conducted in our lab used the dog model of AD to examine whether aged animals treated with atorvastatin show reduced neuropathology and cognitive benefits. Results from this study demonstrated that the statin-treated dogs showed deficits in a task that measures executive function [26]. However, no neuropathological differences existed between the two groups that accounted for impaired cognition. Results from the canine study were consistent with previous reports in hypercholesterolemic aged individuals treated with statins, who showed decreased cognitive performance when compared to a placebo group [24, 25]. These results prompted us to investigate other possible mechanisms that may lead to cognitive decline with statin treatment.
One mechanism of particular interest is the depletion of Coenzyme Q10 (CoQ10) with statin use. CoQ10 is a mitochondrial electron transporter vital for ATP production and a powerful mitochondrial and cellular antioxidant found in all cells [12]. CoQ10 is a potent gene regulator, involved in the expression of hundreds of genes, including those involved in optimal mitochondrial function and inflammatory processes [34]. HMG-CoA reductase is a key enzyme in CoQ10 biosynthesis. Inhibition of HMG-CoA reductase by statins is associated with lower circulating levels of CoQ10 in rodents [43], canines [33] and humans [31]. CoQ10 deficiency results in decreased mitochondrial activity and mitochondrial degradation and increased reactive oxygen species (ROS) and inflammation [38]. CoQ10 levels tend to naturally decline with age and may play a role in both AD-related mitochondrial dysfunction and inflammation [41]. Further, aged canines show impaired mitochondrial function and may be particularly vulnerable to reduced CoQ10 [11].
Methods
Subjects
Twelve beagles ranging in age from 8.9–13.2 years were obtained from either the Lovelace Respiratory Research Institute (Albuquerque, NM) or from Harlan Laboratories (Indianapolis, IN). Based on our previous work, dogs of this age show cognitive decline and significant amounts of brain Aβ peptides [9]. All animals had documented dates of birth, comprehensive medical histories and a veterinary examination ensuring that the animal was in good health prior to the start of the study. At the end of the study, all but one control animal had received treatment for 14.5 months and ranged in age from 10.1–14.6 years. All research was conducted in accordance with approved IACUC protocols.
At baseline animals were ranked by cognitive test scores and placed into equivalent groups with 2 males and 4 females per group. These groups were randomly designated as either the placebo-treated control group or the atorvastatin-treated group. One dog in the control group was euthanized prior to collecting reversal learning error scores.
Drug Treatment
Atorvastatin (Atorvastatin Calcium, also known as Lipitor®, 40 mg tablets) and placebo tablets were kindly provided by Pfizer Inc. (New York, NY). Treated animals received 80 mg per day (2 40mg tablets) and control animals received 2 placebo tablets per day. Atorvastatin was chosen for this study because long term studies using an 80 mg/day dose in dogs did not result in adverse events such as cataracts [30] and this is the highest dose typically used in humans with hypercholesterolemia.
Cognitive Testing
Cognitive tests were used to assess learning and memory. A detailed description of cognitive testing has been described previously [26]. For the current study, scores from a size reversal learning task, a measure of executive function, was used. This task was administered after ~6 months of treatment and resulted in impaired reversal learning in treated dogs. Animals were simultaneously presented with two objects that differed only in size [10]. Once animals were proficient at choosing either the larger or smaller object, the reward contingencies were reversed, requiring animals to select the object that was previously negative. Error scores were used for data analyses.
Serum Samples
Blood samples were collected in 10cc red top tubes, centrifuged, aliquoted and frozen at −80°C. For the current study, blood collected 62 days prior to the end of the study was used for CoQ10 measurement.
Tissue Collection
Animals were sedated by subcutaneous injection with 0.2-mg/kg acepromazine twenty minutes prior to induction of general anesthesia. General anesthesia was induced by inhalation with 5% isoflurane. While maintained under anesthesia, dogs were exsanguinated by cardiac puncture. Within 15 minutes of death, the brain was removed from the skull and bisected midsagitally. The right hemisphere was coronally sectioned (~1 cm) and flash frozen at −80°C. The dissection procedure was completed within 20 minutes, yielding 35–45 minute post mortem interval. For the current study, the coronal section containing the parietal cortex was used for CoQ10 assays and a 50 mg piece of cortex was selected.
CoQ10 Extraction Procedures
CoQ10 was extracted from both serum and parietal cortex samples as previously described [23], with minor modifications. Briefly, 1 mL of serum from each control and atorvastatin treated dog was mixed with 1 mL of ethanol containing 0.1 mM butylatedhydroxytoluene (BHT) and extracted with 3 mL of hexane. For parietal cortex samples, brain tissues from control and atorvastatin treated dogs were homogenized in lysis buffer (pH 7.4) containing Sucrose 320 mM, Tris-HCl (pH=8.8) 9.9 mM, MgCl2 0.098 mM, EDTA 0.076 mM, proteinase inhibitors leupeptin (0.5mg/mL), pepstatin (0.7μg/mL), aprotinin (0.5 mg/mL) and PMSF (40 μg/mL) and phosphatase inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). 1 mL of homogenate was mixed with 1 ml of methanol and extracted with 3 mL of hexane. Each sample was centrifuged at 4000 g for 10 minutes. The hexane phase was evaporated to dryness under nitrogen, residues were dissolved in 25 μL of ethanol, and 20 μL were analyzed by HPLC.
Serum and Parietal Cortex CoQ10 Measurements
The HPLC measures of CoQ10 were conducted as previously described by Mosca et al. [23]. Briefly, the HPLC system consists of a Waters 616 quaternary pump equipped with a Waters 996 Diode array detector. The samples were eluted through a Hypersil GOLD column (C18, 4.6 cm × 25 cm, 5 μm particle size) with a guard column (10 mm) of the same material matrix (Thermo Scientific, Waltham, MA). The elution was performed at a flow rate of 1 mL/min with a gradient consisting of a mixture of solution A (methanol:water 80:20 v/v) and solution B (ethanol:isopropanol 95:5 v/v). The initial conditions were 39% Solution A and 61% Solution B. After 16 minutes, the mobile phase was changed linearly over 2 minutes to 100% Solution B. After 10 min of 100% Solution B, the system was reversed linearly over 2 minutes to the initial conditions. The absorbance was monitored at 275 nm. CoQ10 concentrations were calculated by reference to a standard curve of CoQ10 (0.39–50 μM) in ethanol. By this method, a linear fit (r2 = 0.99) was obtained.
Statistics
CoQ10 levels in serum and brain were compared across groups using independent t-tests. Pearson correlations were used to test the association between brain CoQ10 and either size discrimination or reversal learning error scores. All statistics were conducted using SPSS Statistics 18 (IBM; Armonk, NY).
Results
Previous studies using this cohort of aged dogs indicated that chronic statin treatment increased size reversal learning error scores, a sign of cognitive dysfunction [26]. Overall, the goal of the current study was to determine if a) CoQ10 decreased in serum and brain in atorvastatin treated dogs and; b) whether decreases in CoQ10 were associated with the cognitive deficits observed previously in statin treated dogs.
Serum and Parietal Cortex CoQ10
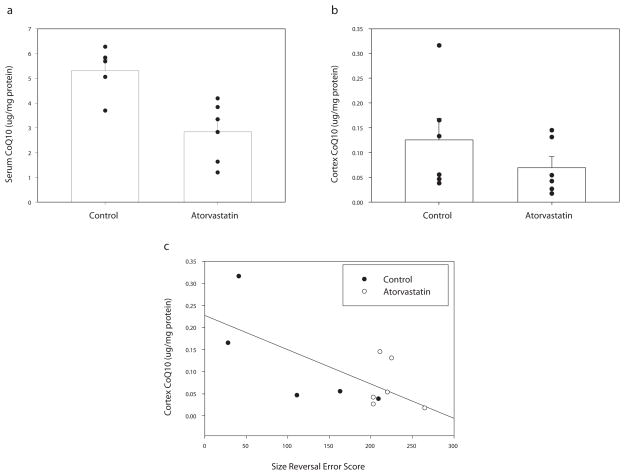
We were interested in determining if CoQ10 concentrations are reduced in parietal cortex following chronic treatment with atorvastatin, given that decreased blood concentrations of CoQ10 are a major side effect of statin use in human clinical studies. However, it remains unclear whether statins decrease brain tissue concentrations of CoQ10 and what role CoQ10 may have in brain function. Here we measured CoQ10 concentration in serum to demonstrate reduction of blood CoQ10 in these dogs. We measured CoQ10 concentration in parietal cortex to examine whether brain CoQ10 is reduced with statin treatment, as seen in blood levels. As expected, total serum CoQ10 was significantly reduced in statin treated dogs compared with controls t(9) = 3.66, p = 0.005 (Figure 1A). In contrast, parietal cortex CoQ10 was not significantly lower in statin treated dogs t(10) = 1.15, p = 0.276 (Figure 1B). We then correlated parietal cortex and serum CoQ10 levels. Interestingly, serum CoQ10 levels were not associated with parietal cortex levels of CoQ10 (r2 = −.010, p = 0.976).
Fig. 1.
CoQ10 in statin treated and control aged dogs a) Serum CoQ10 concentration (y-axis) is decreased in atorvastatin treated dogs. b) Parietal cortex CoQ10 concentration (y-axis) does not differ between groups. c) Reversal learning error scores (x-axis) was inversely correlated with parietal cortex CoQ10 (y-axis).
Cognition and CoQ10 levels
We hypothesized that brain and serum CoQ10 was associated with poorer reversal learning error scores. Reversal learning error scores were inversely correlated with parietal cortex CoQ10 (r2 = −0.68, p = 0.02) (Figure 1C), but not serum CoQ10 (r2 = −0.325 p, = 0.359). Thus, lower levels of CoQ10 in the parietal cortex, but not serum, are associated with deficits in reversal learning ability.
Discussion
Dogs develop Aβ pathology and cognitive deficits with age, similar to humans [9]. Dogs also offer additional information compared with mouse models, given that dog studies utilize chronic doses of statins (over 2 year periods) in doses that are physiologically relevant to those of human [7]. These doses can be administered without the upregulation of HMG-CoA typically observed in rodents [6]. Dogs in this study were given a chronic dose of 80mg per day, the highest dose given to humans with hypercholesterolemia. This dose is ~6–10 mg/kg in a 6–12kg dog, compared to ~1mg/kg in humans. Although the dose was much higher in mg/kg compared to human doses, it was chosen for the initial study parameters to determine whether atorvastatin leads to beneficial changes in the brain of aged dogs with Aβ.
In human clinical studies, plasma levels of CoQ10 decline following statin use [31]. CoQ10 deficits have been linked to inflammation, increased ROS and mitochondrial dysfunction, all of which may impair brain function [37]. Therefore, the aims of the current study were to determine if atorvastatin treatment reduced serum or brain CoQ10 levels and whether CoQ10 was associated with cognitive function. Here we used aged dogs chronically treated with atorvastatin or aged matched controls.
We first examined if CoQ10 was depleted with statin treatment. Although previous studies have reported lower CoQ10 levels in blood in humans following statin treatment, little is known about CoQ10 levels in brain tissue. Statins are prescribed more often in older individuals [19], when CoQ10 levels are naturally declining [41]. Statin treatment in dogs [33] and humans [31] lead to decreased blood levels of CoQ10 and we have also shown similar results in the current study. Interestingly, the results of the current study suggest that atorvastatin treatment does not lead to depletion of CoQ10 in the parietal cortex, and there is no correlation between serum and parietal cortex CoQ10 levels. We next examined whether parietal cortex or serum CoQ10 levels alone are associated with cognitive performance on the reversal learning task [26]. The parietal cortex was used because it is vulnerable to Aβ neuropathology in aged dogs and is a component of the cortical circuit involved with reversal learning [3]. We concluded that lower parietal cortex, but not serum, CoQ10 concentration is associated with poorer reversal learning. Taken together, our data suggest that brain CoQ10 may be linked to cognitive status in aged dogs.
Supplementation with CoQ10 may be beneficial in the aging population, at risk for Alzheimer’s disease. CoQ10 supplements are safe for human consumption in high doses and has already shown potential as a neuroprotectant [38]. CoQ10 treatment of human neuroblastoma cells protect against Aβ toxicity, inhibits formation and extension of Aβ fibrils and destabilizes pre-formed Aβ fibrils [27]. Further, CoQ10 supplementation in AD transgenic mice attenuates brain atrophy [17], decreases production of Aβ [44] and protects against both plaque formation and memory loss [38]. Further studies are needed to determine whether oral doses of CoQ10 increase CoQ10 levels in the cortex. Previous studies have shown improvement in circulating levels of CoQ10 following supplementation in humans [2, 22, 36] and dogs [15, 28, 45], but it is not clear whether CoQ10 levels in brain tissue are also improved. Additionally, it is unclear whether a combination of CoQ10 supplementation and statin treatment would improve cognitive function in domains sensitive to statin treatment. As this may be a concern for human clinical trials, future studies using a larger sample size and a more lipophilic statin should consider further investigating the importance of concurrent statin treatment and CoQ10 supplementation.
Research Highlights.
Serum CoQ10 levels were significantly lower in atorvastatin treated dogs
Parietal cortex CoQ10 was not significantly different in atorvastatin treated dogs
Lower parietal cortex CoQ10 levels were associated with poorer cognition
Acknowledgments
Funded by the Alzheimer’s Association (IIRG 035673 to EH and MPM) and the National Institute of Health (AG-05119 to DB). EB is a Ph.D. student at the Catholic University of the Sacred Heart in Rome and is a recipient of fellowships from the Society of Free Radical Biology and Medicine and the Italian Society of Pharmacology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sarah B. Martin, Email: sbmart2@uky.edu.
Giovanna Cenini, Email: gcenini@gmail.com.
Eugenio Barone, Email: eugeniobarone81@gmail.com.
Amy L.S. Dowling, Email: adowling@uky.edu.
Cesare Mancuso, Email: cmancuso@rm.unicatt.it.
D. Allan Butterfield, Email: dabcns@email.uky.edu.
M. Paul Murphy, Email: mpmurp3@email.uky.edu.
Elizabeth Head, Email: Elizabeth.Head@uky.edu.
References
- 1.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 2.Crane FL. Biochemical functions of coenzyme Q10. J Am Coll Nutr. 2001;20:591–598. doi: 10.1080/07315724.2001.10719063. [DOI] [PubMed] [Google Scholar]
- 3.Cummings BJ, Su JH, Cotman CW, White R, Russell MJ. Beta-amyloid accumulation in aged canine brain: a model of early plaque formation in Alzheimer’s disease. Neurobiol Aging. 1993;14:547–560. doi: 10.1016/0197-4580(93)90038-d. [DOI] [PubMed] [Google Scholar]
- 4.Dufouil C, Richard F, Fievet N, Dartigues JF, Ritchie K, Tzourio C, Amouyel P, Alperovitch A. APOE genotype, cholesterol level, lipid-lowering treatment, and dementia: the Three-City Study. Neurology. 2005;64:1531–1538. doi: 10.1212/01.WNL.0000160114.42643.31. [DOI] [PubMed] [Google Scholar]
- 5.Fassbender K, Simons M, Bergmann C, Stroick M, Lutjohann D, Keller P, Runz H, Kuhl S, Bertsch T, Bergmann Kv, Hennerici M, Beyreuther K, Hartmann T. Simvastatin strongly reduces levels of Alzheimer’s disease beta -amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proc Natl Acad Sci U S A. 2001;98:5856–5861. doi: 10.1073/pnas.081620098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franke C, Noldner M, Abdel-Kader R, Johnson-Anuna LN, Gibson Wood W, Muller WE, Eckert GP. Bcl-2 upregulation and neuroprotection in guinea pig brain following chronic simvastatin treatment. Neurobiol Dis. 2007;25:438–445. doi: 10.1016/j.nbd.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Gerson RJ, MacDonald JS, Alberts AW, Kornbrust DJ, Majka JA, Stubbs RJ, Bokelman DL. Animal safety and toxicology of simvastatin and related hydroxy-methylglutaryl-coenzyme A reductase inhibitors. Am J Med. 1989;87:28S–38S. doi: 10.1016/s0002-9343(89)80596-0. [DOI] [PubMed] [Google Scholar]
- 8.Ghribi O. Preservation of the blood brain barrier integrity may underlie neuroprotective effects of statins in Alzheimer’s disease. J Alzheimers Dis. 2006;10:407–408. doi: 10.3233/jad-2006-10409. [DOI] [PubMed] [Google Scholar]
- 9.Head E. Brain Aging in Dogs: Parallels with Human Brain Aging and Alzheimer’s disease. Vet Therapeutics. 2001;2:247–260. [PubMed] [Google Scholar]
- 10.Head E, Callahan H, Muggenburg BA, Cotman CW, Milgram NW. Visual-discrimination learning ability and beta-amyloid accumulation in the dog. Neurobiol Aging. 1998;19:415–425. doi: 10.1016/s0197-4580(98)00084-0. [DOI] [PubMed] [Google Scholar]
- 11.Head E, Nukala VN, Fenoglio KA, Muggenburg BA, Cotman CW, Sullivan PG. Effects of age, dietary, and behavioral enrichment on brain mitochondria in a canine model of human aging. Exp Neurol. 2009;220:171–176. doi: 10.1016/j.expneurol.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James AM, Smith RA, Murphy MP. Antioxidant and prooxidant properties of mitochondrial Coenzyme Q. Arch Biochem Biophys. 2004;423:47–56. doi: 10.1016/j.abb.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 13.Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet. 2000;356:1627–1631. doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- 14.Khachaturian ZS. Diagnosis of Alzheimer’s disease. Arch Neurol. 1985;42:1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- 15.Kitano M, Watanabe D, Oda S, Kubo H, Kishida H, Fujii K, Kitahara M, Hosoe K. Subchronic oral toxicity of ubiquinol in rats and dogs. Int J Toxicol. 2008;27:189–215. doi: 10.1080/10915810801978060. [DOI] [PubMed] [Google Scholar]
- 16.Kurata T, Miyazaki K, Kozuki M, Panin VL, Morimoto N, Ohta Y, Nagai M, Ikeda Y, Matsuura T, Abe K. Atorvastatin and pitavastatin improve cognitive function and reduce senile plaque and phosphorylated tau in aged APP mice. Brain Res. 2011;1371:161–170. doi: 10.1016/j.brainres.2010.11.067. [DOI] [PubMed] [Google Scholar]
- 17.Li G, Jack CR, Yang XF, Yang ES. Diet supplement CoQ10 delays brain atrophy in aged transgenic mice with mutations in the amyloid precursor protein: an in vivo volume MRI study. Biofactors. 2008;32:169–178. doi: 10.1002/biof.5520320120. [DOI] [PubMed] [Google Scholar]
- 18.Li G, Larson EB, Sonnen JA, Shofer JB, Petrie EC, Schantz A, Peskind ER, Raskind MA, Breitner JC, Montine TJ. Statin therapy is associated with reduced neuropathologic changes of Alzheimer disease. Neurology. 2007;69:878–885. doi: 10.1212/01.wnl.0000277657.95487.1c. [DOI] [PubMed] [Google Scholar]
- 19.Ma J, Sehgal NL, Ayanian JZ, Stafford RS. National trends in statin use by coronary heart disease risk category. PLoS Med. 2005;2:e123. doi: 10.1371/journal.pmed.0020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGuinness B, Passmore P. Can statins prevent or help treat Alzheimer’s disease? J Alzheimers Dis. 2010;20:925–933. doi: 10.3233/JAD-2010-091570. [DOI] [PubMed] [Google Scholar]
- 21.Michikawa M, Yanagisawa K. Apolipoprotein E4 induces neuronal cell death under conditions of suppressed de novo cholesterol synthesis. J Neurosci Res. 1998;54:58–67. doi: 10.1002/(SICI)1097-4547(19981001)54:1<58::AID-JNR7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 22.Mohr D, Bowry VW, Stocker R. Dietary supplementation with coenzyme Q10 results in increased levels of ubiquinol-10 within circulating lipoproteins and increased resistance of human low-density lipoprotein to the initiation of lipid peroxidation. Biochim Biophys Acta. 1992;1126:247–254. doi: 10.1016/0005-2760(92)90237-p. [DOI] [PubMed] [Google Scholar]
- 23.Mosca L, Marcellini S, Perluigi M, Mastroiacovo P, Moretti S, Famularo G, Peluso I, Santini G, De Simone C. Modulation of apoptosis and improved redox metabolism with the use of a new antioxidant formula. Biochem Pharmacol. 2002;63:1305–1314. doi: 10.1016/s0006-2952(02)00867-5. [DOI] [PubMed] [Google Scholar]
- 24.Muldoon MF, Barger SD, Ryan CM, Flory JD, Lehoczky JP, Matthews KA, Manuck SB. Effects of lovastatin on cognitive function and psychological well-being. Am J Med. 2000;108:538–546. doi: 10.1016/s0002-9343(00)00353-3. [DOI] [PubMed] [Google Scholar]
- 25.Muldoon MF, Ryan CM, Sereika SM, Flory JD, Manuck SB. Randomized trial of the effects of simvastatin on cognitive functioning in hypercholesterolemic adults. Am J Med. 2004;117:823–829. doi: 10.1016/j.amjmed.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 26.Murphy MP, Morales J, Beckett TL, Astarita G, Piomelli D, Weidner A, Studzinski CM, Dowling AL, Wang X, Levine H, 3rd, Kryscio RJ, Lin Y, Barrett E, Head E. Changes in cognition and amyloid-beta processing with long term cholesterol reduction using atorvastatin in aged dogs. J Alzheimers Dis. 2010;22:135–150. doi: 10.3233/JAD-2010-100639. [DOI] [PubMed] [Google Scholar]
- 27.Ono K, Hasegawa K, Naiki H, Yamada M. Preformed beta-amyloid fibrils are destabilized by coenzyme Q10 in vitro. Biochem Biophys Res Commun. 2005;330:111–116. doi: 10.1016/j.bbrc.2005.02.132. [DOI] [PubMed] [Google Scholar]
- 28.Prosek M, Butinar J, Lukanc B, Fir MM, Milivojevic L, Krizman M, Smidovnik A. Bioavailability of water-soluble CoQ10 in beagle dogs. J Pharm Biomed Anal. 2008;47:918–922. doi: 10.1016/j.jpba.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Reinoso RF, Sanchez Navarro A, Garcia MJ, Prous JR. Preclinical pharmacokinetics of statins. Methods Find Exp Clin Pharmacol. 2002;24:593–613. [PubMed] [Google Scholar]
- 30.Robertson DG, Urda ER, Rothwell CE, Walsh KM. Atorvastatin is not cataractogenic in beagle dogs. Curr Eye Res. 1997;16:1229–1235. doi: 10.1076/ceyr.16.12.1229.5035. [DOI] [PubMed] [Google Scholar]
- 31.Rundek T, Naini A, Sacco R, Coates K, DiMauro S. Atorvastatin decreases the coenzyme Q10 level in the blood of patients at risk for cardiovascular disease and stroke. Arch Neurol. 2004;61:889–892. doi: 10.1001/archneur.61.6.889. [DOI] [PubMed] [Google Scholar]
- 32.Saheki A, Terasaki T, Tamai I, Tsuji A. In vivo and in vitro blood-brain barrier transport of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors. Pharm Res. 1994;11:305–311. doi: 10.1023/a:1018975928974. [DOI] [PubMed] [Google Scholar]
- 33.Satoh K, Yamato A, Nakai T, Hoshi K, Ichihara K. Effects of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors on mitochondrial respiration in ischaemic dog hearts. Br J Pharmacol. 1995;116:1894–1898. doi: 10.1111/j.1476-5381.1995.tb16679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmelzer C, Lindner I, Rimbach G, Niklowitz P, Menke T, Doring F. Functions of coenzyme Q10 in inflammation and gene expression. Biofactors. 2008;32:179–183. doi: 10.1002/biof.5520320121. [DOI] [PubMed] [Google Scholar]
- 35.Simons M, Keller P, Strooper BD, Beyreuther K, Dotti CG, Simons K. Cholesterol depletion inhibits the generation of beta-amyloid in hippocampal neurons. Proc Natl Acad Sci USA. 1998;95:6460–6464. doi: 10.1073/pnas.95.11.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh RB, Niaz MA, Kumar A, Sindberg CD, Moesgaard S, Littarru GP. Effect on absorption and oxidative stress of different oral Coenzyme Q10 dosages and intake strategy in healthy men. Biofactors. 2005;25:219–224. doi: 10.1002/biof.5520250127. [DOI] [PubMed] [Google Scholar]
- 37.Sohal RS, Forster MJ. Coenzyme Q, oxidative stress and aging. Mitochondrion. 2007;7(Suppl):S103–111. doi: 10.1016/j.mito.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spindler M, Beal MF, Henchcliffe C. Coenzyme Q10 effects in neurodegenerative disease. Neuropsychiatr Dis Treat. 2009;5:597–610. doi: 10.2147/ndt.s5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamai I, Tsuji A. Transporter-mediated permeation of drugs across the blood-brain barrier. J Pharm Sci. 2000;89:1371–1388. doi: 10.1002/1520-6017(200011)89:11<1371::aid-jps1>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 40.Tsuji A, Saheki A, Tamai I, Terasaki T. Transport mechanism of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors at the blood-brain barrier. J Pharmacol Exp Ther. 1993;267:1085–1090. [PubMed] [Google Scholar]
- 41.Turunen M, Olsson J, Dallner G. Metabolism and function of coenzyme Q. Biochim Biophys Acta. 2004;1660:171–199. doi: 10.1016/j.bbamem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 42.Willey JZ, Elkind MS. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors in the treatment of central nervous system diseases. Arch Neurol. 2010;67:1062–1067. doi: 10.1001/archneurol.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willis RA, Folkers K, Tucker JL, Ye CQ, Xia LJ, Tamagawa H. Lovastatin decreases coenzyme Q levels in rats. Proc Natl Acad Sci U S A. 1990;87:8928–8930. doi: 10.1073/pnas.87.22.8928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang X, Yang Y, Li G, Wang J, Yang ES. Coenzyme Q10 attenuates beta-amyloid pathology in the aged transgenic mice with Alzheimer presenilin 1 mutation. J Mol Neurosci. 2008;34:165–171. doi: 10.1007/s12031-007-9033-7. [DOI] [PubMed] [Google Scholar]
- 45.Zaghloul AA, Gurley B, Khan M, Bhagavan H, Chopra R, Reddy I. Bioavailability assessment of oral coenzyme Q10 formulations in dogs. Drug Dev Ind Pharm. 2002;28:1195–1200. doi: 10.1081/ddc-120015352. [DOI] [PubMed] [Google Scholar]