Campylobacter jejuni and Campylobacter coli bacteremia patients were mainly young and without severe underlying diseases. The bacterial isolates were typically susceptible to antimicrobial agents. The outcome was usually good, regardless of appropriate or inappropriate antimicrobial treatment given at the hospital.
Abstract
Background. Campylobacter bacteremia is an uncommon condition, usually diagnosed in elderly and immunocompromised patients.
Methods. Blood culture isolates and clinical information were collected for patients with diagnoses of Campylobacter jejuni or Campylobacter coli bacteremia in Finland from 1998 through 2007. Bacterial species were identified by means of polymerase chain reaction analysis, and minimal inhibitory concentrations for ciprofloxacin, clindamycin, doxycycline, erythromycin, gentamicin, meropenem, and metronidazole were determined with an agar dilution method. Medical records and mortality data within 1 year after the bacteremic episode were reviewed.
Results. The study included 76 patients (median age, 46 years), for whom bacterial isolates (C. jejuni in 73, C. coli in 3) and clinical information were available. Most patients (70%) had no significant underlying diseases. The majority (82%) of the isolates were susceptible for all antimicrobial agents tested. However, antimicrobial therapy seemed to have only a limited effect, because no differences could be detected between patients with appropriate empirical antimicrobial treatment and those with delayed appropriate, inappropriate, or no antimicrobial therapy, either in the duration of hospitalization (median, 4 days for both groups) or in attributable mortality. The outcome of the infection was severe in 4 patients infected with C. jejuni; 2 died within 30 days, spondylodiscitis developed in 1, and Guillain-Barré syndrome developed in 1.
Conclusions. C. jejuni and C. coli bacteremia occurred mainly in moderately young individuals without severe underlying diseases. The bacterial isolates were predominantly susceptible to antimicrobial agents, and the outcome of the disease was typically good, regardless of appropriate or inappropriate antimicrobial treatment given in the hospital.
Campylobacter infection is most frequently caused by Campylobacter jejuni or Campylobacter coli, and it is the most common bacterial enteritis in developed countries [1–3]. The disease is characterized by watery diarrhea, abdominal pain, fever, and sometimes bloody stools [1, 2]. Although the outcome of disease is typically good, some important postinfectious complications occur. Reactive arthritis seems to be comparatively common—a Finnish study suggested up to 7% of individuals infected with C. jejuni or C. coli develop reactive arthritis [4]—whereas Guillain-Barré syndrome is a serious although rare complication occurring after approximately 1–3/10 000 Campylobacter infections [5, 6]. Occasionally, Campylobacter can even be cultured from blood. The incidence of Campylobacter bacteremia has been shown to be <1% of the total incidence of Campylobacter infections [7, 8]. Campylobacter fetus and C. jejuni are the 2 Campylobacter species most frequently isolated from blood [8–11]. However, bacteremic episodes are more typical for C. fetus infections, whereas C. jejuni and C. coli are predominantly detected in fecal samples. Why some C. jejuni or C. coli isolates are more invasive and enter the bloodstream is not well understood.
Underlying conditions known to predispose for Campylobacter bacteremia include HIV infection, liver diseases, and malignancies, and the attributable mortality of Campylobacter bacteremia has ranged between 4% and 16 % [8, 10–13]. Although immunocompromised and elderly patients are especially prone to C. fetus bacteremia [14], some studies have shown that C. jejuni could also be isolated from blood cultures of younger patients without significant underlying diseases [8, 9]. Available data regarding antimicrobial susceptibility of blood isolates are scarce but indicate that resistance to fluoroquinolones is common, resistance to macrolides sometimes occurs, and in general the antimicrobial susceptibility pattern of Campylobacter blood isolates seems to reflect that of the fecal isolates in the country [10, 11, 13]. However, antimicrobial susceptibility has usually been tested with disk diffusion and not with methods giving exact minimal inhibitory concentrations (MICs). Furthermore, the identification of the isolates to the species level has only rarely been verified by molecular methods.
We have reported elsewhere some bacterial characteristics associated with a more severe outcome of C. jejuni enteritis [15, 16]. In the present study, we show, for the first time, a nationwide summary of the clinical characteristics and outcome of C. jejuni and C. coli bacteremia from a 10-year period, as well as antimicrobial susceptibility and species identification, verified by molecular methods, for the corresponding isolates. In addition, to the best of our knowledge, this is the first description of a case of Campylobacter spondylodiscitis in a patient with concurrent C. jejuni bacteremia.
MATERIALS AND METHODS
Collection of Cases and Isolates of Campylobacter jejuni or Campylobacter coli Bacteremia
Because of nationwide mandatory reporting, all clinical microbiology laboratories in Finland (population at end of study period, 5.3 million) report all bacterial findings in blood cultures to the National Infectious Diseases Register, maintained by the National Public Health Institute (later the National Institute for Health and Welfare). Reports of all notified Campylobacter bacteremia cases (including the Finnish personal identification number) in the study period, 1998 through 2007, were received from the National Infectious Diseases Register. In addition, all clinical microbiology laboratories in Finland with blood culture facilities were contacted and asked to send lists of all diagnosed Campylobacter bacteremia cases (including both reported cases and those that should have been reported but were not) during the study period along with the corresponding Campylobacter blood culture isolates routinely stored at −70°C. The study was approved by the Finnish Ministry of Social Affairs and Health.
Identification of Campylobacter Isolates
The characterization of the isolates to species level was confirmed by polymerase chain reaction (PCR) targeting 3 separate genes for C. jejuni and C. coli, respectively. The primer pair C412F and C1228R (annealing temperature [AT], 58°C), targeting a region of the 16S ribosomal RNA gene specific for the genus Campylobacter [17], was used to confirm successful DNA extraction and previous identification to genus level. Multiplex PCR was performed on all isolates positive for the 16S ribosomal RNA, using the MDmapA1 upper and MDmapA2 lower primers [18] targeting the mapA gene of C. jejuni, as well as the COL3 upper [19] and MDCOL2 lower primers [18] targeting the ceuE gene of C. coli (AT, 59°C). Positive C. jejuni isolates were further confirmed by PCR using the primers HIP400F and HIP1134R (AT, 66°C) and targeting the hippuricase gene [20], and positive C. coli isolates were confirmed by PCR using the primers CC18F and CC519R (AT, 60°C) [20] and targeting the aspartokinase gene. Cycling conditions for all PCR reactions were as follows: 10 min at 95°C, 25 cycles of 30 s at 95°C, 90 s at AT (indicated after each primer pair) and 1 min at 72°C, with a final extension step of 10 min at 72°C. The reference strains C. jejuni NCTC11168, C. jejuni 81176, and C. coli LMG6440 were used as positive controls. The C. coli reference strain served as negative control for C. jejuni and the two C. jejuni strains as negative controls for C. coli. In addition a negative control was always present where no template DNA was added. DNA was extracted from Campylobacter isolates harvested from blood agar plates (Columbia agar II containing 8% vol/vol whole horse blood). Bacteria (108 colony-forming units) were dissolved in 500 μL of ddH2O and incubated in a boiling water bath for 10 min. Cell debris were removed by centrifugation at 18,000 g for 2 min. For some isolates, DNA was extracted using the DNeasy Blood & Tissue kit (Qiagen) according to the manufacturer’s instructions.
Susceptibility Testing
The MICs for ciprofloxacin (Bayer HealthCare), doxycycline (Orion Pharma), erythromycin (Amdipharm), gentamicin (Sigma-Aldrich), meropenem (Sandoz), clindamycin (Sigma-Aldrich) and metronidazole (B. Braun Melsungen) were determined by an agar dilution method according to the Clinical and Laboratory Standards Institute (CLSI) guidelines [21]. Mueller-Hinton agar (Oxoid) plates supplemented with defibrinated sheep blood (5%) and incubated for 48 hours at 36°C were used. C. jejuni (American Type Culture Collection [ATCC] 33560), Helicobacter pylori (ATCC 43504) (for metronidazole), and Staphylococcus aureus (ATCC 29213) (for clindamycin) were used as quality control strains. The susceptibility of the isolates for erythromycin (MIC, ≤8 mg/L), ciprofloxacin (MIC, ≤1 mg/L), and doxycycline (MIC, ≤2 mg/L) was interpreted according to the CLSI guidelines [22]. For clindamycin (MIC, ≤2 mg/L), gentamicin (MIC, ≤2 mg/L) and meropenem (MIC, ≤4 mg/L) the susceptibility break points of the National Antimicrobial Resistance Monitoring System (NARMS) were used [23, 24]. For metronidazole, isolates with MICs ≤4 mg/L were considered susceptible, and those with MICs ≥16 mg/L were considered resistant [25].
Review of Patient Data
Medical records concerning the bacteremic episode were reviewed to obtain data on possible underlying diseases, medication, and travel history before the illness, as well as information on clinical picture, laboratory parameters examined, and antimicrobial treatment during the bacteremic episode. Aminoglycosides, carbapenems, fluoroquinolones, macrolides, and tetracyclines were considered appropriate antimicrobial therapy if MICs for the respective isolates indicated susceptibility. Metronidazole and clindamycin were classified as inappropriate therapy irrespective of MICs. Except for empirical antimicrobial treatment, initiated on admission, all antimicrobial therapy administered later at the hospital was regarded as delayed antimicrobial therapy. The Charlson index score was used to categorize comorbid diseases (weighted index of comorbidity; range, 0–6; higher scores indicate more severe underlying diseases) [26]. Mortality data were received from Statistics Finland.
Statistical Analysis
The Mann-Whitney test was used for comparison of continuous variables. The χ2 test and Fisher’s exact test were used for comparison of categorical variables. All tests were 2 sided, and differences were considered statistically significant at P < .05. Analyses were performed with GraphPad Prism software (version 4.03; GraphPad Software).
RESULTS
In the years 1998–2007, there were 35,393 registered Campylobacter infections in Finland, and the annual incidence ranged from 55/100 000 to 78/100 000 [27]. A total of 119 Campylobacter bacteremia cases had been reported during this time (C. jejuni in 86, C. gracilis in 9, C. coli in 6, C. fetus in 4, C. showae in 1, and undetermined Campylobacter species in 13). The ratio between bacteremia and all Campylobacter infections during the study period was 0.3%. A total of 95 Campylobacter bacteremia isolates were received and successfully cultured for further analyses. Four strains that had originally been reported as C. fetus and 1 that had been reported as C. gracilis were excluded, as well as 4 more strains that could not be typed with PCR as either C. jejuni or C. coli. In addition, 5 patients were excluded because no clinical information was found, and 5 patients because of incomplete clinical information (these patients did not receive treatment in the hospital where the blood culture was taken). Included in the final study were 76 patients for whom bacterial isolates were available (C. coli in 3, C. jejuni in 73).
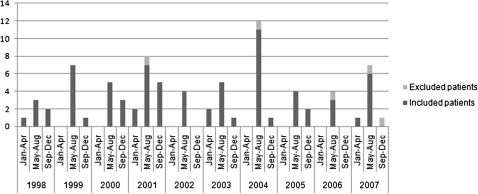
The seasonal variation of C. jejuni and C. coli bacteremia is shown in Figure 1. The annual number of cases ranged from 4 (in 2002 and 2006) to 15 (in 2001), and most cases were reported in May–August. Clinical characteristics and some laboratory test results , grouped by the Charlson index score, are shown in Table 1. A total of 53 patients (70%) had no significant underlying diseases (Charlson index score, 0); only 1 patient was known to have HIV infection. Of the 9 patients with a Charlson index score of 1, 5 had chronic pulmonary diseases, and 4 had either cardiovascular disease, dementia, diabetes or mild liver disease. Included in the group with Charlson index scores ≥2 were 5 patients with malignant diseases, 3 with moderate to severe liver diseases, 1 with renal disease, and 5 with ≥2 significant underlying diseases. None of the severely ill patients (Charlson index score, ≥2) had traveled abroad. The study included 3 children (<18 years of age) and 3 patients >80 years of age.
Figure 1.
Seasonal and annual variation in Campylobacter jejuni and Campylobacter coli bacteremia cases (numbers on left axis) in Finland in 1998–2007; data are shown for included patients (n = 76) and patients excluded because of incomplete clinical information (n = 5).
Table 1.
Characteristics and Laboratory Results for Patients With Campylobacter jejuni or Campylobacter coli Bacteremia Grouped According to Charlson Index Score Classification
| Characteristics | All patients | Charlson index score |
P | ||
| 0 | 1 | ≥2 | |||
| No. (%) of patients | 76 | 53 (70) | 9 (12) | 14 (18) | … |
| Male sex | 56 (74) | 37 (70) | 8 (89) | 11 (79) | NS |
| Age, median (range), years | 46 (1–95) | 41 (2–88) | 54 (40–95) | 57 (1–83) | <.0001,a .0017,b .0035c |
| Foreign travel before bacteremic episoded | 16 (21) | 13 (25) | 3 (33) | 0 | .03e |
| Diarrheaf | 60 (79) | 43 (81) | 8 (89) | 9 (64) | NS |
| Feverg | 64 (84) | 48 (91) | 8 (89) | 8 (57) | .02,a .003c |
| Hospitalization, median (range), days | 4 (0–94) | 3.5 (0–18) | 6 (1–94) | 7.5 (0–45) | .0006,a .042,b .002c |
| ICU treatment | 2 (3) | 1 (2) | 0 | 1 (7) | NS |
| Appropriate empirical antimicrobial treatmenth | 30 (39) | 22 (42) | 4 (44) | 4 (29) | NS |
| Appropriate antimicrobial treatment started in hospitalh | 50 (66) | 32 (60) | 8 (89) | 10 (71) | NS |
| Hemoglobin, initial, g/L, median | 139 | 144 | 143 | 113 | .01,a .0009c |
| Initial leukocyte count, median, cells × 109 | 9.1 | 9.3 | 9 | 7.6 | NS |
| CRP, median, mg/L | |||||
| Initial | 110 | 131 | 97 | 69 | .03a |
| Peak | 152 | 152 | 136 | 160 | NS |
| Mortality, no. of deaths | |||||
| Within 30 days | 2 (3) | 1 (2) | 0 | 1 (7) | NS |
| Within 1 year | 6 (8) | 1 (2) | 1 (11) | 4 (31) | .009,a .006c |
Data represent no. (%) of patients, unless otherwise indicated. Mann-Whitney test was used for comparison of continuous variables, and either the χ2 test or Fisher’s exact test for comparison of categorical variables. Abbreviations: CRP, C-reactive protein; ICU, intensive care unit; NS, not significant (P > .05).
Comparison for Charlson index scores 0 versus ≥1.
Comparison for Charlson index scores 0 versus 1.
Comparison for Charlson index scores 0 versus ≥2.
Patients known to have traveled abroad within 2 weeks before onset of symptoms.
Comparison for Charlson index scores <2 versus ≥2.
Patients known to have had diarrhea, based on hospital treatment records.
Patients known to have had fever (>37.9°C), based on hospital treatment records.
Antimicrobial treatment with aminoglycosides, carbapenems, fluoroquinolones, macrolides, and tetracyclines was considered appropriate based on the susceptibility results (minimum inhibitory concentrations) of the bacterial isolates.
The distributions of MICs for antimicrobial agents are presented in Table 2. The majority of the isolates (82%) were susceptible for all antimicrobials tested. All 5 patients with ciprofloxacin-resistant isolates and 4 of 12 patients with metronidazole-resistant isolates had traveled abroad before the onset of symptoms. Antimicrobial treatment was administered to 91% (69/76) of the patients during hospitalization, and additionally to 4 patients afterward. In total, 96% of patients were treated with antimicrobial drugs, and 39% (30/76) of the patients received appropriate empirical treatment. The variation of hospital stay duration, C-reactive protein levels at discharge from hospital, and mortality data according to antimicrobial therapy are shown in Table 3.
Table 2.
MIC Distribution for 7 Antimicrobials in 76 Isolates of Campylobacter jejuni and Campylobacter coli
| Antimicrobial agent | No. of isolates with given MIC (mg/L) |
|||||||||||||||||
| .002 | .004 | .008 | .015 | .03 | .06 | .125 | .25 | .5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | |
| Ciprofloxacin | 0 | 0 | 0 | 0 | 0 | 2 | 34 | 33 | 2 | 0 | 0 | 1a | 1a | 1a | 1a | 1a | 0 | 0 |
| Clindamycin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 45 | 23 | 6 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Doxycycline | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 52 | 13 | 2 | 0 | 0 | 0 | 1a | 2a | 0 | 0 | 0 |
| Erythromycin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 24 | 39 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gentamicin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 17 | 58 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Meropenem | 1 | 39 | 6 | 23 | 6 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Metronidazole | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 26 | 27 | 5 | 4 | 1a | 1a | 4a | 2a | 4a |
Resistant isolates.
Table 3.
Duration of Hospitalization, C-Reactive Protein (CRP) Level at Discharge from Hospital, and Mortality Data for Patients With Campylobacter jejuni or Campylobacter coli Bacteremia According to Appropriateness of Antimicrobial Therapy During Hospital Treatment Period
| Antimicrobial treatment | Duration of hospitalization, median, days | CRP, mg/La | Deaths within 30 days | Deaths within 1 year |
| 1. Appropriate treatment (n = 50)b | 5 | |||
| A. Empirical (n = 30) | 4 | 35 | 1 | 2 |
| B. Delayed (n = 20) | 6 | 22 | 0 | 3 |
| 2. Inappropriate or no treatment (n = 26) | 3c | 26 | 1 | 1 |
| 3. Delayed, inappropriate, or no treatment (n = 46)d | 4c | 24 | 1 | 4 |
| Pe | .008 (1 vs 2), .03 (1A vs 1B), NS (1A vs 3) | NS | NSf | NSf |
Median levels at hospital discharge, calculated for patients in whom this information was available 87%, 100%, and 77%, respectively, in groups 1A, 1B, and 2.
Appropriateness was defined according to antimicrobial susceptibility results (minimum inhibitory concentrations) of the bacterial isolates for gentamicin, meropenem, ciprofloxacin, erythromycin, and doxycycline.
The exact duration of hospitalization was not known for 1 patient in this group.
Group 3 = Groups 1B and 2 combined.
The Mann-Whitney test was used unless otherwise mentioned. Groups compared are indicated in parentheses; NS, not significant (P > .05).
Fisher’s exact test was used to compare mortality in groups 1 versus 2, 1A versus 1B, and 1A versus 3.
Two patients experienced severe complications attributable to the bacteremic episode while hospitalized, and 2 died within 30 days (Table 4). Thus, the attributable mortality was 3%. Six more patients died within 1 year of admittance to hospital; of these, 4 had severe underlying diseases (malignant diseases in 2, severe kidney disease in 1, and hepatic coma in 1), 1 was 95 years old, and 1 died of traumatic asphyxia. The underlying diseases, duration of hospitalization, antimicrobial treatment, and outcome of disease in the 4 patients with severe complications are described in Table 4. None of the 10 excluded patients died within 30 days after the Campylobacter-positive blood culture.
Table 4.
Serious Complications and Fatal Outcome During Hospitalization Associated With Campylobacter jejuni or Campylobacter coli Bacteremia
| Patient sex (age, years) | Underlying disease (Charlson Index Score); Initial symptoms | Duration of hospitalization, days | Antimicrobial treatment during hospitalization |
Outcome | |
| Empirical | Delayed | ||||
| Male (62) | Hypercholesterolemia, angina pectoris (0); diarrhea, fever | 18 | Metronidazole (MIC, 2 mg/L), roxithromycina (MIC, 1 mg/L for erythromycin) | Metronidazole, roxithromycina | Neurologic symptoms of Guillain-Barré syndrome started 2 weeks after initial symptoms |
| Female (46) | None (0); neck pain, diarrhea, fever | 6 | Cefalexin for 1 day | Azithromycina for 1 day, roxithromycina for up to 2 months (MIC, 2 mg/L for erythromycin) | Spondylodiscitis diagnosed with MR imaging, no bacterial growth in aspirate from surgical drainage |
| b | APECED (0); fever, vomiting, abdominal pain | <1 | Ceftriaxone | None | Necrotic ileus, septic shock, death in hospital |
| Male (22) | Precursor B-cell lymphoblastic leukemia, HBV infection, diabetes (4); fatigue and myalgia | 29 | Amikacina (MIC, 1 mg/L for gentamicin), ceftazidime for 10 days | Amikacina, clarithromycina for 6 days (MIC, 0.5 mg/L for erythromycin), meropenema for 7 days (MIC, 0.008 mg/L) | ARDS, death in hospital |
Abbreviations: APECED, autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy; ARDS, acute respiratory distress syndrome; HBV, hepatitis B virus; MIC, minimum inhibitory concentration. MR, magnetic resonance.
Appropriate antimicrobial treatment.
Information is not shown in order to anonymize the patient, as APECED is an extremely rare disease.
DISCUSSION
In this nationwide retrospective study, we collected C. jejuni and C. coli bacteremia cases from a 10-year-period in Finland. For patients in whom species could be identified and antimicrobial susceptibility of the blood culture isolates could be determined, clinical characteristics were obtained from medical records. Our results suggest that the typical patient with Campylobacter bacteremia is a moderately young and healthy man, who recovers from the bacteremic episode regardless of the timing and appropriateness of antimicrobial treatment. Furthermore, it seems that Campylobacter isolates cultured from blood in Finland are predominantly susceptible to antimicrobial agents and often of domestic origin.
The estimated incidence of Campylobacter bacteremia in relation to enteritis ranges from 0.1% to 1% [7–9], which is in line with the incidence of 0.3% found in the current study. In a large study, 89% of the blood culture isolates were either C. jejuni or C. coli [9]. Similar results were published later [8, 11], although C. fetus was the predominant isolate in a French study [10]. Because molecular typing methods enabling exact identification of isolates to the species level have not usually been used, the distribution of C. jejuni and C. coli in Campylobacter blood culture isolates remains somewhat uncertain. One strength of the present study is that all isolates were verified to the species level by PCR.
Several studies have shown that Campylobacter bacteremia is more common in older patients and in those who are immunocompromised. In particular, patients with HIV infection, malignancies, and liver diseases have been shown to be more prone to the infection [8, 11, 13]. In the present study, patients’ underlying diseases were grouped according to the validated Charlson index score [26]. In contrast to findings in some recent Campylobacter bacteremia studies [8, 10, 11] and an older study [13] but in line with results reported by Skirrow et al [9], we found that the majority of patients (70%) did not have any significant underlying diseases and that the median age for this particular group of patients was as low as 41 years.
Campylobacter infections in Finland show a seasonal peak during summer months [2, 28, 29] and the majority of infections are associated with foreign travel [15, 28, 29]. Although a similar seasonal peak was also evident for Campylobacter bacteremia, only 21% of patients were known to have traveled abroad before the onset of illness. In addition, half of the patients had traveled only in Northern and Central Europe.
Ciprofloxacin resistance is extremely uncommon among domestic Campylobacter isolates in Finland, although isolates obtained from abroad are predominantly resistant [15, 30]. It has been suggested that ciprofloxacin-resistant Campylobacter isolates might cause a more severe disease [31–33], but we have shown opposite results and even reported that isolates highly susceptible to ciprofloxacin (MIC, 0.06–0.25 mg/L) seem to cause a more severe course of disease, characterized by more frequent bloody stools and need for hospitalization [15]. In the current study, 91% of the C. jejuni and C. coli isolates were highly susceptible to ciprofloxacin. It remains to be determined whether this finding merely reflects the high number of domestically obtained infections and closer travel destinations or supports the hypothesis that highly susceptible isolates might cause a more severe disease, such as bacteremia. Furthermore, the present finding that the majority of bacteremia isolates were of domestic origin supports our recent suggestions that Campylobacter isolates acquired in Finland may harbor bacterial factors that lead to a more severe infection [16].
C. jejuni and C. coli infections are typically self-limited, and severe complications are quite rare. Early antimicrobial treatment shortens the duration of diarrhea in enteritis patients, although the effect is marginal [34]. Pacanowski et al reported that failure to administer appropriate antibiotics was associated with fatal outcome in bacteremia caused by Campylobacter species other than C. fetus [10]. However, in a recent Spanish report, mortality was not independently associated with inappropriate empirical therapy [11]. Our results support those of the latter study, because we found no association between inappropriate or delayed appropriate antimicrobial treatment and mortality. Our results could, at least in part, be due to differences in patient characteristics compared with the study by Pacanowski et al [10], including a moderately low median age and the lack of significant underlying diseases in the majority of patients.
The attributable mortality of Campylobacter bacteremia has been estimated to be 4%–16% [8, 10–13]. Our results support those of a recent Danish population-based study [8], because 3% of the patients in the present study died within 30 days of admittance to hospital. The deaths of other patients within 1 year of hospital admission did not seem to be attributable to the bacteremic episode.
Administration of appropriate antimicrobials did not seem to affect the clinical course of disease or duration of hospitalization to any significant extent. Among the patients who had received appropriate antimicrobial treatment while hospitalized, those who had received correct empirical antimicrobial therapy seemed to recover more rapidly. However, there was not a significant difference in the duration of hospitalization between patients with appropriate empirical treatment and those who had received delayed appropriate, inappropriate, or no antimicrobial treatment. Whether clindamycin and metronidazole were classified as appropriate (on the basis of MIC results) or inappropriate (irrespective of MIC results) antimicrobial treatment did not affect the results on the outcome of disease to any greater extent.
We found 2 severe complications during or immediately after bacteremic episodes, Guillain-Barré syndrome and cervical spondylodiscitis in 1 patient each. Although C. coli has been reported to cause spondylodiscitis [35], to the best of our knowledge, this is the first time a patient with C. jejuni bacteremia is reported to have spondylodiscitis. The bacterial aspirate, obtained during surgical drainage of the spinal abscesses, was negative, probably owing to the already initiated appropriate antimicrobial treatment.
The present study is unique in that it is the first nationwide report presenting both clinical characteristics of patients and antimicrobial susceptibility patterns of PCR-verified bacteremia isolates. In Finland, the reporting of bacteremia cases is mandatory. In our study, most of the bacterial isolates had been stored and could be successfully cultivated for further analyses. We also found clinical information for 88% of these patients. This ensures that our material was representative and not biased geographically or with regard to any patient group. Although clinical information was not available for the 10 excluded patients, mortality data was available for all of them. Only 1 of the excluded patients had died within 1 year of the bacteremic episode; thus, the mortality rate did not differ between included and excluded patients. The lack of clinical information suggests that the excluded patients may not have been treated in the hospital, and therefore their possible antimicrobial treatment was not noted in hospital records. It is unlikely that the exclusion of these particular patients had a major impact on the results.
In this nationwide study from a 10-year period, C. jejuni and C. coli bacteremia was uncommon but showed the same seasonal peak as Campylobacter enteritis in Finland. However, in contrast to those with enteritis, patients with bacteremia had predominantly acquired their infection domestically, and bacterial isolates only rarely showed antimicrobial resistance. Furthermore, in contrast to many studies from other countries, the clear majority of patients were moderately young and without significant underlying diseases. Mortality attributable to C. jejuni and C. coli bacteremia was as low as 3%. The outcome of infection was in general good, and antimicrobial treatment did not seem to affect it to any greater extent. Severe complications included single cases of Guillain-Barré syndrome and spondylodiscitis.
Acknowledgments
Acknowledgments. The skilled technical assistance of M. Haverinen and A. Nilsson is gratefully acknowledged. We are sincerely thankful to all clinical microbiology laboratories in Finland for sending us the bacterial isolates.
Financial support. This work was supported by the Academy of Finland (ELVIRA grant to B. F. and H. R.) and the Emil and Ragna Börjesson memorial fund (fellowship grant to P. E.).
Potential conflicts of interest. All authors:No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Blaser MJ. Epidemiologic and clinical features of Campylobacter jejuni infections. J Infect Dis. 1997;176:S103–5. doi: 10.1086/513780. [DOI] [PubMed] [Google Scholar]
- 2.Rautelin H, Hänninen ML. Campylobacters: the most common bacterial enteropathogens in the Nordic countries. Ann Med. 2000;32:440–5. doi: 10.3109/07853890009002018. [DOI] [PubMed] [Google Scholar]
- 3.European Food Safety Authority. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2009. Available at: http://www.efsa.europa.eu/en/efsajournal/pub/2090.htm. Accessed 28 March 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hannu T, Mattila L, Rautelin H, et al. Campylobacter-triggered reactive arthritis: a population-based study. Rheumatology. 2002;41:312–8. doi: 10.1093/rheumatology/41.3.312. [DOI] [PubMed] [Google Scholar]
- 5.McCarthy N, Giesecke J. Incidence of Guillain-Barré syndrome following infection with Campylobacter jejuni. Am J Epidemiol. 2001;153:610–4. doi: 10.1093/aje/153.6.610. [DOI] [PubMed] [Google Scholar]
- 6.Tam CC, Rodrigues LC, Petersen I, Islam A, Hayward A, O'Brien SJ. Incidence of Guillain-Barré syndrome among patients with Campylobacter infection: a general practice research database study. J Infect Dis. 2006;194:95–7. doi: 10.1086/504294. [DOI] [PubMed] [Google Scholar]
- 7.Samuel MC, Vugia DJ, Shallow S, et al. Epidemiology of sporadic Campylobacter infection in the United States and declining trend in incidence, FoodNet 1996–1999. Clin Infect Dis. 2004;38:S165–74. doi: 10.1086/381583. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen H, Hansen KK, Gradel KO, et al. Bacteraemia as a result of Campylobacter species: a population-based study of epidemiology and clinical risk factors. Clin Microbiol Infect. 2010;16:57–61. doi: 10.1111/j.1469-0691.2009.02900.x. [DOI] [PubMed] [Google Scholar]
- 9.Skirrow MB, Jones DM, Sutcliffe E, Benjamin J. Campylobacter bacteraemia in England and Wales, 1981–91. Epidemiol Infect. 1993;110:567–73. doi: 10.1017/s0950268800050986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pacanowski J, Lalande V, Lacombe K, et al. Campylobacter bacteremia: clinical features and factors associated with fatal outcome. Clin Infect Dis. 2008;47:790–6. doi: 10.1086/591530. [DOI] [PubMed] [Google Scholar]
- 11.Fernández-Cruz A, Munoz P, Mohedano R, et al. Campylobacter bacteremia: clinical characteristics, incidence, and outcome over 23 years. Medicine. 2010;89:319–30. doi: 10.1097/MD.0b013e3181f2638d. [DOI] [PubMed] [Google Scholar]
- 12.Reed RP, Friedland IR, Wegerhoff FO, Khoosal M. Campylobacter bacteremia in children. Pediatr Infect Dis J. 1996;15:345–8. doi: 10.1097/00006454-199604000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Pigrau C, Bartolome R, Almirante B, Planes AM, Gavalda J, Pahissa A. Bacteremia due to Campylobacter species: clinical findings and antimicrobial susceptibility patterns. Clin Infect Dis. 1997;25:1414–20. doi: 10.1086/516127. [DOI] [PubMed] [Google Scholar]
- 14.Gazaigne L, Legrand P, Renaud B. Campylobacter fetus bloodstream infection: risk factors and clinical features. Eur J Clin Microbiol Infect Dis. 2008;27:185–9. doi: 10.1007/s10096-007-0415-0. [DOI] [PubMed] [Google Scholar]
- 15.Feodoroff FB, Lauhio AR, Sarna SJ, Hänninen ML, Rautelin HI. Severe diarrhoea caused by highly ciprofloxacin-susceptible Campylobacter isolates. Clin Microbiol Infect. 2009;15:188–92. doi: 10.1111/j.1469-0691.2008.02657.x. [DOI] [PubMed] [Google Scholar]
- 16.Feodoroff B, Ellström P, Hyytiäinen H, Sarna S, Hänninen ML, Rautelin H. Campylobacter jejuni isolates in Finnish patients differ according to the origin of infection. Gut Pathog. 2010;2:22. doi: 10.1186/1757-4749-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linton D, Owen RJ, Stanley J. Rapid identification by PCR of the genus Campylobacter and of five Campylobacter species enteropathogenic for man and animals. Res Microbiol. 1996;147:707–18. doi: 10.1016/s0923-2508(97)85118-2. [DOI] [PubMed] [Google Scholar]
- 18.Denis M, Soumet C, Rivoal K, et al. Development of a m-PCR assay for simultaneous identification of Campylobacter jejuni and C. coli. Lett Appl Microbiol. 1999;29:406–10. doi: 10.1046/j.1472-765x.1999.00658.x. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez I, Grant KA, Richardson PT, Park SF, Collins MD. Specific identification of the enteropathogens Campylobacter jejuni and Campylobacter coli by using a PCR test based on the ceuE gene encoding a putative virulence determinant. J Clin Microbiol. 1997;35:759–63. doi: 10.1128/jcm.35.3.759-763.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linton D, Lawson AJ, Owen RJ, Stanley J. PCR detection, identification to species level, and fingerprinting of Campylobacter jejuni and Campylobacter coli direct from diarrheic samples. J Clin Microbiol. 1997;35:2568–72. doi: 10.1128/jcm.35.10.2568-2572.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clinical and Laboratory Standards Institute/NCCLS. Performance for antimicrobial susceptibility testing: fifteenth informational supplement. M100–S15. Wayne, PA: Clinical and Laboratory Standards Institute; 2005. [Google Scholar]
- 22.Clinical and Laboratory Standards Institute. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria. Approved standard. M45-A. Wayne, PA: Clinical and Laboratory Standards Institute; 2006. [DOI] [PubMed] [Google Scholar]
- 23.National Antimicrobial Resistance Monitoring System. NARMS 2006 executive report. Available at: http://www.fda.gov/AnimalVeterinary/SafetyHealth/AntimicrobialResistance/NationalAntimicrobialResistanceMonitoringSystem/ucm182896.htm. Accessed 14 April 2011. [Google Scholar]
- 24.National Antimicrobial Resistance Monitoring System. 2002. NARMS retail meat annual report. Available at: http://www.fda.gov/AnimalVeterinary/SafetyHealth/AntimicrobialResistance/NationalAntimicrobialResistanceMonitoringSystem/ucm091417.htm. Accessed 14 April 2011. [Google Scholar]
- 25.Hariharan H, Sharma S, Chikweto A, Matthew V, DeAllie C. Antimicrobial drug resistance as determined by the E-test in Campylobacter jejuni, C. coli, and C. lari isolates from the ceca of broiler and layer chickens in Grenada. Comp Immunol Microbiol Infect Dis. 2009;32:21–8. doi: 10.1016/j.cimid.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 27.National Public Health Institute. National infectious diseases register. Available at: http://www3.ktl.fi/stat/. Accessed 5 April 2011. [Google Scholar]
- 28.National Institute for Health and Welfare. Infectious diseases in Finland 1995–2009. Available at: http://www.thl.fi/thl-client/pdfs/d6d63c66-9690-4f4d-9ee1-319bb5648eaf. Accessed 15 April 2011. [Google Scholar]
- 29.Nakari UM, Huovinen E, Kuusi M, Siitonen A. Population-based surveillance study of Campylobacter infections in Finland. Epidemiol Infect. 2010;138:1712–8. doi: 10.1017/S0950268810000567. [DOI] [PubMed] [Google Scholar]
- 30.Schönberg-Norio D, Hänninen ML, Katila ML, et al. Activities of telithromycin, erythromycin, fluoroquinolones, and doxycycline against Campylobacter strains isolated from Finnish subjects. Antimicrob Agents Chemoter. 2006;50:1086–8. doi: 10.1128/AAC.50.3.1086-1088.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engberg J, Neimann J, Nielsen EM, Aerestrup FM, Fussing V. Quinolone-resistant Campylobacter infections: risk factors and clinical consequences. Emerg Infect Dis. 2004;10:1056–63. doi: 10.3201/eid1006.030669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson JM, Smith KE, Vugia DJ. Prolonged diarrhea due to ciprofloxacin-resistant Campylobacter infection. J Infect Dis. 2004;190:1150–7. doi: 10.1086/423282. [DOI] [PubMed] [Google Scholar]
- 33.Helms M, Simonsen J, Olsen KE, Molbak K. Adverse health events associated with antimicrobial drug resistance in Campylobacter species: a registry-based cohort study. J Infect Dis. 2005;191:1050–5. doi: 10.1086/428453. [DOI] [PubMed] [Google Scholar]
- 34.Ternhag A, Asikainen T, Giesecke J, Ekdahl K. A meta-analysis on the effects of antibiotic treatment on duration of symptoms caused by infection with Campylobacter species. Clin Infect Dis. 2007;44:696–700. doi: 10.1086/509924. [DOI] [PubMed] [Google Scholar]
- 35.Lemaire X, Dehecq C, Cattoen C. Spondylodiscitis and an aortic aneurysm due to Campylobacter coli. Ann Clin Microbiol Antimicrob. 2010;9:8. doi: 10.1186/1476-0711-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]