Clinicians are struggling with screening issues related to cognitive impairment given new information noting impairment in about 1/2 of community dwelling human immunodeficiency virus-positive patients. This impairment may impact daily functioning and medication adherence. Unfortunately, major limitations exist in current screening tools.
Abstract
Recent publications estimate the prevalence of human immunodeficiency virus (HIV)–associated neurocognitive disorders (HAND) exceeds 50%, and this rate is likely higher among older patients. Cognitive impairment may impact medication adherence, and symptomatic impairment has been linked to all-cause mortality providing some impetus for early detection. There are currently insufficient data to inform solid recommendations on screening methods. Most HIV-specific tools have poor performance characteristics for all but the most severe form of impairment, which accounts for <5% of cases. Reliance on symptoms is likely to miss a substantial proportion of individuals with HAND due to poor insight, confounding mood disturbances, and lack of well-informed proxies. In the aging HIV-positive population, broader screening tools may be required to allow sensitivity for both HIV and neurodegenerative disorders. We describe the clinical presentation of HAND, review existing data related to screening tools, and provide preliminary and practical recommendations in the absence of more definitive studies.
THE SCOPE OF COGNITIVE DISORDERS IN THE ERA OF COMBINATION ANTIRETROVIRAL THERAPY
Human immunodeficiency virus (HIV) is neurovirulent. Prior to widespread availability of combination antiretroviral therapy (cART), the prevalence of HIV-associated dementia was estimated to be between 6 and 30% among patients with AIDS. With treatment, the frequency of dementia is attenuated, but milder forms of impairment remain highly prevalent and increase with age [1]. Less severe impairment correlates to measurable abnormalities on tasks associated with daily functioning, thus deeming them important clinical syndromes [2]. The presence of symptomatic cognitive impairment predicts non–central nervous system (non-CNS) morbidity and overall HIV mortality [3].
The current diagnostic categories of HAND are designed for research settings and emphasize performance on comprehensive neuropsychological testing batteries [4]. The most severe form of impairment is HIV-associated dementia (HAD), requiring test performance >2 standard deviations below average in 2 cognitive domains (eg, memory and executive function) and marked evidence of impaired daily function. Mild neurocognitive disorder (MND) similarly requires confirmation of impact on daily activities, but to a lesser degree, and involves less-severe neuropsychological impairment. A third diagnostic category, asymptomatic neurocognitive impairment (ANI), is used for participants with impaired neuropsychological performance in the absence of identifiable functional deficits. Such cases are frequent in research settings. A recent survey of 1555 community-dwelling HIV-positive participants in the United States reported HAD frequency at about 2%, but identified non-HAD impairment in 45% of participants who lacked major confounding factors (12% MND and 33% ANI) [1]. Thus, among impaired participants, most were asymptomatic. Using impairment ratings rather than diagnostic categories, 1 group [5] noted no change in the frequency of impairment when comparing pre- and post-cART eras. In short, the frequency of severe impairment has decreased with cART, but the frequency of all severities of cognitive impairment appears to be unchanged. Cognitive impairment is often present among individuals who do not endorse functional symptoms when asked.
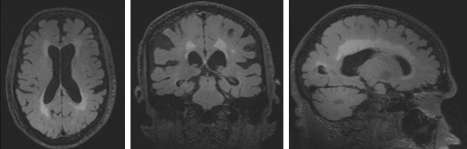
Asymptomatic neuropsychological testing abnormalities may not represent disease in all participants, and there is a substantial lack of clarity regarding the long-term clinical implications of ANI. The frequency of neuropsychological abnormalities among ANI participants exceeds the variability inherent in neuropsychological testing, and studies demonstrating everyday functional abnormalities that correlate to testing impairment do not require symptoms [2]. These testing abnormalities may sometimes be severe and occasionally correlate to MRI changes (Figure 1). Together, this information suggests that ANI does not represent disease-free baseline performance for many individuals. The designation of “asymptomatic” may more accurately reflect our inability to document impaired function, possibly related to lack of insight, frontal lobe processing deficits, limited daily activity (floor effect), or poor objective proxy information. Undetected cognitive impairment may increase the risk of poor adherence, lead to financial errors, or result in other adverse outcomes [2, 6]. Because functional impairment is the cornerstone of diagnosing dementia, optimal assessments may require objective evaluation of everyday function.
Figure 1.
Brain magnetic resonance imaging (MRI) of an asymptomatic 79-year-old human immunodeficiency virus (HIV)–positive patient with extensive neuropsychological testing abnormalities. Images demonstrate global brain atrophy and diffuse white matter injury.
AGING AND COGNITIVE DISORDERS IN HIV
Nearly all studies investigating the effects of age on the frequency of HAND identify increased risk, although data are mixed when considering only neuropsychological testing performance [7]. Few studies include sufficient numbers of cases over age 60 and none have systematically addressed potential non-HIV etiologies such as Alzheimer’s disease (AD). In older participants, comorbidities such as cumulative cerebrovascular disease may have significant contributions.
The risk for acceleration of neurodegeneration in HIV has been raised, though the issue currently remains largely theoretical. Proteins associated with age-related neurodegenerative disorders, such as ubiquitin, amyloid, and tau, have been noted in neuropathological tissues with some studies noting a higher frequency of these proteins in the era of cART [8]. Prior to cART, pathologists noted accumulation of amyloid protein in diffuse but nonneuritic plaques, with neuritic plaques being the hallmark of AD [9]. A report using an amyloid-specific positron emission tomography (PET) ligand, Pittsburgh compound B (PIB), in younger, healthy and nonimpaired HIV-positive participants failed to identify a pattern of uptake that would be typical for AD [10]. The PIB compound identifies only neuritic and not diffuse plaques, and this work must be extended to a population of older participants where sufficient baseline risk for AD exists. Ultimately, longitudinal evaluations may be essential, because HAND is more likely to present as static impairment with some fluctuation rather than the relentless insidious deterioration that is the cornerstone of neurodegenerative disorders [4].
CLINICAL CHARACTERISTICS OF HIV-ASSOCIATED DEMENTIA—IMPACT ON SCREENING TOOLS
HIV enters the central nervous system (CNS) during acute infection; however, neurons remain largely uninfected and the primary mechanism of brain injury relates to extensive inflammation leading to neuronal dysfunction and synaptodendritic injury [8]. Early studies identified HIV staining concentrated in subcortical and deep grey matter structures, an anatomy that informs the clinical presentation of HAND, with motor, behavioral, and predominantly subcortical cognitive features [11]. Early neuropsychological screening batteries were designed with a focus on these subcortical features, targeting psychomotor speed and information processing as well as executive functioning and memory. The clinical–anatomic correlations are further bolstered by findings associating cognitive impairment and apathy to deep grey matter structures, principally the caudate nucleus and the nucleus accumbens, respectively. Extrapyramidal motor features are often observed and increase with age [12]. Although memory deficits occur, the pattern typically involves inefficient learning and problems with executive functioning and retrieval rather than pure difficulty in formulating a memory itself (encoding deficits) [13]. These features contrast sharply with the cortical deficits typical of AD where encoding of new memories is common. Screening instruments intended for older HIV-positive populations will need to consider both presentations. The motor deficits observed in HIV are generally not seen in early stage AD but may be seen in Parkinson disease–spectrum disorders. The added increased frequency of cerebrovascular comorbidity in HIV may further complicate the clinical scenario.
SCREENING RECOMMENDATIONS FOR DEMENTIA IN HIV-NEGATIVE POPULATIONS
Most agencies provide mixed or limited support for routine screening for dementia in HIV-negative populations, despite knowledge that dementia remains frequently underdiagnosed [14]. Citing limited outcome data for clinical intervention, effects related to stigma associated with cognitive disease, and lack of specificity for existing screening instruments, the most recent (2003) United States Preventative Service Task Force (USPSTF) guidelines concluded that there is insufficient evidence to recommend for or against routine screening in the absence of known impairments [15]. The recommendations suggest screening for patients who exhibit functional changes. The American Academy of Neurology recommends increased screening for patients with mild cognitive impairment (MCI) due to heightened risk for progression to dementia; however, for asymptomatic elders, it concurs with the USPSTF [16]. Past recommendations from the American Geriatric Society, the American Medical Association, and the American Academy of Family Physicians similarly focus on symptomatic disease rather than screening asymptomatic participants [17].
HIV DEMENTIA SCREENING TOOLS
The most commonly referenced HIV screening tool is the HIV Dementia Scale (HDS) [18]. This tool provides a rapid assessment of eye movements, motor skills, simple learning, and attention. The HDS performance characteristics are modest with acceptable sensitivity only for the most severe disease. For example, among hospitalized patients, 12% of whom met clinical criteria for HAD, the sensitivity at a cut-point of 10 (out of 16) was 0.92 and the specificity was 0.71, resulting in a positive predictive value (PPV) of 0.3 and a negative predictive value (NPV) of 0.98 [19]. The performance characteristics are much worse for detecting milder impairment [20, 21]. A study of HIV-positive individuals interested in returning to work noted sensitivity and specificity for all impairment to be only 39% and 85%, respectively [22].
Adjusting for age and education substantially improves performance but simultaneously complicates interpretation for primary care settings. Even with adjustment, the HDS demonstrates only modest ability to identify impairment (70% sensitivity) [23]. A modified version (mHDS) excludes the antisaccade eye movement task, which may be the most sensitive aspect of the HDS. The mHDS was no better than a simple test of psychomotor speed and manual dexterity (the grooved pegboard test) in identifying dementia [24]. Substantial educational attainment effects have also been noted [25]. Taken together, these data are concerning that the HDS is suboptimal for most HAND patients given that HAD constitutes <5% of impaired cases among community-dwelling adults.
Reliance on patient self-report of symptoms is not ideal. Studies have revealed limited correlation between self-reported memory impairments and performance on objective neuropsychological tests, and tighter association with depressive symptoms. The Patient Assessment of Own Functioning (PAOF), for example, identified depressive symptoms and psychomotor inefficiency rather than neuropsychological testing performance, and when applied to HIV-negative substance abusers, there was no correlation to neuropsychological testing performance [26, 27]. Importantly, screening for symptoms would not identify ANI, the largest subset of impaired participants. It is possible that, combined with a clinical risk identification schema that includes risk factors such as CD4 nadir count, plasma viral load, and age, these metrics could be improved, given reports that such clinical variables may have some utility [28].
Computer-delivered cognitive assessments have a theoretical benefit in that they alleviate the time burden on clinical staff. In the pre-cART era, the Sequential and Choice Reaction Time Program (CALCAP), a measure of reaction time, attention, psychomotor speed, and memory, identified advanced disease but not milder disease [29, 30]. More recently, the CogState was evaluated and similarly performed well in identifying advanced dementia [31]. A pilot study revealed some promise in using the Computer-based Assessment of Mild Cognitive Impairment (CAMCI); however, the study design and size limited interpretation for the clinical setting [32]. In general, computer approaches have several important limitations including an inability to test verbal learning efficiency, complicated outputs requiring interpretation, cost burdens, limitations in the face of literacy and non-English speakers, and a need to train staff.
Understanding which subtests within comprehensive batteries best correlate to cognitive impairment could provide pivotal information for screening tool development. A domestic study noted that a combination of tests tapping verbal memory (Hopkins Verbal Learning Test—revised total recall) and psychomotor speed (Grooved Pegboard or digit symbol modalities) emerged as best predictors of rater-determined impairment [33]. The combined tests outperformed the HDS. A second international trial noted that verbal learning efficiency (WHO-UCLA auditory verbal learning sum of trials 1–5), psychomotor speed (digit symbol modalities test) and motor speed (timed gait) together best distinguished HAD from non-HAD cases among cART-naive advanced HIV-positive participants [34]. However, the time needed to perform the list-learning task (verbal learning efficiency) in each study effectively precludes its use in simple screening instruments. A shorter battery that was limited to tests of psychomotor speed (digit symbol, Trails A) and cognitive flexibility (Trails B) generally performed worse in accurately categorizing impairment (60% of cases when less stringent cut-points were used) [35].
There are considerable differences between screening tools for HIV and those with utility for other disorders encountered in older age. The most widely used screening tool for AD, the Mini Mental State Exam (MMSE), does not test executive function or motor skill, rendering it less sensitive to subcortical neuropathology, and limited investigations confirm poor sensitivity to HAND [36, 37]. Among the first 75 cases enrolled into the UCSF Memory and Aging HIV Cohort (all with age >60 years), the MMSE did not appear to provide sufficient variability by cognitive group to help diagnostically [mean score out of 30 possible points (sample size, standard deviation) were as follows: normal cognition: 29.3 (37, 0.75); ANI: 28.7 (13, 1.49); MND: 28.3 (20, 1.26) and HAD: 23 (4, 11.37) (unpublished data). Notably, the HAD group included 1 patient thought to have comorbid advanced AD and an MMSE score of 6]. Overall, the narrow distribution of these scores demonstrates poor likelihood of utility even in aged HIV-positive participants.
In summary, studies related to screening have broad limitations, are often underpowered, and attempt to identify only the most severe form of impairment (Table 1). Nearly all have been completed in younger populations; it is anticipated that the performance characteristics would be even less favorable in older participants. There are insufficient data to make firm recommendation on optimal screening tools, but it appears clear that the MMSE is not a good choice.
Table 1.
Screening Tools for Human Immunodeficiency Virus–Associated Neurocognitive Disorders
| Description | Benefits | Limitations | Recommendation | Estimated time needed to complete | |
| HIV dementia scale (HDS) [Powers, JAIDS 1995] |
5-item set of tests completed by clinician include a memory task, motor speed task, cube drawing, and evaluation of eye movements | Validated in HIV, quick to perform | Requires training; questionable consistency for saccadic eye movement portion of evaluation; less sensitive for non–HIV related impairment/neurodegenerative disorders and all but the most severe form of HAND | Prefer use of modified HDS or international HDS over HDS given high proportion of non-Caucasians in US HIV epidemic and potential challenges with consistent interpretation of eye movements | 10 minutes |
| Modified HIV dementia scale (mHDS) [Davis AIDS Reader, 2002] |
HDS with eye movement portion removed | Validated in HIV, quick to perform | Less sensitive for non–HIV related impairment/neurodegenerative disorders and all but the most severe form of HAND | Reasonable to consider in younger age (<65 years) recognizing limitations in all but severe disease | 5 minutes |
| International HIV dementia scale (iHDS) [Sacktor AIDS, 2005] |
4-item set of tests completed by clinician include memory task, finger tapping, a sequential motor task (Luria Sequence) and recall | Validated in HIV (internationally), quick to perform | Requires less training than saccadic eye movements, may not be sensitive to non–HIV related impairment/neurodegenerative disorders | Reasonable to consider in younger age (<65 years) recognizing limitations in all but severe disease, may have less cultural influence than mHDS in US setting | 5 minutes |
| Mini mental state exam (MMSE) [Folstein J Psychiatr Res, 1975] |
30-item test heavily weighted on orientation (10 items) rather than psychomotor speed | Familiar to many clinicians | Copyright protected, not sensitive to HIV-related injury | Not recommended as not likely to identify HIV-related impairment | 10 minutes |
| Assessment of symptoms using standardized questions such as the Medical Outcomes Survey | Subjective reporting of cognitive symptoms | Easy to perform, can be done by patients in waiting room | Only identifies symptomatic disease (likely to miss most participants with impaired testing performance); not sensitive for neuropsychological testing impairment | May be useful in concert with objective screening instruments or considered as an initial screen with follow-up testing (will not identify ANI) | Variable |
| Neuropsychological testing | Tailored set of tests that can test a broad area of cognitive domains | Comprehensive assessment of function, likely most sensitive to cognitive impairment | Impractical for primary care setting due to time needed to perform testing and specialized training to interpret | Best reserved for referral of concerned cases and research | 1–4 hours |
| Montreal cognitive assessment (MoCA) [Nasreddine JAGS, 2005] |
30-item test that taps multiple domains subserved by cortical and subcortical regions | May have broader applicability to milder impairment in the era of cART and to heterogeneity of disease potentially seen in elder HIV patients; free, available online and translated into multiple languages | Not validated in HIV; initial pilot study with less than optimal performance characteristics | Reasonable choice but more work is needed | 10 minutes |
| Computerized assessments | Several available and test multiple domains including attention, reaction time, memory and psychomotor speed | Can be done in doctor’s office with little supervision, time-saving comprehensive evaluation than simple screening instruments | Often costly, limitations in assessing learning efficiency; sometimes require trained interpretation | More work is needed before recommendations can be made | Variable |
Summary of neuropsychological screening tools and their sensitivity to detection of cognitive impairment in HIV.
Abbreviations: HAND, human immunodeficiency virus--associated neurocognitive disorders; HIV, human immunodeficiency virus.
SHOULD WE SCREEN FOR COGNITIVE IMPAIRMENT AND IF SO, HOW?
We could find no published dementia screening guidelines in the setting of HIV. The American Academy of HIV Medicine recently convened a panel to address clinical recommendations for aged HIV-positive participants. These recommendations, still unpublished, will likely focus on symptomatic cases (personal communication as a panel member). It is critical that practical recommendations consider competing priorities in complex HIV care settings. The use of comprehensive neuropsychological batteries is not feasible for screening. The USPSTF compiled a series of key questions to consider in developing cognitive screening recommendations, considered in the context of a predominantly HIV-negative population (Table 2). Although these may serve as a template for future studies, because most of these questions are inadequately addressed in HIV, recommendations must necessarily be based on empiric evidence and expert opinion.
Table 2.
Questions That May Inform Dementia Screening in Aged Human Immunodeficiency Virus (HIV) Participants (Adapted From United States Preventative Service Task Force [USPSTF] Evidence Review for Dementia Screening in HIV-Negative Adults)
| 1) Does screening for cognitive impairment in primary care settings affect clinical outcomes? |
| 2) What is the prevalence of undiagnosed cognitive impairment in primary care patients? |
| 3) Does a reliable and valid screening test exist to detect cognitive impairment in older HIV patients? |
| 4) Do pharmacological or non-pharmacological interventions, including care-giver interventions improve outcomes? |
| 5) What are the adverse effects of screening for cognitive impairment? |
| 6) What are the costs and cost-effectiveness of screening for cognitive impairment? |
| 7) What are the side effects of treatment? |
Abbreviations: HAND, HIV-associated neurocognitive disorders; ANI, asymptomatic neurocognitive impairment.
Prevalence estimates of cognitive impairment differ substantially among studies owing to differing methodological approaches and study populations. More impairment is typically noted in studies with more untreated participants, persons with AIDS, or older participants. Choice of normative data may also influence prevalence estimates. When compared with coenrolled risk and demographically matched HIV-negative groups, the differences in neuropsychological testing performance associated with HIV appear less than would be anticipated in comparison with studies that utilize published normative data. This suggests that some of the impairment noted in HIV-positive populations is influenced by other coexisting factors that are absent in published control data sets. In the current era, the prevalence of cognitive impairment in HIV infection appears to be high, estimated at 46% in cases without severe confounding factors when published controls are used for comparison [1]. Among these impaired participants, most are asymptomatic (meet ANI criteria). Neuropsychological impairment has been linked to medication adherence; however, there are no studies demonstrating that early identification will impact adherence. Based on our knowledge from HIV-negative populations, other potential benefits of early recognition may ensue, including protection from unsafe situations and financial abuse, and increased quality of life through information sharing and improved life planning [38]. Generally, providing information to patients is an important aspect of clinical care and serves as a mechanism to develop or implement compensatory strategies to maintain independence.
Medication treatment strategies for impairment have been disappointing. The potential benefits of CNS-penetrant antiretroviral drugs in the setting of long-term plasma suppression of virus are unclear despite published case series where intervention to change antiretroviral choice is required [39]. Should clear data emerge recommending specific treatment strategies, or should adjuvant treatments prove to be efficacious, the need for early identification would be greater. Identification of cognitive impairment could provide a stimulus for starting cART in ART-naive patients, or signal the need for intensified adherence counseling in those patients already receiving treatment. Among impaired participants with comorbid conditions that may lead to cognitive impairment (eg, obstructive sleep apnea, coexisting neurovirulent infections such as syphilis, vitamin deficiencies, and metabolic derangements), it is reasonable to believe that treatment may improve cognition. Counseling to avoid psychoactive medications and illicit drugs may also be advantageous. In summary, it is likely that identifying impairment may impact treatments aimed at improving quality of life, but intervention trials are lacking and this effort must be considered within the context of competing priorities for primary care settings. Given the frequency of cognitive impairment in the HIV-positive population, screening is likely to identify far more truly impaired cases than false-positive cases.
GENERAL RECOMMENDATIONS AND CONCLUSIONS
At this point, only general recommendations can be made regarding appropriate screening tools for HAND. Both HIV and cerebrovascular disease–specific pathology would be expected to demonstrate preferential subcortical involvement, thereby negating the utility of screening measures that do not adequately tap cognitive domains and effectively excluding use of the MMSE as a screening tool. Currently available HIV-targeted screening instruments, such as the HDS, have marked limitations in that they are sensitive only for the most severe forms of impairment.
Screening tools that tap both cortical and subcortical processes exist, but have not been broadly used in HIV. Among such tools is the Montreal Cognitive Assessment (MoCA), which is free (http://www.mocatest.org/) and translated (but not necessarily validated) in many languages. The MoCA provides some coverage of executive function, motor skill, language fluency, and verbal learning. To date, the MoCA has been tested in a pilot study of 119 relatively young (mean age 43 years) participants identifying 45 of 78 impaired participants (59%) at a cut-point of ≤25 (PPV: 85%, NPV: 53%). Shifting the cut point to 27 increased sensitivity but overall performance characteristics were not reported [40]. A recent publication identified that the MoCA may benefit from modifications designed to increase difficulty of some items for application in typical HIV clinics [41]. Other tests that combine list-learning tasks with tasks of cognitive flexibility and psychomotor speed may be superior. Individuals with symptoms may require more in-depth neuropsychological testing if screening is negative, because false-negative cases occur with all screening tests. This should be combined with a screen for depression (eg, Back Depression Scale-II). These can be completed in about 10 minutes. Use of only symptom-based screening tools is likely to miss >50% of impaired cases. The addition of a neurological examination focused on motor speed, tone, and reflexes and integration with clinical variables (eg, CD4 nadir, viral load) may add specificity.
Overall, there are important considerations for early detection of cognitive impairment, particularly among older HIV-positive patients. Unlike the general healthy population, older HIV-positive patients are managing a complex medical condition that disrupts CNS integrity and cognitive function and may be life threatening if not adequately controlled through medical intervention. With HIV, treatment failure and broad antiretroviral medication resistance are potential outcomes of cognitive impairment. As such, unlike in the healthy older population, failure to identify cognitive deficits in the HIV-positive population may directly influence successful management of the disease. In balance, there are important clinical needs that can be addressed by research aimed at demonstrating whether improved detection results in improved outcomes.
Acknowledgments
Acknowledgments. We thank Zhaoyan Diao for assistance with statistical analyses.
Financial support. This work supported by the National Institutes of Health (grant numbers K23-AG032872 to V. V., P50-AG023501 to B. M., R01-MH085604 to R. P.).
Potential conflicts of interest. V. V. serves as a consultant for the American Academy of HIV Medicine and a faculty member for the International Antiviral Society-USA. B. M. has received grant support from Novartis, is a consultant for TauRx and Allon Therapeutics and is a board member on the John Douglas French Alzheimer’s Foundation and the Larry L. Hillblom Foundation. All other authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Heaton RK, Clifford DB, Franklin DR, Jr, et al. CHARTER Group. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–96. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heaton RK, Marcotte TD, Mindt MR, et al. HNRC Group. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10:317–31. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- 3.Vivithanaporn P, Heo G, Gamble J, et al. Neurologic disease burden in treated HIV/AIDS predicts survival: A population-based study. Neurology. 2010;75:1150–8. doi: 10.1212/WNL.0b013e3181f4d5bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thames AD, Kim MS, Becker BW, et al. Medication and finance management among HIV-infected adults: The impact of age and cognition. J Clin Exp Neuropsychol. 2011;33:200–9. doi: 10.1080/13803395.2010.499357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valcour V, McMurtray A. Interactions betweeen advanced age and HIV cognitive impairment. In: Paul R, Sacktor N, Valcour V, Tashima K, editors. HIV and the brain: New challenges in the modern era. Towota, NJ: Humana Press; 2008. [Google Scholar]
- 8.Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: Neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- 9.Esiri MM, Biddolph SC, Morris CS. Prevalence of Alzheimer plaques in AIDS. J Neurol Neurosurg Psychiatry. 1998;65:29–33. doi: 10.1136/jnnp.65.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ances BM, Christensen JJ, Teshome M, et al. Cognitively unimpaired HIV-positive subjects do not have increased 11C-PiB: A case-control study. Neurology. 2010;75:111–5. doi: 10.1212/WNL.0b013e3181e7b66e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray F, Adle-Biassette H, Chretien F, Lorin de la Grandmaison G, Force G, Keohane C. Neuropathology and neurodegeneration in human immunodeficiency virus infection. Pathogenesis of HIV-induced lesions of the brain, correlations with HIV-associated disorders and modifications according to treatments. Clin Neuropathol. 2001;20:146–55. [PubMed] [Google Scholar]
- 12.Valcour V, Watters MR, Williams AE, Sacktor N, McMurtray A, Shikuma C. Aging exacerbates extrapyramidal motor signs in the era of highly active antiretroviral therapy. J Neurovirol. 2008;5:362–7. doi: 10.1080/13550280802216494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woods SP, Scott JC, Dawson MS, et al. Construct validity of Hopkins Verbal Learning Test—Revised component process measures in an HIV-1 sample. Arch Clin Neuropsychol. 2005;20:1061–71. doi: 10.1016/j.acn.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Borson S, Scanlan JM, Watanabe J, Tu SP, Lessig M. Improving identification of cognitive impairment in primary care. Int J Geriatr Psychiatry. 2006;21:349–55. doi: 10.1002/gps.1470. [DOI] [PubMed] [Google Scholar]
- 15.U.S. Preventive Services Task Force. Screening for dementia: recommendation and rationale. Ann Intern Med. 2003;138:925–6. doi: 10.7326/0003-4819-138-11-200306030-00014. [DOI] [PubMed] [Google Scholar]
- 16.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: Early detection of dementia: Mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133–42. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 17.American Medical Association. Practical guide for the primary care physician on the diagnosis, management and treatment of dementia. Chicago, IL: Program on Aging and Community Health; 2001. [Google Scholar]
- 18.Power C, Selnes OA, Grim JA, McArthur JC. HIV Dementia Scale: A rapid screening test. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:273–8. doi: 10.1097/00042560-199503010-00008. [DOI] [PubMed] [Google Scholar]
- 19.Berghuis JP, Uldall KK, Lalonde B. Validity of two scales in identifying HIV-associated dementia. J Acquir Immune Defic Syndr. 1999;21:134–40. [PubMed] [Google Scholar]
- 20.Bottiggi KA, Chang JJ, Schmitt FA, et al. The HIV Dementia Scale: Predictive power in mild dementia and HAART. J Neurol Sci. 2007;260:11–5. doi: 10.1016/j.jns.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 21.Richardson MA, Morgan EE, Vielhauer MJ, Cuevas CA, Buondonno LM, Keane TM. Utility of the HIV dementia scale in assessing risk for significant HIV-related cognitive-motor deficits in a high-risk urban adult sample. AIDS Care. 2005;17:1013–21. doi: 10.1080/09540120500100858. [DOI] [PubMed] [Google Scholar]
- 22.Smith CA, van Gorp WG, Ryan ER, Ferrando SJ, Rabkin J. Screening subtle HIV-related cognitive dysfunction: the clinical utility of the HIV dementia scale. J Acquir Immune Defic Syndr. 2003;33:116–8. doi: 10.1097/00126334-200305010-00018. [DOI] [PubMed] [Google Scholar]
- 23.Morgan EE, Woods SP, Scott JC, et al. Predictive validity of demographically adjusted normative standards for the HIV Dementia Scale. J Clin Exp Neuropsychol. 2008;30:83–90. doi: 10.1080/13803390701233865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis HF, Skolasky RL, Jr, Selnes OA, Burgess DM, McArthur JC. Assessing HIV-associated dementia: Modified HIV dementia scale versus the Grooved Pegboard. AIDS Read. 2002;12:29–31, 38. [PubMed] [Google Scholar]
- 25.Waldrop-Valverde D, Nehra R, Sharma S, et al. Education effects on the international HIV dementia scale. J Neurovirol. 2010;16:264–7. doi: 10.3109/13550284.2010.497808. [DOI] [PubMed] [Google Scholar]
- 26.Rourke SB, Halman MH, Bassel C. Neurocognitive complaints in HIV-infection and their relationship to depressive symptoms and neuropsychological functioning. J Clin Exp Neuropsychol. 1999;21:737–56. doi: 10.1076/jcen.21.6.737.863. [DOI] [PubMed] [Google Scholar]
- 27.Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev. 2009;19:152–68. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cysique LA, Murray JM, Dunbar M, Jeyakumar V, Brew BJ. A screening algorithm for HIV-associated neurocognitive disorders. HIV Med. 2010;11:642–9. doi: 10.1111/j.1468-1293.2010.00834.x. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez R, Heaton RK, Moore DJ, et al. Computerized reaction time battery versus a traditional neuropsychological battery: Detecting HIV-related impairments. J Int Neuropsychol Soc. 2003;9:64–71. doi: 10.1017/s1355617703910071. [DOI] [PubMed] [Google Scholar]
- 30.Worth JL, Savage CR, Baer L, Esty EK, Navia BA. Computer-based neuropsychological screening for AIDS dementia complex. AIDS. 1993;7:677–81. doi: 10.1097/00002030-199305000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Cysique LA, Maruff P, Darby D, Brew BJ. The assessment of cognitive function in advanced HIV-1 infection and AIDS dementia complex using a new computerised cognitive test battery. Arch Clin Neuropsychol. 2006;21:185–94. doi: 10.1016/j.acn.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Becker JT, Dew MA, Aizenstein HJ, Lopez OL, Morrow L, Saxton J. Concurrent validity of a computer-based cognitive screening tool for use in adults with HIV disease. AIDS Patient Care STDS. 2011;25:351–7. doi: 10.1089/apc.2011.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carey CL, Woods SP, Rippeth JD, et al. Initial validation of a screening battery for the detection of HIV-associated cognitive impairment. Clin Neuropsychol. 2004;18:234–48. doi: 10.1080/13854040490501448. [DOI] [PubMed] [Google Scholar]
- 34.Valcour VG, Shiramizu BT, Sithinamsuwan P, et al. HIV DNA and cognition in a Thai longitudinal HAART initiation cohort: The SEARCH 001 Cohort Study. Neurology. 2009;72:992–8. doi: 10.1212/01.wnl.0000344404.12759.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellis RJ, Evans SR, Clifford DB, et al. Clinical validation of the NeuroScreen. J Neurovirol. 2005;11:503–11. doi: 10.1080/13550280500384966. [DOI] [PubMed] [Google Scholar]
- 36.Ganasen KA, Fincham D, Smit J, Seedat S, Stein D. Utility of the HIV Dementia Scale (HDS) in identifying HIV dementia in a South African sample. J Neurol Sci. 2008;269:62–4. doi: 10.1016/j.jns.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 37.Skinner S, Adewale AJ, DeBlock L, Gill MJ, Power C. Neurocognitive screening tools in HIV/AIDS: Comparative performance among patients exposed to antiretroviral therapy. HIV Med. 2009;10:246–52. doi: 10.1111/j.1468-1293.2008.00679.x. [DOI] [PubMed] [Google Scholar]
- 38.Milne A. Dementia screening and early diagnosis: The case for and against. Health Risk Soc. 2010;12:65–76. [Google Scholar]
- 39.Marra CM, Zhao Y, Clifford DB, et al. AIDS Clinical Trials Group 7736 Study Team. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS. 2009;23:1359–66. doi: 10.1097/QAD.0b013e32832c4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Overton E, Ances B, Grubb J, et al. Paper presented at: 18th Conference on Retroviruses and Opportunistic Infections. 27 February–8 March: 2011. Novel screening tools for HIV-associated neurocognitive disorders. Boston, MA. Paper 401. [Google Scholar]
- 41.Koski L, Brouillette MJ, Lalonde R, et al. Computerized testing augments pencil-and-paper tasks in measuring HIV-associated mild cognitive impairment. HIV Med. 2011;12:472–80. doi: 10.1111/j.1468-1293.2010.00910.x. [DOI] [PubMed] [Google Scholar]