Abstract
Context
Exercise guidelines for individuals with diabetes include both aerobic and resistance training although few studies have directly examined this exercise combination.
Objective
To examine the benefits of aerobic training alone, resistance training alone, and a combination of both on hemoglobin A1c (HbA1c) in individuals with type 2 diabetes.
Design, Setting, and Participants
A randomized controlled trial in which 262 sedentary men and women in Louisiana with type 2 diabetes and HbA1c levels of 6.5% or higher were enrolled in the 9-month exercise program between April 2007 and August 2009.
Intervention
Forty-one participants were assigned to the nonexercise control group, 73 to resistance training 3 days a week, 72 to aerobic exercise in which they expended 12 kcal/kg per week; and 76 to combined aerobic and resistance training in which they expended 10 kcal/kg per week and engaged in resistance training twice a week.
Main Outcome
Change in HbA1c level. Secondary outcomes included measures of anthropometry and fitness.
Results
The study included 63.0% women and 47.3% nonwhite participants who were a mean (SD) age of 55.8 years (8.7 years) with a baseline HbA1c level of 7.7% (1.0%). Compared with the control group, the absolute mean change in HbA1c in the combination training exercise group was −0.34% (95% confidence interval “CI”, −0.64% to −0.03%; P=.03). The mean changes in HbA1c were not statistically significant in either the resistance training (−0.16%; 95% CI, −0.46% to 0.15%; P=.32) or the aerobic (−0.24%; 95% CI, −0.55% to 0.07%; P=.14) groups compared with the control group. Only the combination exercise group improved maximum oxygen consumption (mean, 1.0 mL/kg per min; 95% CI, 0.5-1.5, P<.05) compared with the control group. All exercise groups reduced waist circumference from −1.9 to −2.8 cm compared with the control group. The resistance training group lost a mean of −1.4 kg fat mass (95% CI, −2.0 to −0.7 kg; P<.05) and combination training group lost a mean of −1.7 (−2.3 to −1.1 kg; P<.05) compared with the control group.
Conclusions
Among patients with type 2 diabetes mellitus, a combination of aerobic and resistance training compared with the nonexercise control group improved HbA1c levels. This was not achieved by aerobic or resistance training alone.
Although it is Generally Accepted that regular exercise provides substantial health benefits to individuals with type 2 diabetes, the exact exercise prescription in terms of type (aerobic vs resistance vs both) is unclear.1-6
A 2001 meta-analysis of 12 aerobic training studies and 2 resistance training studies concluded that exercise training decreases hemoglobin A1c (HbA1c) by 0.66% in individuals with type 2 diabetes.5 A 2006 meta-analysis composed of 27 studies found exercise to be associated with a mean reduction in HbA1c of 0.80% with no difference in magnitude of change in HbA1c between aerobic, resistance, or combination training.7 None of the studies in either of the meta-analyses were adequately powered to directly compare aerobic, resistance, and combination training. In 2007 Sigal et al8 published the findings from the Diabetes Aerobic and Resistance Exercise (DARE) study, which is the first adequately powered and controlled study comparing aerobic training, resistance training, or both with change in HbA1c in individuals with type 2 diabetes. All exercise groups had a reduction in HbA1c compared with the control group, but the combination group had a larger reduction (−1.0%) compared with the resistance training (−0.4%) and aerobic (−0.5%) groups. However, the combination-exercise group performed both the aerobic and resistance training for 135 minutes a week for each activity type, resulting in approximately 270 minutes per week of exercise. It is unclear whether the additional benefit observed was due to the combination of resistance and aerobic training or to the extra exercise time.
Given that the 2008 Federal Physical Activity Guidelines recommend aerobic exercise in combination with resistance training, the unanswered question as to whether for a given amount of time the combination of aerobic and resistance exercise is better than either alone has significant clinical and public health importance. The goal of the Health Benefits of Aerobic and Resistance Training in individuals with type 2 diabetes (HART-D) study was designed to compare aerobic training, resistance training, and a combination of both on HbA1c in sedentary women and men with type 2 diabetes while maintaining similar weekly training durations.
Methods
Participants and Enrollment Process
We recruited all of our participants from the greater Baton Rouge, Louisiana, area using media, mailers, and community events between April 2007 and August 2009 (Figure 1). After the telephone screen, eligible participants were invited to an orientation where participants were provided a detailed study overview, provided consent, and were screened for additional inclusion criteria. If eligible, participants were invited to partake in an educational run-in period consisting of 6 one-hour visits over 2 weeks. During the run-in period, HbA1c was measured, and at each visit a brief video education session was provided. We randomized 262 sedentary 30- to 75-year-old adults with type 2 diabetes and HbA1c levels of 6.5% to 11.0%. Sedentary was defined as not exercising more than 20 minutes on 3 or more days a week. Diabetes status was confirmed by medical history review. Exclusion criteria included body mass index (calculated as weight in kilograms divided by height in meters squared) of 48.0 or higher, blood pressure of 160/100 mm Hg or higher, fasting triglycerides 500 mg/dL or higher, use of an insulin pump, urine protein greater than 100 mg/dL, serum creatinine greater than 1.5 mg/dL, history of stroke, advanced neuropathy or retinopathy, or any serious medical condition that prevented participants from adhering to the protocol or exercising safely. The protocol was approved by the institutional review board of the Pennington Biomedical Research Center, and all participants gave written informed consent.
Figure 1. Participant Flow Chart.
To convert triglycerides from mg/dL to mmol/L, multiply by 0.0113 and creatinine from mg/dL to μmol/L, multiply by 88.4.
Study Design
HART-D was a randomized 9-month exercise intervention with a control and 3 different exercise groups: aerobic training only, resistance training only, and a combination of aerobic and resistance training.
The nonexercise control group was offered weekly stretching and relaxation classes and was asked to maintain current activity during the 9-month study period.
We designed interventions to have approximately equal time requirements. All exercise sessions were supervised. We standardized the exercise prescription to body weight and estimated that 150 minutes per week of moderate intensity exercise is equivalent to 10 to 12 kcal/kg of body weight per week. Exercise intensity was 50% to 80% of maximum oxygen consumption. We selected an aerobic dose of 12 kcal/kg per week for the aerobic group and 10 kcal/kg per week for the combination exercise group.
Participants were weighed weekly to calculate their kcal/kg per week target. During weeks 12 and 24 the exercise dose was reduced by one-third to provide a recuperation week. Each session had a 5-minute warm-up and cool-down period. American College of Sports Medicine equations were used to estimate caloric expenditure rate and time required per session.9
Resistance training participants exercised 3 days per week with each session consisting of 2 sets of 4 upper body exercises (bench press, seated row, shoulder press, and pull down), 3 sets of 3 leg exercises (leg press, extension, and flexion) and 2 sets each of abdominal crunches and back extensions. Those in the combination exercise group had 2 resistance training sessions per week with each session consisting of 1 set of each of the above 9 exercises. For the combination and resistance training groups, each set consisted of 10 to 12 repetitions. Once the participant was able to complete 12 repetitions for each set of exercises on 2 consecutive exercise sessions, the prescribed weight was increased.
To ensure safety, participants had monthly visits with a certified diabetes educator who conducted foot checks for blisters and pressure sores and reviewed fasting glucose records to identify hypoglycemic risk. These visits included documentation of medication changes and measuring weight and HbA1c levels. The certified diabetes educator had no role in the intervention.
The primary outcome of HART-D, change in HbA1c levels, was assessed monthly. Secondary outcomes include measures of anthropometry, fitness, strength, and changes in diabetes medications that were assessed at baseline and follow-up only.
Hemoglobin A1c assessed at the monthly visits with the diabetes educator was from a finger prick sample run on an automated glycosylated hemoglobin analyzer (DCA 2000+, Bayer, Dublin, Ireland). At baseline and follow-up clinic visits, blood was collected and processed after an overnight fast. Lipid (total cholesterol, high-density lipoprotein and low-density lipoprotein cholesterol, and triglyceride) and glucose levels were analyzed on a DXC 600 Pro (Beckman Coulter Inc, Brea, California). Insulin was measured using an immunoassay on the Siemens 2000 (Siemens, Deerfield, Illinois).
Exercise testing was conducted on a treadmill (Trackmaster 425, Carefusion, Newton, Kansas) with respiratory gases sampled using a True Max 2400 Metabolic Measurement Cart (ParvoMedics, Salt Lake City, Utah). Participants self-selected a walking pace and the grade increased by 2% every 2 minutes until exhaustion. The same speed was used for baseline and follow-up testing. Maximal estimated metabolic equivalent tasks (METs) achieved was calculated from speed and grade.9 We performed all muscular strength assessments using a Biodex System 3 dynamometer (Biodex Medical Systems, Shirley, New York). Concentric isokinetic knee flexion and extension were tested to determine peak torque (60°/s) and total work (300°/s). Peak torque was determined as the best of 5 maximal repetitions and divided by lean mass. Total work was determined as the total work accomplished for 30 repetitions of maximal effort.
Diabetes medication type and dosage were assessed by detailed questionnaire with visual confirmation of prescription bottles. Participants were categorized as either increased, decreased, or no change in diabetes medications based on baseline and follow-up medication dosages. When the type of diabetes medication was changed, a pharmacist was consulted to categorize the change.
Weight was measured on a GSE 450 electronic scale (GSE Scale Systems, Novi, Michigan) and height was measured using a standard stadiometer. Dual-energy x-ray absorptiometry scans were performed using the QDR 4500A whole-body scanner (Hologic Inc, Bedford, Massachusetts). Diet was assessed at baseline and follow-up using the Block Food Frequency Questionnaire.10 Race/ethnicity was obtained through written self-report to examine the effect of ethnicity on exercise training response.
Strategies to reduce dropout and maintain adherence included a 2-week prerandomization run-in period, behavioral contracts, and consistent support from staff members. Participants were reimbursed $100 ($50 each) for baseline and follow-up assessments, and an additional $100 based on adherence. For the control group, adherence was based on attending monthly diabetes educational sessions, returning monthly stepcount forms, and completing medical symptoms questionnaires. For each month missed, $10 was deducted. For the exercise group, compensation was reduced by $1 for every 1% decrease in attendance beyond 90%.
Power and sample size calculations were based on a predicted HbA1c difference of 0.66 HbA1c units with an SD of effect of 1.2 HbA1c units, α = .05, 1-β = .85 and an expected dropout rate of 15%.5 The calculation yielded 70 participants per group. Members of the HART-D scientific advisory committee recommended that the control group be monitored closely. Safety monitoring included blinded monitoring of HbA1c levels. The control group was unblinded after 7 participants (17.1%) had an increase in HbA1c level of 1.0% or higher. With the data safety monitoring board recommendation, we decided to stop randomizing individuals into the control group. As a result, a total of 41 individuals were randomized to the control group. At that point, the SD of effect was 1.0 HbA1c units instead of 1.2; thus, our power for comparison of exercise groups vs control was still 1-β = .83.
Eligible participants were randomized after completing run-in and baseline assessments. The randomization sequence was computer generated using randomly permuted blocks of equal length with fixed numbers of treatment allotments each, to balance treatment enrollments over time. After the control group was closed early, a new randomization sequence was prepared.
There were separate intervention and assessment teams and all assessment staff were blinded to participant randomization assignment. The clinical testing and exercise training laboratories were in separate buildings, and participants were reminded frequently not to disclose their group assignment to assessment staff.
Statistical Analysis
Primary outcome analyses used the intention-to-treat principle and included all participants as randomized. All available data were examined using linear mixed-effects models for repeated measures over time. For the dependent variables, HbA1c, weekly step counts, and exercise training monthly data were available, whereas for the independent variables, assessing medications, fitness, strength, and anthropometry, only baseline and follow-up data were available. Covariates included baseline value, age, sex, duration of diabetes, and race/ethnicity with the fitness variables also adjusted for maximum heart rate during exercise testing at baseline and follow-up. Results are presented as least-squares adjusted means with 95% confidence intervals (CIs). Additionally, because HART-D was designed as an efficacy study, we performed per-protocol analyses limited to a subgroup of participants composed of all control participants and only the exercise group participants who met the criteria of at least 70% adherence to their exercise prescription for at least 6 months.
Significance of between-group differences in medication changes (no change, increase, or decrease) were assessed using the χ2 test with the linear association across groups assessed by the Mantel-Haenszel test. To assess concomitant reductions in hypoglycemic medication use and reductions in HbA1c, levels, a composite dichotomous outcome variable was created. Individuals who decreased diabetes medication or reduced HbA1c by 0.5% without increasing medications were defined as successfully achieving the HbA1c-diabetes medication composite outcome. The likelihood of achieving the composite outcome was assessed using logistic regression with adjustment for baseline HbA1c, age, sex, duration of diabetes, and race/ethnicity.
P≤.05 (2-tailed) was used to identify statistical significance. All analyses were performed using SAS version 9.1 (SAS Institute Inc, Cary, North Carolina).
Results
Table 1 shows that the study participants were 63.0% women and 47.3% nonwhite, participants who were a mean (SD) age of 55.8 (8.7) years, had a body mass index of 34.9 (5.9), had HbA1c level of 7.7% (1.0%), and duration of diabetes of 7.1 (5.5) years. There were 97.3% taking diabetes medications with 18.3% taking insulin. Many participants had a history of comorbidities. Baseline systolic blood pressure and LDL cholesterol were well controlled, most likely because of the high percentage of patients taking medications.
Table 1. Baseline Participant Characteristicsa.
| Characteristics | All Participants (N = 262) | Randomization Groups | |||
|---|---|---|---|---|---|
| Control (n = 41) | Exercise Training Type | ||||
| Resistance (n = 73) | Aerobic (n = 72) | Combination (n = 76) | |||
| Age, mean (SD), y | 55.8 (8.7) | 58.6 (8.2) | 56.9 (8.7) | 53.7 (9.1) | 55.4 (8.3) |
|
| |||||
| Women, No. (%) | 165 (63.0) | 28 (68.3) | 43 (58.9) | 45 (62.5) | 49 (64.5) |
|
| |||||
| Race/ethnicity, No. (%) | |||||
|
| |||||
| White | 138 (52.7) | 22 (53.7) | 41 (56.2) | 39 (54.2) | 36 (47.4) |
|
| |||||
| African American | 114 (43.5) | 17 (41.5) | 30 (41.1) | 33 (45.8) | 34 (44.7) |
|
| |||||
| Hispanic/other | 10 (3.8) | 2 (4.9) | 2 (2.7) | 0 (0.0) | 6 (7.9) |
|
| |||||
| Smoking history, No. (%) | |||||
|
| |||||
| Current | 10 (3.8) | 2 (4.9) | 3 (4.1) | 2 (2.8) | 3 (4.0) |
|
| |||||
| Former | 77 (29.4) | 13 (31.7) | 25 (34.3) | 21 (29.2) | 18 (23.7) |
|
| |||||
| Diabetes factors, mean (SD) | |||||
|
| |||||
| HbA1c, % | 7.7 (1.0) | 7.9 (1.3) | 7.6 (0.9) | 7.6 (1.0) | 7.6 (1.0) |
|
| |||||
| Fasting glucose, mg/dL | 151.0 (36.4) | 158.4 (40.7) | 153.8 (39.3) | 146.4 (30.6) | 148.8 (35.9) |
|
| |||||
| Duration of diabetes, y | 7.1 (5.5) | 7.2 (5.2) | 7.2 (5.5) | 7.4 (6.0) | 6.7 (5.4) |
|
| |||||
| Fasting insulin, median (IQR), μUI/mL | 15.3 (9.9-23.1) | 12.6 (10.7-21.5) | 16.0 (12.5-23.0) | 13.9 (8.3-22.7) | 16.9 (9.9-25.3) |
|
| |||||
| Hypoglycemic medications, No. (%) | |||||
|
| |||||
| Any | 255 (97.3) | 40 (97.6) | 73 (100) | 68 (94.4) | 74 (97.4) |
|
| |||||
| Biguanide | 170 (64.9) | 23 (56.1) | 46 (63.0) | 48 (66.7) | 53 (69.7) |
|
| |||||
| Thiazolidinediones | 46 (17.6) | 10 (24.4) | 11 (15.1) | 14 (19.4) | 11 (14.5) |
|
| |||||
| Sulfonylurea | 63 (24.1) | 11 (26.8) | 18 (24.7) | 17 (23.6) | 17 (22.4) |
|
| |||||
| Incretin mimetics | 27 (10.3) | 3 (7.3) | 10 (13.7) | 6 (8.3) | 8 (10.5) |
|
| |||||
| DPP-4 inhibitors | 13 (5.0) | 1 (2.4) | 6 (8.2) | 4 (5.6) | 2 (2.6) |
|
| |||||
| Meglitinides | 9 (3.4) | 2 (4.9) | 3 (4.1) | 1 (1.4) | 3 (4.0) |
|
| |||||
| Insulin | 48 (18.3) | 7 (17.1) | 9 (12.3) | 15 (20.8) | 17 (22.4) |
|
| |||||
| Combination drugs | 43 (16.4) | 8 (19.5) | 14 (19.2) | 9 (12.5) | 12 (15.8) |
|
| |||||
| Anthropometrics, mean (SD) | |||||
|
| |||||
| Weight, kg | 98.2 (18.8) | 97.0 (20.0) | 96.9 (16.6) | 97.5 (18.6) | 100.6 (20.4) |
|
| |||||
| BMI | 34.9 (5.9) | 34.8 (6.2) | 34.1 (5.4) | 34.7 (6.1) | 35.8 (6.2) |
|
| |||||
| Waist circumference, cm | 112.1 (13.8) | 110.6 (14.4) | 110.9 (12.2) | 111.3 (14.2) | 114.9 (14.5) |
|
| |||||
| Body fat, %b | 37.8 (7.3) | 38.5 (7.0) | 37.0 (7.6) | 37.1 (7.7) | 38.8 (6.8) |
|
| |||||
| Fat body mass, kgb | 37.1 (10.7) | 37.9 (11.8) | 36.1 (10.1) | 35.7 (10.1) | 39.0 (11.3) |
|
| |||||
| Lean body mass, kgb | 57.7 (11.0) | 56.9 (11.8) | 58.2 (10.7) | 57.3 (10.7) | 57.9 (11.3) |
|
| |||||
| Exercise test variables, mean (SD) | |||||
|
| |||||
| Peak relative V̇O2, mL/kg per min | 19.2 (4.2) | 18.5 (3.9) | 19.6 (4.3) | 19.9 (5.0) | 18.6 (3.4) |
|
| |||||
| Time on treadmill, min | 10.7 (2.5) | 10.6 (2.1) | 10.7 (2.5) | 10.8 (2.8) | 10.7 (2.5) |
|
| |||||
| Speed/grade estimated METs | 6.9 (1.3) | 6.7 (1.4) | 7.0 (1.3) | 7.0 (1.5) | 6.7 (1.2) |
|
| |||||
| Muscular work, Nm | 2268 (913) | 2017 (889) | 2327 (944) | 2355 (937) | 2264 (867) |
|
| |||||
| Muscular torque, Nm/kg | 2.2 (0.6) | 2.1 (0.5) | 2.2 (0.6) | 2.3 (0.6) | 2.2 (0.6) |
|
| |||||
| Cardiovascular disease factors, mg/dL | |||||
|
| |||||
| Cholesterol, mean (SD) | |||||
|
| |||||
| Low-density lipoprotein | 95.5 (29.4) 1 | 02.6 (25.8) | 94.1 (30.4) | 92.5 (28.3) | 95.9 (31.1) |
|
| |||||
| High-density lipoprotein | 49.3 (11.5) | 50.4 (9.5) | 49.4 (12.1) | 49.0 (13.4) | 49.1 (10.0) |
|
| |||||
| Triglycerides, median (IQR) | 132 (88-188) | 143 (106-206) | 135 (99-198) | 132 (85-77) | 122 (85-173) |
|
| |||||
| C-reactive protein, median (IQR) | 3.4 (1.3-7.5) | 3.1 (0.8-7.5) | 2.7 (1.1-6.3) | 3.4 (1.2-8.1) | 4.4 (2.2-7.6) |
|
| |||||
| Blood pressure, mean (SD), mm Hg | |||||
|
| |||||
| Systolic | 126.2 (13.1) 1 | 27.1 (13.9) | 124.1 (12.5) | 124.4 (12.5) | 129.4 (13.5) |
|
| |||||
| Diastolic | 75.6 (8.5) | 76.4 (8.0) | 75.1 (8.2) | 75.8 (9.4) | 75.3 (8.3) |
|
| |||||
| Other medication use, No. (%) | |||||
|
| |||||
| Blood pressure | 208 (79.4) | 31 (75.6) | 59 (80.8) | 56 (77.8) | 62 (81.6) |
|
| |||||
| Cholesterol | 168 (64.1) | 22 (53.7) | 53 (72.6) | 45 (62.5) | 48 (63.2) |
|
| |||||
| Antidepressant | 47 (17.9) | 10 (24.4) | 16 (21.9) | 12 (16.7) | 9 (11.8) |
|
| |||||
| Other medical history, No. (%) | |||||
|
| |||||
| Abnormal exercise ECG | 49 (18.7) | 5 (12.2) | 13 (17.8) | 14 (19.4) | 17 (22.4) |
|
| |||||
| Myocardial infarction | 7 (2.7) | 0 (0) | 5 (6.9) | 0 (0) | 2 (2.6) |
|
| |||||
| Heart catheterization | 45 (17.2) | 5 (12.2) | 15 (20.6) | 13 (18.1) | 12 (15.8) |
|
| |||||
| Coronary artery bypass surgery | 14 (5.0) | 1 (2.4) | 3 (4.1) | 5 (6.9) | 5 (6.6) |
|
| |||||
| Cancer | 29 (11.1) | 3 (7.3) | 9 (12.3) | 10 (13.9) | 7 (9.2) |
|
| |||||
| Neuropathy | 46 (17.6) | 7 (17.1) | 18 (24.7) | 12 (16.7) | 9 (11.8) |
Abbreviations: BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; DPP, dipeptidyl peptidase 4, ECG, electrocardiogram; MET, metabolic equivalent; N m, Newton meters; V̇O2, volume of oxygen consumed.
SI conversions: To convert low-density lipoprotein and high-density lipoprotein to mmol/L, multiply by 0.0259; triglycerides to mmol/L, multiply by 0.0113; glucose to mmol/L, multiply by 0.0555, and insulin to pmol/L, multiply by 6.945.
Percentages may not sum to 100 due to rounding.
Due to equipment malfunction, data for 9 participants were not collected.
Table 2 summarizes the exercise training data by month for individuals who met the per-protocol criteria. The aerobic and combination training groups performed their aerobic exercise at about 65% of maximum oxygen consumption. For both the aerobic and combination training groups the mean treadmill grade and METs progressively increased during the intervention period resulting in shorter exercise sessions over the course of the intervention. The total exercise dose ranged from 623.7 to 681.9 MET/min per week for the aerobic group and 532.0 to 572.8 MET/min per week in the combination training group. Taking into account the exercise intervention time and warm-up and cool-down time, we estimate that participants in the aerobic group typically spent mean 140 min/wk (range, 130-150 min/wk) on the treadmill, the resistance training group averaged 141 min/wk, and the combination training group spent a mean 110 min/wk (range, 100-120 min/wk) on the treadmill and between 30 and 40 min/wk lifting weights. There was no change in outside steps for any of the groups including the control group. The range in weekly steps for the control group was from 4180 to 4376. The mean change in kilocalories per day intake at follow-up was −93 (95% CI, −219 to 33) for the control, −162 (95% CI, −256 to −68) for the resistance training, −179 (95% CI−275 to −82) for the aerobic, and −127 (95% CI, −220 to −34) for the combination training groups, with no significant between-group differences.
Table 2. Exercise Intervention Data for Individuals Who Met the Per-Protocol Criteria.
| Mean (SE) by Intervention Month | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Resistance group | |||||||||
|
| |||||||||
| Total weight lifted, lb/wka | 41 472 (2363) | 55 192 (2363) | 61 355 (2365) | 67 061 (2363) | 68 440 (2363) | 68 850 (2365) | 71 799 (2363) | 69 366 (2363) | 72 433 (2375) |
|
| |||||||||
| Daily steps | 4912 (356) | 5121 (356) | 5109 (356) | 4874 (357) | 4829 (360) | 4702 (363) | 4861 (368) | 5067 (375) | 5136 (383) |
|
| |||||||||
| Weight, kg | 100.8 (2.4) | 99.7 (2.4) | 99.5 (2.4) | 99.0 (2.4) | 98.9 (2.4) | 99.0 (2.4) | 99.0 (2.4) | 99.0 (2.4) | 99.1 (2.4) |
|
| |||||||||
| Aerobic group | 1 | 2 | 3d | 4 | 5 | 6d | 7 | 8 | 9 |
|
| |||||||||
| Sessions/wk, No. | 3.0 (.02) | 3.1 (.02) | 3.1 (.02) | 3.1 (.02) | 3.1 (.02) | 3.0 (.02) | 3.1 (.02) | 3.1 (.02) | 3.0 (.02) |
|
| |||||||||
| Intensity, % of peak V ˙O2 | 60.1 (2.4) | 64.3 (2.4) | 63.8 (2.4) | 64.8 (2.4) | 64.5 (2.4) | 66.3 (2.4) | 66.9 (2.4) | 66.7 (2.4) | 65.6 (2.4) |
|
| |||||||||
| Treadmill grade, % | 2.0 (.3) | 3.7 (.3) | 4.5 (.3) | 5.0 (.3) | 5.4 (.3) | 5.7 (.3) | 6.0 (.3) | 6.1 (.3) | 6.8 (.3) |
|
| |||||||||
| Treadmill speed, mph | 2.9 (.1) | 3.1 (.1) | 3.1 (.1) | 3.1 (.1) | 3.1 (.1) | 3.2 (.1) | 3.1 (.1) | 3.1 (.1) | 3.1 (.1) |
|
| |||||||||
| Mean METs level | 4.1 (.2) | 5.0 (.2) | 5.3 (.2) | 5.6 (.2) | 5.8 (.2) | 5.9 (.2) | 6.0 (.2) | 6.1 (.2) | 6.4 (.2) |
|
| |||||||||
| Time aerobic, min/wkb | 123.4 (3.2) | 140.0 (3.2) | 122.5 (3.2) | 127.7 (3.3) | 122.3 (3.3) | 109.6 (3.2) | 115.0 (3.3) | 118.3 (3.3) | 112.9 (3.3) |
|
| |||||||||
| MET/min per wk | 498.3 (4.1) | 674.4 (4.1) | 627.5 (4.2) | 681.8 (4.2) | 679.5 (4.3) | 623.7 (4.2) | 670.5 (4.4) | 681.9 (4.3) | 680.3 (4.5) |
|
| |||||||||
| Daily steps | 4365 (352) | 4448 (354) | 4336 (354) | 4419 (358) | 4355 (361) | 4358 (366) | 4397 (369) | 4365 (372) | 4410 (378) |
|
| |||||||||
| Weight, kg | 99.0 (2.4) | 98.8 (2.4) | 98.5 (2.4) | 98.6 (2.4) | 98.7 (2.4) | 98.4 (2.4) | 98.3 (2.4) | 98.2 (2.4) | 98.3 (2.4) |
|
| |||||||||
| Combination group | 1 | 2 | 3d | 4 | 5 | 6d | 7 | 8 | 9 |
|
| |||||||||
| Sessions/wk | 3.0 (.02) | 3.0 (.02) | 3.0 (.02) | 3.0 (.02) | 3.0 (.02) | 3.0 (.02) | 3.0 (.02) | 3.0 (.02) | 3.0 (.02) |
|
| |||||||||
| Intensity, % of peak V̇O2 | 58.5 (2.2) | 63.6 (2.2) | 63.4 (2.2) | 65.8 (2.2) | 65.4 (2.2) | 65.4 (2.2) | 64.8 (2.2) | 65.4 (2.2) | 66.1 (2.2) |
|
| |||||||||
| Treadmill grade, % | 1.8 (.3) | 3.5 (.3) | 4.3 (.3) | 4.8 (.3) | 5.1 (.3) | 5.4 (.3) | 5.6 (.3) | 5.7 (.3) | 6.0 (.3) |
|
| |||||||||
| Treadmill speed, mph | 2.8 (.1) | 3.0 (.1) | 3.0 (.1) | 3.1 (.1) | 3.1 (.1) | 3.1 (.1) | 3.1 (.1) | 3.1 (.1) | 3.1 (.1) |
|
| |||||||||
| Mean METs level | 3.9 (.1) | 4.7 (.1) | 5.2 (.1) | 5.4 (.1) | 5.5 (.1) | 5.7 (.1) | 5.8 (.1) | 5.9 (.1) | 6.0 (.2) |
|
| |||||||||
| Time aerobic, min/wkb | 102.4 (3.0) | 125.2 (3.0) | 107.6 (3.0) | 108.6 (3.0) | 106.6 (3.0) | 98.0 (3.0) | 103.7 (3.0) | 101.3 (3.1) | 101.4 (3.1) |
|
| |||||||||
| MET/min per wk | 395.0 (3.8) | 571.3 (3.8) | 532.4 (3.8) | 561.6 (3.8) | 566.6 (3.8) | 532.0 (3.9) | 570.2 (4.0) | 565.4 (4.1) | 572.8 (4.1) |
|
| |||||||||
| Total weight lifted, lb/wka | 12 387 (2324) | 16 988 (2324) | 17 921 (2324) | 18 546 (2324) | 19 119 (2324) | 19 122 (2326) | 19 996 (2332) | 20 076 (2351) | 20 961 (2367) |
|
| |||||||||
| Daily stepsc | 5207 (372) | 5141 (372) | 5167 (374) | 5002 (377) | 5167 (378) | 5185 (380) | 5147 (383) | 4966 (387) | 5388 (393) |
|
| |||||||||
| Weight, kg | 98.0 (2.6) | 97.5 (2.6) | 97.2 (2.6) | 97.4 (2.6) | 97.4 (2.6) | 97.1 (2.6) | 97.1 (2.6) | 96.8 (2.6) | 96.7 (2.6) |
Abbreviations: MET, metabolic equivalent task; V̇O2 oxygen consumption per unit time.
Weekly total weight lifted is the mean of the sum of the total weight lifted during each week for each participant.
Does not include 5-minute warm-up and 5-minute cool down.
Step counters were removed during exercise intervention; thus, daily steps do not include steps obtained during intervention treadmill walking.
During the weeks of 12 (month 3) and 24 (month 6) the aerobic dose was reduced by one-third.
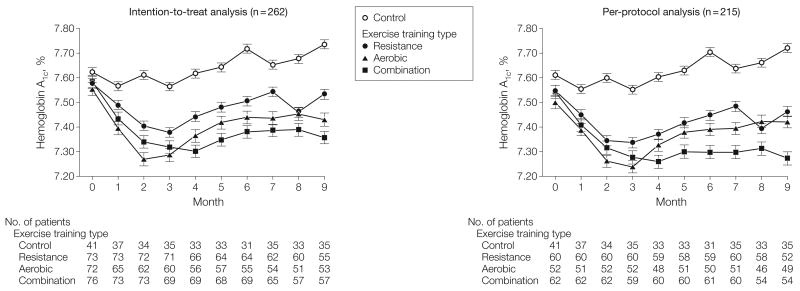
Figure 2 depicts monthly HbA1c levels across the groups for the intention-to-treat and per-protocol analyses derived from a linear mixed model that included the covariates age, sex, race/ethnicity, diabetes duration, and baseline HbA1c. The group (P = .01) and month (P< .001) effects were statistically significant. Similar results were observed in the per-protocol analyses, which included all controls and only individuals in the exercise groups who obtained at least 70% adherence to their exercise prescription for at least 6 months.
Figure 2.
Monthly Hemoglobin A1c Levels Monthly mean hemoglobin A1c (HbA1c) levels, derived from a mixed-linear model containing the covariates of age, sex, race/ethnicity, diabetes duration, and baseline HbA1c, are presented for group with the intention-to-treat analysis (n=262) and the per-protocol analysis (n = 215). For the intention-to-treat model, the group (P=.01) and month (P< .001) effect were statistically significant. Error bars represent standard errors.
Table 3 summarizes results for baseline, follow-up and change in HbA1c across the groups. The absolute change in HbA1c in the combination training group vs the control group was −0.34% (95% CI, −0.64% to −0.03%; P=.03). In neither the resistance training (−0.16%, 95% CI, −0.46% to 0.15%; P=.32) nor the aerobic (−0.24%; 95% CI−0.55% to 0.07%; P=.14) groups were changes in HbA1c significant compared with those in the control group. In a subgroup analysis limited to participants with a baseline HbA1c 7.0% or higher, both the aerobic (−0.50%; 95% CI, −0.90% to −0.11%; P=.01) and combination training (−0.53%; 95% CI, −0.92% to −0.14%; P=.008) groups experienced significant reductions in HbA1c vs the control. In both analyses the intention-to-treat and per-protocol analyses produced similar results.
Table 3. Baseline, Follow-up, and Change in Hemoglobin A1ca.
| Intervention Group | No. of Participants | Mean (SE), % | Mean (95% Confidence Interval), % | Pair-Wise P Value | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline Value | Follow-up Value | Within-Group Changes | Between-Group Comparison vs Control Group Changes | |||||
| Intention-to-Treat Analysis (n = 262) | ||||||||
| Control | 41 | 7.62 (0.10) | 7.74 (0.10) | 0.11 (−0.13 to 0.36) | ||||
|
| ||||||||
| Resistance | 73 | 7.58 (0.07) | 7.53 (0.08) | −0.04 (−0.23 to 0.14) | −0.16 (−0.46 to 0.15) | .32 | ||
|
| ||||||||
| Aerobic | 72 | 7.56 (0.07) | 7.43 (0.08) | −0.12 (−0.31 to 0.07) | −0.24 (−0.55 to 0.07) | .14 | ||
|
| ||||||||
| Combination | 76 | 7.59 (0.07) | 7.36 (0.08) | −0.23 (−0.41 to −0.05) | −0.34 (−0.64 to −0.03) | .03 | ||
|
| ||||||||
| Per-Protocol Analysis (n = 215) | ||||||||
| Control | 41 | 7.61 (0.10) | 7.72 (0.10) | 0.11 (−0.13 to 0.35) | ||||
|
| ||||||||
| Resistance | 60 | 7.55 (0.08) | 7.46 (0.08) | −0.09 (−0.28 to 0.11) | −0.19 (−0.51 to 0.12) | .29 | ||
|
| ||||||||
| Aerobic | 52 | 7.50 (0.09) | 7.42 (0.09) | −0.08 (−0.29 to 0.13) | −0.19 (−0.51 to 0.13) | .32 | ||
|
| ||||||||
| Combination | 62 | 7.54 (0.08) | 7.27 (0.08) | −0.27 (−0.46 to −0.08) | −0.38 (−0.69 to −0.07) | .027 | ||
|
| ||||||||
| Intention-to-Treat Analysis Limited to Participants With Baseline Hemoglobin A1c ≥7.0% (n = 119) | ||||||||
| Control | 24 | 8.00 (0.012) | 8.18 (0.12) | 0.18 (−0.12 to 0.48) | ||||
|
| ||||||||
| Resistance | 30 | 7.97 (0.09) | 7.83 (0.10) | −0.14 (−0.38 to 0.10) | −0.33 (−0.72 to 0.06) | .10 | ||
|
| ||||||||
| Aerobic | 31 | 7.97 (0.09) | 7.64 (0.10) | −0.32 (−0.58 to −0.07) | −0.50 (−0.90 to −0.11) | .01 | ||
|
| ||||||||
| Combination | 34 | 7.99 (0.09) | 7.64 (0.10) | −0.34 (−0.58 to −0.11) | −0.53 (−0.92 to −0.14) | .008 | ||
|
| ||||||||
| Per-Protocol Analysis Limited to Participants With Baseline Hemoglobin A1c ≥7.0% (n = 94) | ||||||||
| Control | 24 | 7.99 (0.12) | 8.17 (0.13) | 0.18 (−0.12 to 0.48) | ||||
|
| ||||||||
| Resistance | 25 | 7.95 (0.10) | 7.78 (0.10) | −0.17 (−0.42 to 0.09) | −0.34 (−0.75 to 0.05) | .09 | ||
|
| ||||||||
| Aerobic | 20 | 7.89 (0.11) | 7.64 (0.12) | −0.25 (−0.53 to 0.03) | −0.43 (−0.84 to −0.02) | .04 | ||
|
| ||||||||
| Combination | 25 | 7.97 (0.10) | 7.52 (0.11) | −0.45 (−0.71 to −0.19) | −0.63 (−1.03 to −0.23) | .002 | ||
Values are expressed as fitted mean and all are adjusted for baseline value, age, sex, duration of diabetes, and race/ethnicity. Blank cells indicate not applicable.
Table 4 summarizes results for fitness, strength, and body composition. The combination training group improved peak oxygen consumption per unit time compared with the control and the resistance training groups. All groups improved time on treadmill compared with the control group. Work per extension over 30 repetitions increased in the resistance training group compared with all other groups and in the combination training group compared with the control and aerobic groups. At follow-up, the combination training group had a decrease in mean weight compared with the control and resistance training group. Participants in the resistance training group had reduction in fat mass compared with the control, whereas the combination training group had a reduction in fat mass compared with the control and aerobic groups. The mean lean mass in the resistance training group increased compared with the aerobic group and combination groups. All exercise groups had reduction in waist circumferences compared with the control group. The findings from the per-protocol analysis closely matched the intent-to-treat analysis.
Table 4. Change in Fitness, Body Composition, and Strength Variablesa.
| Characteristics | Mean (95% Confidence Interval)a | |||
|---|---|---|---|---|
| Control | Resistance | Aerobic | Combination | |
| Intention-to-Treat Analysis (n = 262) | ||||
| Peak V̇O2, mL/kg per min | −0.3 (−1.0 to 0.4) | 0.0 (−0.5 to 0.6) | 0.5 (0.01 to 1.0) | 1.0 (0.5 to 1.5)g |
|
| ||||
| Peak lean V̇O2, mL/kg muscle per min | −0.3 (−1.4 to 0.8) | −0.5 (−1.3 to 0.3) | 0.7 (−0.2 to 1.5) | 1.2 (0.4 to 2.0)g |
|
| ||||
| Time on treadmill, minutes | −0.6 (−1.4 to 0.1) | 0.4 (−0.2 to 1.0)e | 2.2 (1.6 to 2.7)g | 2.4 (1.9 to 3.0)g |
|
| ||||
| Speed/grade estimated MET | −0.2 (−0.5 to 0.1) | 0.2 (−0.01 to 0.3)e | 0.8 (0.6 to 1.0)g | 0.8 (0.6 to 1.0)g |
|
| ||||
| Muscular work, N mb | 89 (−48 to 226) | 542 (441 to 644)d | 115 (10 to 221) | 273 (174 to 372)d |
|
| ||||
| Muscular torque, n m/kg lean massb | 0.04 (−.08 to 0.16) | 0.18 (0.09 to 0.27)f | −0.07 (−0.17 to 0.02) | 0.15 (0.06 to 0.24)f |
|
| ||||
| Body mass, kg | 0.4 (−0.7 to 1.5) | −0.3 (−1.1 to 0.5) | −0.8 (−1.6 to 0.1) | −1.5 (−2.3 to −0.7)g |
|
| ||||
| Fat mass, kgc | 0.1 (−0.7 to 1.0) | −1.4 (−2.0 to −0.7)e | −0.6 (−1.2 to −0.1) | −1.7 (−2.3 to −1.1)e,f |
|
| ||||
| Lean mass, kgc | 0.1 (−0.5 to 0.8) | 0.8 (0.4 to 1.3) | −0.5 (−1.0 to 0.1)h | 0.0 (−0.5 to 0.4)h |
|
| ||||
| Waist circumference, cm | 0.7 (−0.6 to 1.9)d | −1.9 (−2.8 to −1.0) | −1.5 (−2.5 to −0.6) | −2.8 (−3.7 to −1.9) |
|
| ||||
| Per-Protocol Analysis (n = 215) | ||||
| Peak V̇O2, mL/kg per min | −0.3 (−1.0 to 0.4) | 0.1 (−0.4 to 0.7) | 0.6 (−0.1 to 1.2) | 1.1 (0.6 to 1.7)g |
|
| ||||
| Peak lean V̇O2, mL/kg muscle per min | −0.3 (−1.4 to 0.8) | −0.4 (−1.3 to 0.5) | 0.7 (−0.3 to 1.6) | 1.4 (0.5 to 2.3)g |
|
| ||||
| Time on treadmill, minutes | −0.6 (−1.4 to 0.2) | 0.5 (−0.1 to 1.1)e | 2.3 (1.6 to 3.0)g | 2.5 (1.8 to 3.1)g |
|
| ||||
| Speed/grade estimated MET | −0.2 (−0.5 to 0.1) | 0.3 (−0.5 to 0.1)e | 0.9 (0.6 to 1.1)g | 0.8 (0.5 to 1.0)g |
|
| ||||
| Muscular work, N m | 89 (−54 to 231) | 585 (471 to 699)d | 105 (−15 to 226) | 280 (169 to 392)d |
|
| ||||
| Muscular torque, N m/kg lean mass | 0.04 (−.08 to 0.16) | 0.21 (0.11 to 0.30)e,f | −0.10 (−0.20 to 0.00) | 0.14 (0.04 to 0.24)f |
|
| ||||
| Body mass, kg | 0.4 (−0.7 to 1.5) | −0.2 (−1.1 to 0.7) | −0.8 (−1.8 to 0.1) | −1.6 (−2.4 to −0.7)g |
|
| ||||
| Fat mass, kgc | 0.1 (−0.7 to 1.0) | −1.3 (−1.9 to −0.6)e | −0.7 (−1.4 to 0.0) | −1.8 (−2.5 to −1.1)e,f |
|
| ||||
| Lean mass, kgc | 0.1 (−0.5 to 0.7) | 0.9 (0.4 to 1.3) | −0.4 (−1.0 to 0.1)h | 0.0 (−0.5 to 0.5)h |
|
| ||||
| Waist circumference, cm | 0.7 (−0.6 to 2.0)d | −2.0 (−3.1 to −1.0) | −1.6 (−2.7 to −0.4) | −2.7 (−3.7 to −1.7) |
Abbreviations: MET, metabolic equivalent task; N m, Newton-meters; V̇O2, volume of oxygen consumed.
Values are expressed as fitted mean (95% CI) and all are adjusted for baseline value, age, sex, duration of diabetes and race/ethnicity with the fitness variables also adjusted for maximum heart rate during exercise testing at baseline and follow-up.
Peak torque was determined as the best of 5 maximal repetitions and then divided by lean mass. Total work (N m) was determined as the total work accomplished for 30 repetitions of maximal effort.
Due to equipment failure n = 253 for the Intention-to-Treat analysis and n = 209 for Per-Protocol analysis.
Significantly (P≤ .05) different from all other groups.
Significantly (P≤ .05) different from control group.
Significantly (P≤ .05) different from aerobic training group.
Significantly (P≤ .05) different from control group and resistance training group.
Significantly (P≤ .05) different from resistance training group.
The prevalence of increases in hypoglycemic medications were 39% in the control, 32% in the resistance training, 22% in the aerobic, and 18% in the combination training groups with the Mantel-Haenszel test for linear association being significant (P=.005). The prevalence of decreases in hypoglycemic medications were 15% in the control, 22% in the resistance training, 19% in the aerobic, and 26% in the combination training groups (P=.20). The prevalence of individuals who achieved the composite outcome of either decreasing hypoglycemic medication or reducing HbA1c by 0.5% without increasing medications were 22% in the control group, 26% in the resistance training, 29% in the aerobic, and 41% in the combination training groups (Mantel-Haenszel χ2, P=.02). The odds ratio of achieving the composite outcome compared with the control group was 1.5 (95% CI, 0.6-3.8) for the resistance training, 1.8 (95% CI, 0.7-4.70) for the aerobic, and 2.9 (95% CI, 1.2-7.0) for the combination training groups.
Twenty-one events qualified as serious adverse events with the prevalence similar across groups (control, 3 events; resistance training, 8 events; aerobic, 6 events; and combination taring, 4 events). The nature of the adverse events was diverse and included diverticulitis, emergency hysterectomy, lung cancer, 5 cardiovascular disease events (all unrelated to intervention), blood clot, and others. No serious adverse event occurred during exercise training and only 1 was considered associated with exercise.
Comment
The primary finding from this randomized, controlled exercise trial involving individuals with type 2 diabetes is that although both resistance and aerobic training provide benefits, only the combination of the 2 were associated with reductions in HbA1c levels. Furthermore, cumulative benefit across all outcomes was greater in the combination training group compared with either aerobic or resistance training alone. To our knowledge, this is the first large randomized trial involving individuals with type 2 diabetes to directly test exercise prescriptions that are consistent with the 2008 Physical Activity Guidelines of 500 to 1000 MET-minutes per week combined with 2 days of resistance training.4 The 2 most important goals in the development of the exercise doses for HART-D were to keep the total duration of weekly exercise similar across groups while ensuring that the aerobic prescriptions met current guidelines. We achieved both goals: total time spent exercising across the groups was approximately 140 min/wk, and the aerobic and the combination training groups performed approximately 680 and 570 MET/min per week, respectively. Our findings strongly support the 2008 Physical Activity Guidelines recommendation that optimal physical activity programs consist of regular physical activity combined with resistance training.
An absolute decrease of 1% in HbA1c levels has been associated with a 15% to 20% decrease in major cardiovascular disease events and 37% decrease in microvascular complications.11-13 Thus, our observed reduction of −0.3 to −0.4% HbA1c levels might be expected to produce a 5% to 7% reduction in cardiovascular disease risk and a 12% reduction in risk of microvascular complications. Furthermore, subgroup analysis showed that individuals with elevated baseline HbA1c (≥7.0%) had an HbA1c difference of −0.5% to −0.6%, which would be expected to decrease risk of cardiovascular disease events by 7% to 10% and microvascular complications by 18%. However, these risk reduction estimates are likely conservative because they are derived from medication studies and do not take into account improvements in cardiorespiratory fitness and strength and reductions in fat mass and waist circumference. For example, our findings that both the aerobic and combination training groups had a 1 MET increase in fitness compared with controls is of public health significance given that each MET is associated with 15% to 20% lower cardiovascular disease mortality risk.14-16
It also is important to appreciate that the follow-up difference in HbA1c between the combination training group and the control group occurred even though the control group had increased its use of diabetes medications while the combination training group decreased its diabetes medication uses. This is illustrated by the HbA1c-diabetes medication composite outcome achieved by 40% of individuals in the combination treatment group compared with only 22% in the control group.
Although the pattern of HbA1c change in the HART-D and DARE trials was similar to the aerobic and resistance training groups having approximately half the improvement in HbA1c reductions as the combination training group, the magnitude of change in each of the groups is smaller in HART-D than in the DARE trial. The difference in change in HbA1c in the combination groups (−0.3% vs −1.0%) may be explained by the larger resistance training dose performed by DARE participants, but it does not explain differences in the aerobic (−0.2 vs −0.5%) and resistance (−0.2 vs −0.4%) groups because these doses were similar in the 2 studies.
There are differences in the HART-D and DARE study populations and methods that may account for the different training responses. Compared with DARE participants, our participants had a longer duration of diabetes (7.1 vs 5.4 years), included more women (63% vs 36%), had a higher prevalence of non-white participants (47% vs 8%), and included 18.3% of the HART-D participants who were treated with insulin, whereas insulin use was an exclusion criterion in the DARE trial. The most important methodological difference between the trials may be the handling of the changes in hypoglycemic medications. In the DARE trial, steps were taken to minimize hypoglycemic medication changes resulting in a low prevalence of change in medications, whereas in HART-D, changes in hypoglycemic medication were left to the discretion of the participants and their physicians, resulting in substantial changes in hypoglycemic medication across groups.
All exercise groups in HART-D had a reduction of waist circumference compared with the control group. Only the combination training group lost weight, and while both the combination and resistance training groups lost fat mass compared with the control group, the aerobic group did not. The lack of weight loss in the resistance training group can be explained by the increase in lean mass. The failure of the aerobic group to lose a substantial amount of weight (or fat) has been reported in numerous aerobic exercise trials, which may be due to aerobic training resulting in energy intake, expenditure compensation, or both.18-20 However, in this instance the combination training group performed a similar dose of aerobic exercise yet lost weight and fat mass, leaving the possibility that the resistance training contributed to the weight loss. The potential role and mechanisms of resistance training in preventing lean mass loss and producing weight loss in combination of aerobic exercise is an area deserving of future research.
Strengths and Limitations
Strengths of the HART-D study include that this is an efficacy study, using tightly controlled exercise regimens, with all exercise completed in a laboratory with extensive monitoring of training. However, these ideal circumstances also represent a limitation in terms of dissemination. The population was diverse in age, sex, ethnicity, medication use, and comorbidities making our findings generalizable. Despite a population with many medical concerns, we obtained good exercise adherence and a low dropout rate. Furthermore, the exercise prescriptions performed are easily obtainable and well tolerated by individuals with diabetes, which has important implications for refining future physical activity recommendations. We used a food frequency questionnaire at baseline and follow-up to assess changes in diet, which limits our ability to identify changes in caloric intake and diet composition.
Conclusions
Among patients with type 2 diabetes mellitus, a combination of aerobic and resistance training compared with non-exercise control improved HbA1c levels; this was not achieved by aerobic or resistance training alone.
Acknowledgments
Funding/Support: This work was supported by grant DK068298 from the National Institutes of Health.
Role of Sponsor: The funding sponsor had no role in the design, protocol development, or in the conducting the trial, data collection, data analysis or preparing the manuscript.
Footnotes
Trial Registration clinicaltrials.gov Identifier: NCT00458133
Author Contributions: Dr Church had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Church, Blair, Mikus, Nauta.
Acquisition of data: Church, Cocreham, Johannsen, Kramer, Myers, Nauta, Rodarte, Sparks, Thompson, Earnest.
Analysis and interpretation of data: Church, Johannsen, Johnson, Kramer, Earnest.
Drafting of the manuscript: Church, Johannsen, Kramer, Myers, Nauta, Rodarte, Earnest.
Critical revision of the manuscript for important intellectual content: Church, Blair, Cocreham, Johannsen, Johnson, Mikus, Myers, Nauta, Rodarte, Sparks, Thompson, Earnest.
Statistical analysis: Church, Johnson, Thompson.
Obtained funding: Church, Blair, Mikus.
Administrative, technical, or material support: Church, Blair, Cocreham, Mikus, Myers, Nauta, Rodarte, Sparks, Earnest.
Study supervision: Church, Cocreham, Johannsen, Nauta, Sparks.
Financial Disclosures: Dr Church reported that he receives honoraria for lectures from scientific, educational, and lay groups; has received research funding from the American Heart Association and the National Institutes of Health, oversaw study sites for large pharmaceutical trials funded by Sanofi Aventis, Orexigen, Arena, and Amylin; is a member of the Jenny Craig medical advisory board; has served as a consultant to Technogym, Trestle Tree, Neuliven Health, and Coca-Cola; and serves as a paid expert for EverydayHealth.com. Dr Blair reported that he receives book royalties (<$5000 annually) from publisher Human Kinetics; honoraria for service on the scientific/medical advisory Boards for Alere, Technogym, Santech, and Jenny Craig; and honoraria for lectures and consultations from scientific, educational, and lay groups. During the past 5-year period he reported that he has received research grants from the National Institutes of Health, Department of Defense, Body Media, and Coca-Cola. Dr Earnest reported that he receives honoraria for lectures from scientific, educational, and lay groups. No other disclosures were reported.
Additional Contributions: We thank all HART-D participants and the Pennington Biomedical Research Center staff members Gina Billiot, Sheletta Donatto, RD, and Ronald Monce, PA-C, for their hard work and commitment. In addition we thank the HART-D data and safety monitoring board and Scientific Advisory Board, and Pennington Biomedical Research Center faculty members William Cefalu, MD, Steve Smith, MD (now at Burnham Institute in Orlando, Florida), and Jennifer Rood, PhD, for their scientific guidance both in planning and conducting the study. Part of Ms Donatto and Mr Monce's salaries were supported through the HART-D grant, and Dr. Rood's laboratory was reimbursed for clinical laboratory measures. Members of the data and safety monitoring board were provided modest honorariums for their time. Drs Cefalu and Dr Smith and the Scientific Advisory Board received no compensation for their contributions.
References
- 1.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Standards of medical care in diabetes—2007. Diabetes Care. 2007;30(suppl 1):S4–S41. doi: 10.2337/dc07-S004. [DOI] [PubMed] [Google Scholar]
- 3.Sherwin RS, Anderson RM, Buse JB, et al. American Diabetes Association; National Institute of Diabetes and Digestive and Kidney Diseases. Prevention or delay of type 2 diabetes. Diabetes Care. 2004;27(suppl 1):S47–S54. doi: 10.2337/diacare.27.2007.s47. [DOI] [PubMed] [Google Scholar]
- 4.US Department of Health and Human Services 2008 Physical Activity Guidelines for Americans. [Accessed August 2010];2008 http://www.health.gov/paguidelines/pdf/paguide.pdf.
- 5.Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001;286(10):1218–1227. doi: 10.1001/jama.286.10.1218. [DOI] [PubMed] [Google Scholar]
- 6.Marwick TH, Hordern MD, Miller T, et al. Council on Clinical Cardiology, American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; Council on Nutrition, Physical Activity, and Metabolism; Interdisciplinary Council on Quality of Care and Outcomes Research. Exercise training for type 2 diabetes mellitus: impact on cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119(25):3244–3262. doi: 10.1161/CIRCULATIONAHA.109.192521. [DOI] [PubMed] [Google Scholar]
- 7.Snowling NJ, Hopkins WG. Effects of different modes of exercise training on glucose control and risk factors for complications in type 2 diabetic patients: a meta-analysis. Diabetes Care. 2006;29(11):2518–2527. doi: 10.2337/dc06-1317. [DOI] [PubMed] [Google Scholar]
- 8.Sigal RJ, Kenny GP, Boulé NG, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147(6):357–369. doi: 10.7326/0003-4819-147-6-200709180-00005. [DOI] [PubMed] [Google Scholar]
- 9.American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. 7th. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 10.Block G, Thompson FE, Hartman AM, Larkin FA, Guire KE. Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. J Am Diet Assoc. 1992;92(6):686–693. [PubMed] [Google Scholar]
- 11.Selvin E, Marinopoulos S, Berkenblit G, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141(6):421–431. doi: 10.7326/0003-4819-141-6-200409210-00007. [DOI] [PubMed] [Google Scholar]
- 12.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agel J, Arendt EA, Bershadsky B. Anterior cruciate ligament injury in national collegiate athletic association basketball and soccer: a 13-year review. Am J Sports Med. 2005;33(4):524–530. doi: 10.1177/0363546504269937. [DOI] [PubMed] [Google Scholar]
- 14.Church TS, Cheng YJ, Earnest CP, et al. Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes Care. 2004;27(1):83–88. doi: 10.2337/diacare.27.1.83. [DOI] [PubMed] [Google Scholar]
- 15.Church TS, LaMonte MJ, Barlow CE, Blair SN. Cardiorespiratory fitness and body mass index as predictors of cardiovascular disease mortality among men with diabetes. Arch Intern Med. 2005;165(18):2114–2120. doi: 10.1001/archinte.165.18.2114. [DOI] [PubMed] [Google Scholar]
- 16.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301(19):2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 17.Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359(20):2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 18.King NA, Hopkins M, Caudwell P, Stubbs RJ, Blundell JE. Individual variability following 12 weeks of supervised exercise: identification and characterization of compensation for exercise-induced weight loss. Int J Obes (Lond) 2008;32(1):177–184. doi: 10.1038/sj.ijo.0803712. [DOI] [PubMed] [Google Scholar]
- 19.Ross R, Janssen I. Physical activity, total and regional obesity: dose-response considerations. Med Sci Sports Exerc. 2001;33((6)(suppl)):S521–S527. doi: 10.1097/00005768-200106001-00023. [DOI] [PubMed] [Google Scholar]
- 20.Church TS, Martin CK, Thompson AM, Earnest CP, Mikus CR, Blair SN. Changes in weight, waist circumference and compensatory responses with different doses of exercise among sedentary, overweight postmenopausal women. PLoS One. 2009;4(2):e4515. doi: 10.1371/journal.pone.0004515. [DOI] [PMC free article] [PubMed] [Google Scholar]