Abstract
Context
Analyses of smooth pursuit eye movement parameters in patients with schizophrenia provide information about the integrity of neural networks mediating motion perception, sensorimotor transformation, and cognitive processes such as prediction. Although pursuit eye tracking deficits have been widely reported in schizophrenia, the integrity of discrete components of pursuit responses and the effect of second-generation antipsychotic medication on them are not well established.
Objective
To examine different components of smooth pursuit performance in antipsychotic-naive patients with schizophrenia before and after treatment with second-generation antipsychotic medication.
Design, Setting, and Participants
Thirty-three antipsychotic-naive patients with schizophrenia performed 3 different smooth pursuit paradigms designed to evaluate specific components of the pursuit response. All of the patients were retested after 6 weeks of treatment with risperidone or olanzapine. Testing was also performed with 39 matched healthy individuals. Thirteen patients and 21 healthy participants were retested after 26 and 52 weeks.
Main Outcome Measures
Pursuit initiation, maintenance gain (ratio of eye velocity over target velocity), and frequency of catch-up saccades during pursuit maintenance.
Results
Prior to treatment, pursuit gain when tracking less predictable ramp targets tended to be reduced, latency of pursuit initiation was speeded, and catch-up saccade frequency was increased during predictive pursuit. After antipsychotic treatment initiation, pursuit gain decreased with ramp targets, indicating treatment-emergent impairments in sensorimotor processing. No changes were observed for predictive pursuit. Exploratory analyses in the subgroup with follow-up to 1 year revealed that these effects continued through long-term follow-up with some partial normalization at 1 year. Deficits were unrelated to drug dosage and clinical ratings.
Conclusions
Impaired sensorimotor function was observed after initiation of second-generation antipsychotic medications, which may be explained by their serotonergic antagonism of brainstem sensorimotor systems. Predictive mechanisms supported by frontostriatal-cerebellar circuitry were not affected by treatment initiation and appear able to compensate for treatment-emergent sensorimotor impairments during predictive tracking.
Smooth pursuit eye movements enable us to focus our eyes on moving objects by using well-established sensorimotor and cognitive mechanisms. Pursuit deficits in schizophrenia were first reported in 1908, making them perhaps the oldest biological marker for a major mental illness.1 Genetic linkage studies indicate that pursuit deficits may represent a useful intermediate phenotype for schizophrenia.2,3 Further, novel quantitative smooth pursuit measurements provide information about disturbances of distinct neurocognitive processes, including visual motion processing, sensorimotor transformation, and the use of predictive mechanisms.4–7
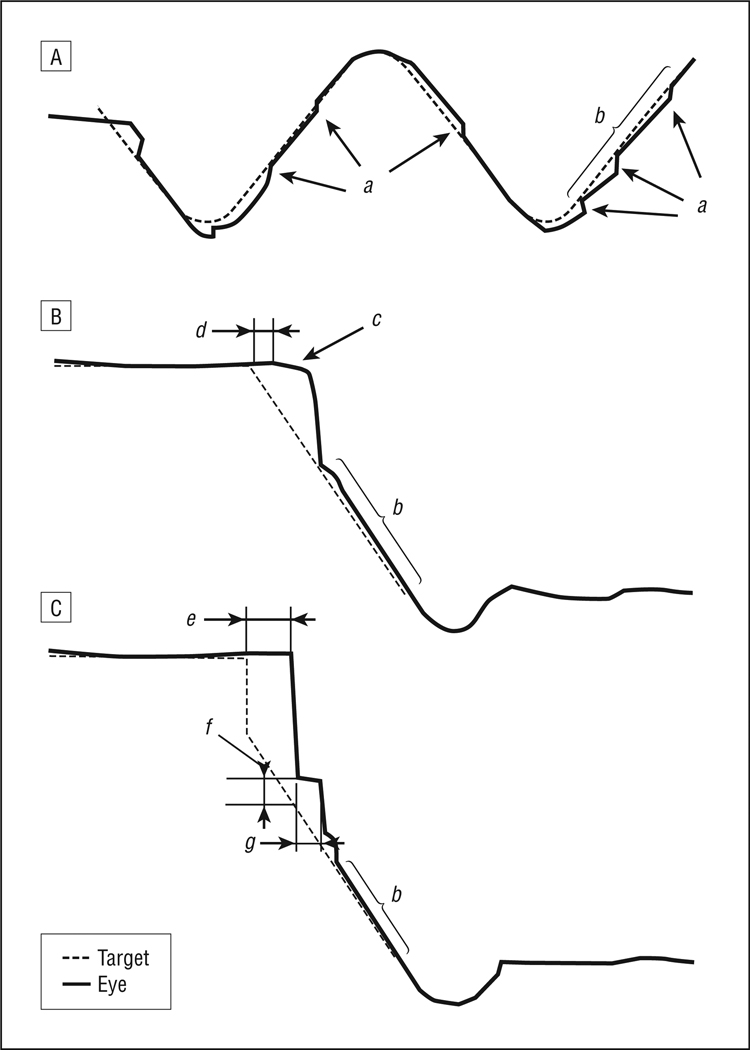
The maintenance of smooth pursuit is driven by a combination of the prediction of target velocity and visual feed-back about performance quality, with their loadings depending on the extent of experience with the pattern of target motion and the predictability of the stimulus.7–9 Small position errors due to slow pursuit velocity are corrected by catch-up saccades (CUS). Different pursuit tasks can isolate distinct components of the pursuit response (Figure 1), such as visual motion processing during pursuit initiation after saccades (step ramps), pursuit latency and maintenance when tracking unpredictable target ramps (pure ramps), and pursuit maintenance when tracking predictable target patterns (oscillating targets). Pursuit maintenance in unpredictable ramp tasks relies heavily on immediate visual feedback rather than prediction because of the unpredictability and brevity of target movement. In contrast, maintenance velocity when tracking regularly oscillating targets depends primarily on prediction derived from learning a repetitive pattern.
Figure 1.
Paradigms used to evaluate smooth pursuit performance: target and eye position data for the oscillating (A), pure ramp (B), and foveofugal step ramp (C) tasks. a indicates corrective catch-up saccades; b, period when pursuit maintenance was assessed; c, pursuit initiation in the pure ramp task; d, pursuit initiation latency; e, latency of the initial catch-up saccade; f, saccade error of the initial catch-up saccade; and g, pursuit initiation in the step ramp task.
Functional brain imaging and neurophysiological studies have defined the neural mechanisms subserving different components of smooth pursuit in humans.8,10–15 Extrastriatal area V5 is crucial for motion perception and processing, and the frontal eye fields generate oculomotor commands and contribute to predictive pursuit.16–18 The V5 lesions have especially pronounced effects on motion processing during pursuit initiation,19,20 whereas frontal eye field lesions slow pursuit initiation and impair maintenance but leave motion processing unimpaired.18,21 Other areas mediate sensorimotor, cognitive, and motor processes, including supplementary and parietal eye fields, anterior cingulate, dorsolateral prefrontal cortex, cerebellum, and basal ganglia.8,12,22,23 These areas directly or indirectly project to the dorsolateral pontine nuclei and the nucleus reticularis tegmenti pontis in the brainstem and then to the cerebellum.24
The quantitative evaluation of pursuit performance on different pursuit tasks in antipsychotic-naive patients with schizophrenia can help unravel the complex sensorimotor and cognitive system deficits associated with the disorder, independent from potential medication confounds. Further, assessing the effect of antipsychotic medication on pursuit systems in previously untreated patients may be informative about drug effects on multiple brain systems subserving smooth pursuit. Previous comparisons of antipsychotic-naive or treatment-withdrawn patients and patients treated with mostly first-generation antipsychotics revealed few consistent differences in global eye tracking measures, maintenance velocity, or CUS frequency.25–32 Longitudinal studies found pursuit performance to be relatively unaffected by first-generation an-tipsychotic medication.6,33,34 Some studies with patients receiving first-generation antipsychotics reported greater deficits in treated than untreated chronically ill patients, but selection bias rather than drug effects may have accounted for those observations.35–38
Investigations of saccadic eye movements indicate that oculomotor responses may be more affected by second-generation than first-generation antipsychotics,39–42 but the effects on pursuit have not been systematically studied. Some evidence indicates that clozapine impairs pursuit, but studies included only small sample sizes and examined only pursuit maintenance.28,30 Effects of second-generation antipsychotics on serotonergic as well as dopaminergic systems might account for this difference.43
The aims of our study were to use several pursuit paradigms to assess sensorimotor and cognitive mechanisms involved in pursuit control in antipsychoticnaive patients with schizophrenia and to examine the differential effects of second-generation antipsychotic treatment on pursuit performance.
METHODS
PARTICIPANTS
Thirty-three antipsychotic-naive patients (24 men, 9 women; mean [SD] age, 25.0 [7.0] years; mean [SD] premorbid IQ, 97.3 [8.1]) from inpatient and outpatient services met DSM-IV criteria for schizophrenia (n=30), schizoaffective disorder, depressed subtype (n=2), or schizophreniform disorder (n=1). Diagnoses were confirmed at consensus conferences using all of the available clinical data, including responses on the Structured Clinical Interview for DSM-III-R.44 Time since the first psychotic symptom until testing was on average 1 year (median, 12.2 months). Thirty-nine healthy control participants (24 men, 15 women; mean [SD] age, 23.5 [4.3] years; mean [SD] premorbid IQ, 99.0 [6.3]) without any history of Axis I disorders (according to the Structured Clinical Interview for DSM-III-R) and without any known history of psychotic or mood disorder with psychotic features in their first-degree relatives (participant’s report) were recruited from the surrounding community via advertisements. Groups were matched on age (t70=−1.13; P=.27) and premorbid IQ (t70=1.02; P=.31) estimated by vocabulary test performance.45 Inclusion criteria for both groups included the following: (1) age between 16 and 45 years; (2) premorbid IQ greater than 80; (3) no known systemic or neurological disease; (4) no history of head trauma with loss of consciousness for more than 10 minutes; (5) no lifetime history of substance dependence and no substance abuse for at least 3 months; (6) no alcohol (24 hours), coffee, tea, or cigarettes (1 hour) prior to testing according to self-report and clinical observation; (7) no lifetime exposure to lithium carbonate, lithium citrate, mood stabilizers, stimulants, or anti-cholinergics; and (8) no benzodiazepines or antidepressants (5 half-lives) before testing (as an exception, 1 patient was tested after 4 days [3.5 half-lives] of withdrawal from 50 mg of sertraline hydrochloride). Follow-up studies were conducted 6 weeks after baseline testing for all of the participants. Additionally, 13 patients and 21 control participants were retested after 26 and 52 weeks. The study was approved by the University of Pittsburgh Institutional Review Board, and all of the participants provided informed consent.
Clinical ratings were obtained for patients at each time of testing (Table): (1) the Brief Psychiatric Rating Scale46; (2) the Schedule for the Assessment of Negative Symptoms47; (3) the Schedule for the Assessment of Positive Symptoms47; and (4) the Extrapyramidal Side-Effects Scale.48 Medication assignment was not randomized as treatment choice was guided by standard clinical practice. Medication dosages were stable in the week prior to each testing.
Table.
Clinical Ratings and Antipsychotic Medication for Patients With Schizophrenia at Baseline and 6-, 26-, and 52-Week Follow-upa
| Mean (SD) |
||||
|---|---|---|---|---|
| Scale and Medication |
Baseline (n=33) |
6 wk (n=33) |
26 wk (n=13) |
52 wk (n=13) |
| Scale score | ||||
| BPRS | 49.0 (8.6) | 41.5 (8.1)b | 38.4 (9.9) | 34.1 (9.4) |
| SANS | 14.2 (3.1) | 13.0 (2.2)b | 13.0 (3.2) | 11.8 (3.1) |
| SAPS | 9.1 (3.4) | 5.5 (3.2)b | 4.4 (3.5) | 3.9 (3.2) |
| EPS | NA | 3.0 (3.2) | 3.0 (3.2) | 3.1 (2.9) |
| Risperidone | NA | (n = 29) | (n = 11) | (n = 11) |
| Dose, mg | NA | 3.9 (2.1) | 3.2 (1.1) | 3.2 (1.7) |
| Olanzapine | NA | (n=4) | (n=2) | (n=2) |
| Dose, mg | NA | 11.25 (6.3) | 15.00 (7.1) | 15.00 (7.1) |
Abbreviations: BPRS, Brief Psychiatric Rating Scale; EPS, Extrapyramidal Side-Effects Scale; NA, not applicable; SANS, Schedule for the Assessment of Negative Symptoms; SAPS, Schedule for the Assessment of Positive Symptoms.
Comparisons of change in clinical scores and medication dosages from 6 to 52 weeks yielded no significant changes.
Comparisons of baseline to 6 weeks (paired t tests): t30=5.07, P<.001 for the BPRS; t32=3.49, P=.001 for the SANS; and t32=5.34, P<.001 for the SAPS.
A significant reduction of psychopathological symptoms was observed after introduction of antipsychotic medication, and this persisted at 26 and 52 weeks (Table).
STIMULUS PRESENTATION AND ASSESSMENT OF EYE MOVEMENTS
Visual acuity testing assured a minimum of 20/40 far acuity, and visual acuity was corrected to that threshold if necessary. Eye movement studies were performed in a darkened black room. Subjects were seated at the center of a circular black arc (1-m radius) with their head immobilized by a chin rest and forehead and occipital restraints. The visual stimulus was a laser spot (3 mm) projected by a mirror and mounted on a rotary stage platform that moved the target across the display arc under computer control (New England Affiliated Technologies, Lawrence, Massachusetts). Subjects were instructed via intercom to always follow the moving target with their eyes as precisely as possible. Eye movement data were inspected online during testing, and re-alerting instructions were given if subjects became inattentive. Eye movement recording was performed using infrared sensors mounted on spectacle frames (model 210; Applied Science Laboratories, Inc, Bedford, Massachusetts). Fixation targets were presented for 5 seconds at 0°, ±3°, ±6°, ±9°, ±12°, and ±15° before or after each task to calibrate the eye movement data.
ANALYSIS OF EYE MOVEMENTS
Eye movement data were digitized at 500 Hz with a 14-bit A/D converter. Signals were smoothed and filtered with a nonlinear transition band between 20 and 65 Hz for eye velocity and between 30 and 65 Hz for acceleration data. Data from each trial were visually inspected to eliminate blinks and artifacts. Saccade onset was defined as the point when eye acceleration exceeded 1000°/s2, and saccade end points were identified at 25% of peak deceleration. Measurement resolution allowed detection of saccades with amplitudes on the order of 0.20° to 0.25°. All types of saccades were excluded from data before calculating smooth pursuit gain, which reflects the ratio of eye velocity to target velocity. Additionally, a 10-millisecond interval following the end point of saccades was excluded when calculating pursuit gain. All of the eye movement data were scored blinded to group membership.
OSCILLATING TASK
The oscillating task assessed sustained smooth pursuit of predictable target motion (Figure 1A). The paradigm was similar to an oscillating sinusoidal waveform except that across the center of the display screen the target moved at a constant speed. This was done for 2 reasons. First, it facilitated measurement of maintenance gain by allowing comparison of average pursuit velocity with a constant stimulus velocity. Second, it eliminated demands during the constant velocity epochs for ongoing dynamic adjustment of pursuit velocity as is required when tracking sinusoidal target motion. We presented predictable oscillating targets moving back and forth across the display arc covering ±17° in total. Beyond the positions of ±12°, target speed gradually decelerated to reverse its direction at ±17°, at which point it immediately accelerated again until it reached a constant speed at ±12°. One trial consisted of 1 full sweep of the target from ±17° to the opposing 17°. Blocks of target oscillation with a particular constant target speed (8°/s, 16°/s, 24°/s, or 32°/s) between ±12° included 13, 15, 23, and 31 trials, respectively. The primary parameter of interest was maintenance gain between ±10° of target sweeps (b in Figure 1A). We further determined the frequency of CUS and mean CUS amplitudes during this epoch (a in Figure 1A).
PURE RAMP TASK
In the pure ramp task, subjects tracked targets that moved at an unpredictable time, direction, and speed from center fixation to assess pursuit initiation latency and tracking of less predictable targets (Figure 1B). Each trial started with an initial central fixation for 2 to 4 seconds before targets moved at a constant speed to either the left or the right at 1 of 5 target speeds (4°/s, 8°/s, 16°/s, 24°/s, or 32°/s). The target was extinguished after reaching ±15° and reappeared at the central fixation position after a 1-second delay to begin the next trial. Target conditions were presented in a randomized order with each condition presented 4 times, resulting in a total of 40 trials (4 repetitions × 5 speeds × 2 directions). Whenever subjects reduced pursuit velocity near the end of ramps, such data were removed from analyses before calculating gain. Parameters of interest were maintenance gain (b in Figure 1B) and latency of pursuit initiation (time for pursuit velocity to reach 2°/s for ≥ 20 milliseconds if that preceded the first CUS) (d in Figure 1B).
FOVEOFUGAL STEP RAMP TASK
The foveofugal step ramp task was similar to the pure ramp task except that after initial central fixation for 2 to 4 seconds, the target stepped 3° to the left or right before continuing in that direction at 4°/s, 8°/s, 16°/s, or 24°/s (Figure 1C). This task assessed the use of visual motion information in the “open-loop period” before visual feedback could influence performance. As in the pure ramp task, each ramp moved 15° from central fixation. The task consisted of 32 trials (4 repetitions × 4 speeds × 2 directions) presented in a fixed pseudorandom order. Parameters of interest were the position error of the initial CUS (f in Figure 1C) and pursuit initiation gain during the first 100 milliseconds after the initial CUS (g in Figure 1C). These parameters together provide data regarding how the pursuit movement uses visual motion information.19 Maintenance gain in the remaining interval after the 100-millisecond “open-loop period” (b in Figure 1C) and the latency of the initial CUS following the target step (e in Figure 1C) were also measured.
STATISTICAL ANALYSIS
Measures of parameters of interest were averaged across identical trials. Because we did not find evidence for an interaction of target direction with group using 4-way repeated-measures analyses of variance (target direction × target speed × time of testing × group), leftward and rightward tracking performance was combined in all of the analyses. Two-way mixed analyses of variance (target speed × group) were computed using baseline data to test for group differences before treatment. In the full sample of 33 patients and 39 control participants, a 3-way repeated-measures analysis of variance (target speed × time of testing × group) was performed to identify treatment-emergent effects during the 6-week short-term follow-up. Exploratory analyses of the long-term follow-up data using 3-way mixed repeated-measures analyses of variance were conducted to determine persistence or normalization of treatment-emergent effects, with data from 6-, 26-, and 52-week follow-up included in the analyses. Subjects in this subgroup did not differ from the original sample in sociodemographic or clinical ratings or in baseline eye movement performance. The robust effects of target speed on tracking performance are not presented in detail except when interactions with group or time of testing effects were significant. Means and standard deviations of all of the parameters for each task are presented in eTables 1, 2, 3, and 4 (http://www.archgenpsychiatry.com).
RESULTS
OSCILLATING TASK
Baseline
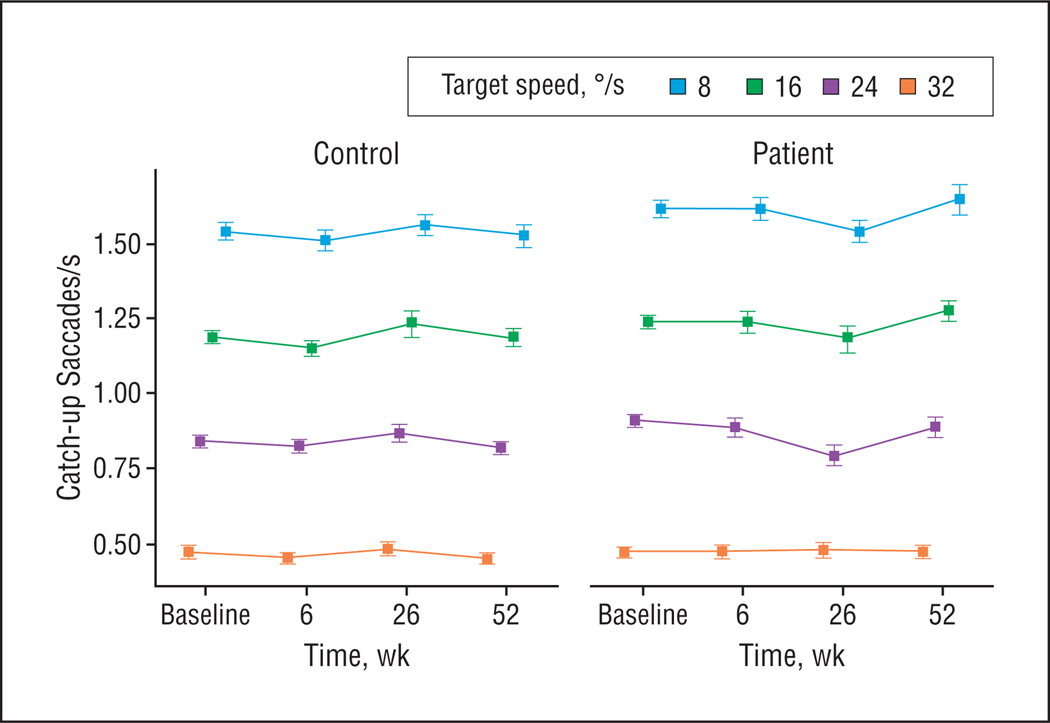
Maintenance gain in the oscillating task did not differ between antipsychotic-naive patients and control participants (Figure 2A). Patients exhibited higher CUS rates than control participants (F1,70[group] =6.09; P = .02) (Figure 3), and group differences increased with higher target speeds (F3,68[speed × group] =4.37; P = .007).
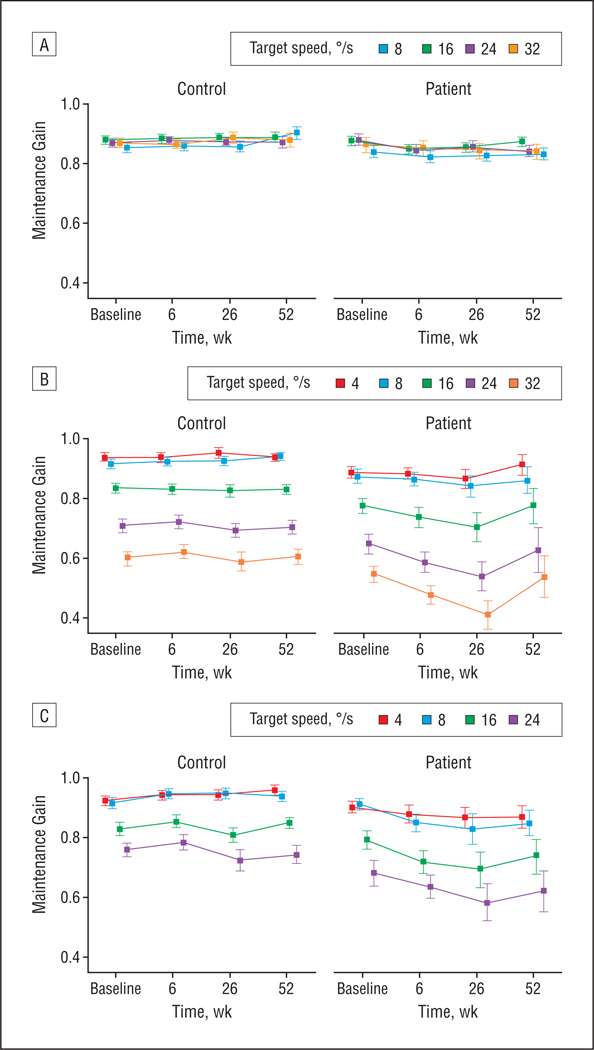
Figure 2.
Mean smooth pursuit maintenance gain with the oscillating (A), pure ramp (B), and foveofugal step ramp (C) tasks at different target speeds measured in 33 patients with schizophrenia and 39 control participants at baseline and 6-week follow-up. The 26- and 52-week follow-up data are from 13 patients and 21 control participants from the original group who were available for long-term follow-up testing. Values represent group means and standard error.
Figure 3.
Frequency of catch-up saccades per second in the oscillating task at different target speeds measured in 33 patients with schizophrenia and 39 control participants at baseline and 6-week follow-up. The 26- and 52-week follow-up data are from 13 patients and 21 control participants from the original group who were available for long-term follow-up testing. Values represent group means and standard errors.
Short-term Follow-up
There was no change in maintenance gain or CUS rate after treatment initiation in patients compared with control participants (Figure 2A). Elevated CUS rates in patients persisted during the short-term follow-up (F1,70[group] =8.92; P= .004), as did the increased group difference with higher target speeds (F3,68[speed × group] =5.34; P = .002) (Figure 3).
Long-term Follow-up
The CUS rates in patients increased from the 6- to 52-week follow-up but remained the same in control participants (F2,27[time × group] =3.57; P=.04) (Figure 3). There were no significant changes in maintenance gain.
For the CUS amplitudes (overall mean [SD], 1.53° [0.30°] in patients and 1.54° [0.24°] in control participants), no effects were found at baseline, short-term follow-up, or long-term follow-up.
PURE RAMP TASK
Baseline
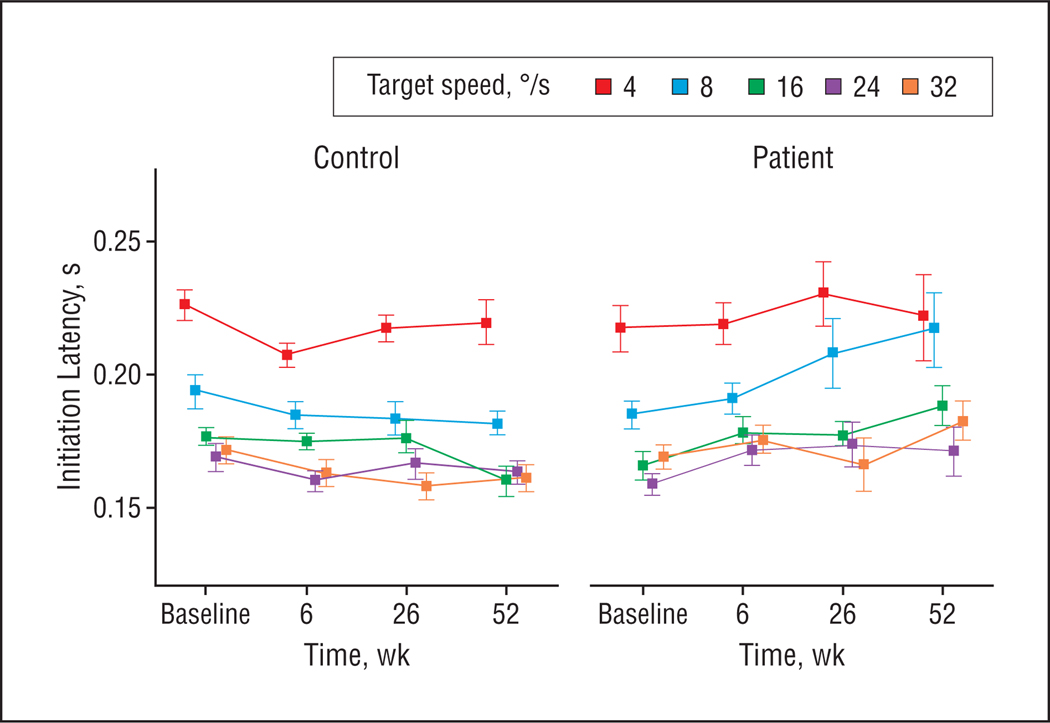
During the less predictable ramp task, there was a trend for patients’ maintenance gain to be lower than that of control participants (F1, 70[group] =3.85; P=.05) (Figure 2B). Patients initiated pursuit faster than control participants (F1,48[group] =5.56;P=.02) (Figure 4).
Figure 4.
Smooth pursuit initiation latency in the pure ramp task at different target speeds measured in 30 patients with schizophrenia and 35 control participants at baseline and 6-week follow-up. The 26- and 52-week follow-up data are from 12 patients and 18 control participants from the original group who were available for long-term follow-up testing. Values represent group means and standard errors.
Short-term Follow-up
Maintenance gain was decreased in patients compared with control participants (F1,70[group] =8.07; P = .008), an effect that occurred from baseline to 6-week follow-up (F1,70[time × group] =5.52; P = .02). The gain decrease in patients tended be greater at higher target speeds (F4,67[speed × time × group] =2.50; P=.05) (Figure 2B). During the 6-week follow-up, pursuit initiation latencies slowed in patients relative to control participants (F1,41[time × group] =9.54; P = .004), eliminating group differences observed at baseline (Figure 4).
Long-term Follow-up
Maintenance gain was reduced in patients compared with control participants during long-term follow-up (F1,32[group] =7.60; P=.01), with different changes in patients than in control participants over time (F2,31[time × group] =4.60; P=.02) (Figure 2B). Maintenance gain in patients continued to be decreased from the 6-week follow-up to the 26-week follow-up but increased by 1 year (F1,12[time] =5.50; P= .04), whereas in control participants it remained stable over time. These maintenance gain changes in patients were greater at higher target speeds (F8,25 [speed × time × group] =2.96; P=.02). For pursuit initiation latency, no group differences were observed during long-term follow-up (Figure 4).
FOVEOFUGAL STEP RAMP TASK
Baseline
Pursuit initiation gain (first 100 milliseconds) tended to be lower in antipsychotic-naive patients than control participants (F1,69[group] =3.26; P=.08) (Figure 5A). The position error of the initial CUS (Figure 5B) and maintenance gain (Figure 2C) did not differ between groups, but patients tended to make their initial CUS faster than control participants (F1,56[group] =3.07; P=.08) (Figure 5C).
Figure 5.
Smooth pursuit initiation gain (A) and catch-up saccades (CUS) error (B) and latency (C) in the foveofugal step ramp task at different target speeds measured in 33 patients with schizophrenia and 39 control participants at baseline and 6-week follow-up. The 26- and 52-week follow-up data are from 13 patients and 21 control participants from the original group who were available for long-term follow-up testing. Values represent group means and standard errors.
Short-term Follow-up
Pursuit initiation gain in patients was decreased compared with control participants (F1,67 [group] = 6.55;P=.01), an effect that tended to be more pronounced after treatment initiation (F1,67[time × group] =3.15; P=.08) (Figure 5A). The position error of the initial CUS (Figure 5B) did not change after treatment initiation. Latencies of initial CUS in patients increased during 6 weeks of treatment relative to control participants (F1,70[time × group] =9.49; P=.003), eliminating the pretreatment group difference (Figure 5C). During the 6-week follow-up, maintenance gain decreased in patients relative to control participants (F1,70[time × group] =8.56; P=.005) (Figure 2C) so that it was lower than in control participants (F1,70[group] =5.70; P=.02). This effect parallels findings for initiation gain from the foveofugal step ramp task and for maintenance gain from the pure ramp task.
Long-term Follow-up
Pursuit initiation gain (F1,31 [group] =11.76; P=.002) (Figure 5A) and maintenance gain (F1,32[group] =10.87; P=.002) (Figure 2C) were reduced in patients compared with control participants, representing persistence of short-term treatment-emergent effects. Changes in maintenance gain (F2,31[time × group] =4.44; P=.02) and a similar trend in initiation gain (F2,30 [time × group] = 3.08; P=.06) suggested some normalization of function by 52 weeks in patients. Analysis of position errors of the initial CUS revealed lower accuracy of saccades in patients (F1,32[group] =4.22; P=.048) (Figure 5B). No changes in latencies of initial CUS were observed (Figure 5C).
OCULOMOTOR PARAMETERS AND CLINICAL CHARACTERISTICS
Spearman rank correlations between the Schedule for the Assessment of Negative Symptoms, Schedule for the Assessment of Positive Symptoms, and Extrapyramidal Side-Effects Scale scores and oculomotor parameters did not reveal significant relationships between clinical ratings and eye movement parameters at any time. Duration of time since the onset of psychotic symptoms prior to testing was not correlated with any oculomotor parameter, making illness duration unlikely to be related to changes in smooth pursuit performance observed after treatment initiation. Because the group of patients treated with olanzapine was too small to conduct any medication-specific analysis, we performed analysis of eye movement data with risperidone-treated patients only. This yielded no different findings for any task compared with those reported for the whole sample. Although the medication dose range in patients receiving risperidone was rather narrow, it did not correlate with any oculomotor parameter.
COMMENT
This is the first longitudinal study evaluating sensorimotor and cognitive components of smooth pursuit initiation and maintenance in antipsychotic-naive patients with schizophrenia. Our findings document modest impairments of the pursuit system in these patients at baseline, including trends for reduced pursuit gain of less predictable ramp targets, speeded pursuit latency, and increased CUS rates during predictable pursuit. Initiation of second-generation antipsychotic medication consisting of risperidone or olanzapine yielded an effective reduction of psychotic symptoms after 6 weeks. Pursuit gain of less predictable targets decreased significantly during these first weeks of treatment and pursuit latency normalized, but tracking of predictable oscillating targets did not change. Exploratory long-term follow-up in a subset of participants indicated that these effects continued during the first 6 months of treatment with partial normalization by 1 year. There were no systematic correlations between oculomotor parameters and psychopathological symptoms or extrapyramidal side effects. The treatment-related changes with less predictable ramp tasks that require sensorimotor processing to a high extent document a selective effect of second-generation antipsychotic drugs on sensorimotor control of pursuit responses. This is in accordance with earlier reports of pursuit gain reduction associated with clozapine treatment28,30 and saccade latency prolongation with risperidone.39,40 Furthermore, the observation of unchanged pursuit of predictable oscillating targets relying heavily on prediction and rule-based learning but less on sensorimotor processing implies that higher-order cognitive or predictive signals were not affected by treatment initiation. These findings provide novel information about the effect of second-generation antipsychotic drugs on functional brain systems in patients with schizophrenia.
SMOOTH PURSUIT PERFORMANCE IN ANTIPSYCHOTIC-NAIVE PATIENTS
We observed trends for pursuit gain reductions with less predictable ramp targets prior to treatment, in accordance with previous studies of antipsychotic-naive patients with schizophrenia.5,6,25,26,30,32,36,37 Increased CUS rates in the predictive oscillating task are also consistent with earlier reports.27,28,30,49,50 Unimpaired initial CUS accuracy in the step ramp task suggests that CUS efficiently corrected for small position errors when tracking moving targets. The presence of increased CUS rates together with unimpaired maintenance gain in the oscillating task might be explained by an instability of the pursuit response, resulting in increased aggregate CUS rates due to brief periods of slow pursuit rather than consistently slowed pursuit maintenance velocity.49
Differences in task demands in our oscillating target task, relative to previous studies using triangular or sinusoidal waveforms to evaluate pursuit, might explain why we did not observe maintenance gain impairment with that task. Patients with schizophrenia might have greater difficulty dynamically adjusting pursuit velocity and acceleration required for sinusoidal target tasks or have difficulty predicting abrupt reversals in target movement direction or motion onset as with triangular or trapezoidal waveforms.31 When these additional task demands were removed, patients with schizophrenia were able to match eye velocity to that of a predictable constant target similar to healthy subjects, even at high target speeds. This indicates that at least some cognitive predictive mechanisms for sustained pursuit are spared in schizophrenia51,52 even though the ability to initiate and maintain predictive pursuit with a combined reliance on temporal and velocity memory signals in the absence of a visual target may be impaired.31
Normal saccadic CUS amplitude errors in the step ramp task before treatment imply sufficient integrity of visual motion information processing in extrastriatal area V5,5,19,53,54 although pursuit initiation gain also depending on visual motion signals tended to be impaired. Interestingly, we did find evidence for faster pursuit initiation in schizophrenia before treatment, indicating speeded sensorimotor processing. These results differ from our previous study5 where we did not find evidence for speeded pursuit latency. This discrepancy might be due to different stimulus presentation because in our previous study, pursuit was not driven by a continuously moving visual target such as in the present investigation but by a sequentially illuminated line of light-emitting diodes. Faster pursuit initiation as observed in the present investigation might be related to a reduction in top-down inhibitory prefrontal regulation of attentional processes that has been proposed to account for speeded latencies of visually guided saccades in antipsychoticnaive patients.40 Consistent with this pattern, we found a trend for speeded initial CUS latencies with the step ramp task at baseline.
ADVERSE EFFECTS OF SECOND-GENERATION ANTIPSYCHOTIC MEDICATION ON SMOOTH PURSUIT OF LESS PREDICTABLE TARGETS
Ramp tasks impose a higher demand for online sensorimotor processing than sustained pursuit of oscillating targets,7,9 which is reflected in the dramatically lower maintenance gains for faster-moving ramp targets than oscillating targets (Figure 2). Significantly decreased gain for pursuit initiation and maintenance on ramp tasks at 6-week follow-up that continued during the first 6 months of treatment without a parallel effect on sustained predictive pursuit therefore implies an adverse influence of second-generation antipsychotic medication on sensorimotor systems rather than on cognitive or predictive pursuit control. Exploratory analyses suggest partial normalization by 1 year, which could be due to the development of partial tolerance to drug effects. Because we did not find significant changes in psychopathological symptoms or medication dosages during long-term follow-up, illness recovery or decreased medication dosages are unlikely to explain the partial normalization of sensorimotor function. However, the size of the follow-up sample was small, and blood drug-level measurements were not obtained to ensure that reduced treatment compliance did not account for reduced pursuit deficits at 1 year. Further studies are required to confirm the presence and time course of tolerance development. Consistent with the reported effects on sensorimotor systems when tracking less predictable targets, latencies of pursuit and saccade initiation increased after medication initiation, yielding post-treatment findings similar to those of previous studies with antipsychotic-treated patients.5,6,39,40,55,56
Worsening of pursuit after second-generation antipsychotic treatment with clozapine has been attributed to its antagonism of serotonin 2A receptors, a property shared by risperidone and olanzapine.28,30,43,57,58 Seroton-ergic effects on pursuit have only rarely been studied. A single dose of MK-212, a direct serotonin agonist, was reported to increase maintenance gain and reduce CUS frequency in 10 healthy subjects, suggesting that activation of some serotonin receptors facilitates pursuit59; however, no effect was found in another study with sertraline, a serotonin reuptake inhibitor.60 In our previous study using ramp tasks with first-episode patients before and after treatment, first-generation antipsychotic medication did not impair sensorimotor aspects of pursuit control.6 This difference from the present study is consistent with an important role for serotonergic mechanisms in the sensorimotor changes reported here. Similarly, risperidone but not haloperidol has been reported to impair sensorimotor control of visually guided saccades to stationary targets.39–42 These latter effects have been attributed to serotonergic antagonism on saccade-regulating neurons in the brainstem, which are under inhibitory serotonergic regulation from the dorsal raphe nucleus.39,61 Altered serotonergic activity might result in a disturbance of the precisely temporally integrated synchronicity of these neurons, which could reduce saccadic velocities and slow latencies.61 Similar effects on the dorsolateral pontine nuclei and the nucleus reticularis tegmenti pontis in the brainstem that encode a variety of pursuit-related signals could potentially disrupt the pursuit system as well.24,62 More translational research is needed to investigate whether second-generation antipsychotic agents cause adverse treatment-emergent effects on visual sensorimotor systems via serotonin mechanisms.
MINIMAL EFFECTS OF SECOND-GENERATION ANTIPSYCHOTIC MEDICATION ON PREDICTIVE PURSUIT
The absence of treatment-induced maintenance gain impairment with oscillating targets suggests that cognitive or predictive mechanisms may be able to sufficiently compensate for treatment-emergent sensorimotor deficits during predictive pursuit.
Recent studies support the hypothesis of a greater reliance on predictive signals during pursuit in patients with schizophrenia. Higher pursuit velocity in treated patients with schizophrenia compared with healthy subjects was reported after a target was switched off during predictable sinusoidal target movements,51 and patients showed closer adherence to predictive, repetitive target patterns as revealed with predictive saccade tasks.54,63,64 Predictive sustained pursuit requires motor learning mechanisms mediated by frontostriatal and cerebellar systems.9,12,22,65 Accordingly, a recent imaging study revealed greater activation during predictive pursuit in medicated patients with schizophrenia than in healthy control subjects in the dorsolateral prefrontal cortex, thalamus, and cerebellar hemispheres, a network that modulates planning and rule-based learning during pursuit.8,11,13,14,22,52,66–69 Furthermore, greater activation of the frontal eye fields and the anterior cingulate during pursuit was found in patients when pursuit had to be generated during intervals of target blanking, which is consistent with an increased reliance on predictive systems for pursuit control in schizophrenia.22,52 Predictive modulation of pursuit in our study could be maintained by sufficiently intact components in frontostriatal-cerebellar circuitry. Alternatively, serotonergic effects may protect striatal systems to allow compensation for adverse treatment-emergent effects on visual sensorimotor systems as has been shown for neuropsychological functions depending on the same neural systems.70
In conclusion, treatment with low to moderate dosages of second-generation antipsychotic agents such as risperidone and olanzapine led to treatment-emergent sensorimotor impairments in patients with schizophrenia. Exploratory analyses suggest that partial normalization may have developed to some of these sensorimotor effects by 1 year of treatment. In comparison, cognitive mechanisms such as prediction seemed sufficiently unaffected by treatment initiation to be able to provide compensation for these sensorimotor disturbances.
Because serotonin 2A antagonists may preserve striatal function and minimize extrapyramidal side effects,71 our findings imply that brainstrem sensorimotor mechanisms under serotonergic control may be adversely affected. More studies are needed to further specify these effects with regard to potential differences between individual drugs and drug classes, their neuropharmaco-logical mechanisms, and their time course. The effects of second-generation antipsychotics on pursuit systems, as previously established with saccade paradigms, as well as the modest pursuit disturbances evident prior to treatment using the specific paradigms in this study suggest that the severity of disorder-related impairment in pursuit tracking may be overestimated when patients treated with second-generation antipsychotic medication are assessed.
Acknowledgments
Funding/Support: This work was supported by grants MH62134, MH45156, and MH01433 from the National Institutes of Health, grant M01 RR00056 from the General Clinical Research Centers, National Center for Research Resources, National Institutes of Health, grant RIS-INT-35 from Janssen, and a Feodor Lynen Fellowship provided by the Alexander von Humboldt Foundation (Dr Lencer).
Footnotes
Financial Disclosure: None reported.
Additional Information: The eTables are available at http://www.archgenpsychiatry.com.
Additional Contributions: Gretchen Haas, PhD, Cameron Carter, MD, Debra Montrose, PhD, and the clinical core staff of the Center for the Neuroscience of Mental Disorders (under director David Lewis, MD) provided assistance in diagnostic and psychopathological assessments.
REFERENCES
- 1.Diefendorf AR, Dodge R. An experimental study of the ocular reactions on the nsane from photographic records. Brain. 1908;31:451–489. [Google Scholar]
- 2.Arolt V, Lencer R, Purmann S, Schürmann M, Müller-Myhsok B, Krecker K, Schwinger E. Testing for linkage of eye tracking dysfunction and schizophrenia to markers on chromosomes 6, 8, 9, 20, and 22 in families multiply affected with schizophrenia. Am J Med Genet. 1999;88(6):603–606. [PubMed] [Google Scholar]
- 3.Matthysse S, Holzman PS, Gusella JF, Levy DL, Harte CB, Jørgensen A, Møller L, Parnas J. Linkage of eye movement dysfunction to chromosome 6p in schizophrenia: additional evidence. Am J Med Genet B Neuropsychiatr Genet. 2004;128B(1):30–36. doi: 10.1002/ajmg.b.30030. [DOI] [PubMed] [Google Scholar]
- 4.Levy DL, Lajonchere CM, Dorogusker B, Min D, Lee S, Tartaglini A, Lieberman JA, Mendell NR. Quantitative characterization of eye tracking dysfunction in schizophrenia. Schizophr Res. 2000;42(3):171–185. doi: 10.1016/s0920-9964(99)00122-x. [DOI] [PubMed] [Google Scholar]
- 5.Sweeney JA, Luna B, Haas GL, Keshavan MS, Mann JJ, Thase ME. Pursuit trackng impairments in schizophrenia and mood disorders: step-ramp studies with unmedicated patients. Biol Psychiatry. 1999;46(5):671–680. doi: 10.1016/s0006-3223(99)00132-8. [DOI] [PubMed] [Google Scholar]
- 6.Sweeney JA, Luna B, Srinivasagam NM, Keshavan MS, Schooler NR, Haas GL, Carl JR. Eye tracking abnormalities in schizophrenia: evidence for dysfunction in the frontal eye fields. Biol Psychiatry. 1998;44(8):698–708. doi: 10.1016/s0006-3223(98)00035-3. [DOI] [PubMed] [Google Scholar]
- 7.Becker W, Fuchs AF. Prediction in the oculomotor system: smooth pursuit during transient disappearance of a visual target. Exp Brain Res. 1985;57(3):562–575. doi: 10.1007/BF00237843. [DOI] [PubMed] [Google Scholar]
- 8.Burke MR, Barnes GR. Brain and behavior: a task-dependent eye movement study. Cereb Cortex. 2008;18(1):126–135. doi: 10.1093/cercor/bhm038. [DOI] [PubMed] [Google Scholar]
- 9.Burke MR, Barnes GR. Sequence learning in two-dimensional smooth pursuit eye movements in humans. J Vis. 2007;7(1):5. doi: 10.1167/7.1.5. [DOI] [PubMed] [Google Scholar]
- 10.Petit L, Haxby JV. Functional anatomy of pursuit eye movements in humans as revealed by fMRI. J Neurophysiol. 1999;82(1):463–471. doi: 10.1152/jn.1999.82.1.463. [DOI] [PubMed] [Google Scholar]
- 11.Berman RA, Colby CL, Genovese CR, Voyvodic JT, Luna B, Thulborn KR, Sweeney JA. Cortical networks subserving pursuit and saccadic eye movements in humans: an FMRI study. Hum Brain Mapp. 1999;8(4):209–225. doi: 10.1002/(SICI)1097-0193(1999)8:4<209::AID-HBM5>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagel M, Sprenger A, Zapf S, Erdmann C, Kompf D, Heide W, Binkofski F, Lencer R. Parametric modulation of cortical activation during smooth pursuit with and without target blanking: an fMRI study. Neuroimage. 2006;29(4):1319–1325. doi: 10.1016/j.neuroimage.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 13.Lencer R, Nagel M, Sprenger A, Zapf S, Erdmann C, Heide W, Binkofski F. Cortical mechanisms of smooth pursuit eye movements with target blanking: an fMRI study. Eur J Neurosci. 2004;19(5):1430–1436. doi: 10.1111/j.1460-9568.2004.03229.x. [DOI] [PubMed] [Google Scholar]
- 14.Tanabe J, Tregellas J, Miller D, Ross RG, Freedman R. Brain activation during smooth-pursuit eye movements. Neuroimage. 2002;17(3):1315–1324. doi: 10.1006/nimg.2002.1263. [DOI] [PubMed] [Google Scholar]
- 15.O’Driscoll GA, Wolff AL, Benkelfat C, Florencio PS, Lal S, Evans AC. Functional neuroanatomy of smooth pursuit and predictive saccades. Neuroreport. 2000;11(6):1335–1340. doi: 10.1097/00001756-200004270-00037. [DOI] [PubMed] [Google Scholar]
- 16.Dukelow SP, DeSouza JF, Culham JC, van den Berg AV, Menon RS, Vilis T. Distinguishing subregions of the human MT+ complex using visual fields and pursuit eye movements. J Neurophysiol. 2001;86(4):1991–2000. doi: 10.1152/jn.2001.86.4.1991. [DOI] [PubMed] [Google Scholar]
- 17.Rosano C, Krisky CM, Welling JS, Eddy WF, Luna B, Thulborn KR, Sweeney JA. Pursuit and saccadic eye movement subregions in human frontal eye field: a high-resolution fMRI investigation. Cereb Cortex. 2002;12(2):107–115. doi: 10.1093/cercor/12.2.107. [DOI] [PubMed] [Google Scholar]
- 18.Heide W, Kurzidim K, Kompf D. Deficits of smooth pursuit eye movements after frontal and parietal lesions. Brain. 1996;119(pt 6):1951–1969. doi: 10.1093/brain/119.6.1951. [DOI] [PubMed] [Google Scholar]
- 19.Newsome WT, Wurtz RH, Komatsu H. Relation of cortical areas MT and MST to pursuit eye movements, II: differentiation of retinal from extraretinal inputs. J Neurophysiol. 1988;60(2):604–620. doi: 10.1152/jn.1988.60.2.604. [DOI] [PubMed] [Google Scholar]
- 20.Komatsu H, Wurtz RH. Modulation of pursuit eye movements by stimulation of cortical areas MT and MST. J Neurophysiol. 1989;62(1):31–47. doi: 10.1152/jn.1989.62.1.31. [DOI] [PubMed] [Google Scholar]
- 21.MacAvoy MG, Gottlieb JP, Bruce CJ. Smooth pursuit eye movement representation in the primate frontal eye field. Cereb Cortex. 1991;1(1):95–102. doi: 10.1093/cercor/1.1.95. [DOI] [PubMed] [Google Scholar]
- 22.Schmid A, Rees G, Frith C, Barnes G. An fMRI study of anticipation and learning of smooth pursuit eye movements in humans. Neuroreport. 2001;12(7):1409–1414. doi: 10.1097/00001756-200105250-00023. [DOI] [PubMed] [Google Scholar]
- 23.Lindner A, Haarmeier T, Erb M, Grodd W, Thier P. Cerebrocerebellar circuits for the perceptual cancellation of eye-movement-induced retinal image motion. J Cogn Neurosci. 2006;18(11):1899–1912. doi: 10.1162/jocn.2006.18.11.1899. [DOI] [PubMed] [Google Scholar]
- 24.Ono S, Das VE, Economides JR, Mustari MJ. Modeling of smooth pursuit-related neuronal responses in the DLPN and NRTP of the rhesus macaque. J Neurophysiol. 2005;93(1):108–116. doi: 10.1152/jn.00588.2004. [DOI] [PubMed] [Google Scholar]
- 25.Siever LJ, van Kammen DP, Linnoila M, Alterman I, Hare T, Murphy DL. Smooth pursuit eye movement disorder and its psychobiologic correlates in unmedicated schizophrenics. Biol Psychiatry. 1986;21(12):1167–1174. doi: 10.1016/0006-3223(86)90223-4. [DOI] [PubMed] [Google Scholar]
- 26.Campion D, Thibaut F, Denise P, Courtin P, Pottier M, Levillain D. SPEMimpairment in drug-naive schizophrenic patients: evidence for a trait marker. Biol Psychiatry. 1992;32(10):891–902. doi: 10.1016/0006-3223(92)90178-3. [DOI] [PubMed] [Google Scholar]
- 27.Litman RE, Hommer DW, Clem T, Rapaport MH, Pato CN, Pickar D. Smooth pursuit eye movements in schizophrenia: effects of neuroleptic treatment and caffeine. Psychopharmacol Bull. 1989;25(3):473–478. [PubMed] [Google Scholar]
- 28.Litman RE, Hommer DW, Radant A, Clem T, Pickar D. Quantitative effects of typical and atypical neuroleptics on smooth pursuit eye tracking in schizophrenia. Schizophr Res. 1994;12(2):107–120. doi: 10.1016/0920-9964(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 29.Spohn HE, Coyne L, Spray J. The effect of neuroleptics and tardive dyskinesia on smooth-pursuit eye movement in chronic schizophrenics. Arch Gen Psychiatry. 1988;45(9):833–840. doi: 10.1001/archpsyc.1988.01800330059007. [DOI] [PubMed] [Google Scholar]
- 30.Friedman L, Jesberger JA, Meltzer HY. Effect of typical antipsychotic medications and clozapine on smooth pursuit performance in patients with schizophrenia. Psychiatry Res. 1992;41(1):25–36. doi: 10.1016/0165-1781(92)90015-u. [DOI] [PubMed] [Google Scholar]
- 31.Thaker GK, Ross DE, Buchanan RW, Adami HM, Medoff DR. Smooth pursuit eye movements to extra-retinal motion signals: deficits in patients with schizophrenia. Psychiatry Res. 1999;88(3):209–219. doi: 10.1016/s0165-1781(99)00084-0. [DOI] [PubMed] [Google Scholar]
- 32.Hutton SB, Crawford TJ, Puri BK, Duncan LJ, Chapman M, Kennard C, Barnes TR, Joyce EM. Smooth pursuit and saccadic abnormalities in first-episode schizophrenia. Psychol Med. 1998;28(3):685–692. doi: 10.1017/s0033291798006722. [DOI] [PubMed] [Google Scholar]
- 33.Muir WJ, St Clair DM, Blackwood DH, Roxburgh HM, Marshall I. Eye-tracking dysfunction in the affective psychoses and schizophrenia. Psychol Med. 1992;22(3):573–580. doi: 10.1017/s0033291700038034. [DOI] [PubMed] [Google Scholar]
- 34.Schlenker R, Cohen R. Smooth-pursuit eye-movement dysfunction and motor control in schizophrenia: a follow-up study. Eur Arch Psychiatry Clin Neurosci. 1995;245(2):125–126. doi: 10.1007/BF02190739. [DOI] [PubMed] [Google Scholar]
- 35.Bartfai A, Levander SE, Nybäck H, Berggren BM, Schalling D. Smooth pursuit eye tracking, neuropsychological test performance, and computed tomography in schizophrenia. Psychiatry Res. 1985;15(1):49–62. doi: 10.1016/0165-1781(85)90039-3. [DOI] [PubMed] [Google Scholar]
- 36.Sweeney JA, Haas GL, Li S, Weiden PJ. Selective effects of antipsychotic medications on eye-tracking performance in schizophrenia. Psychiatry Res. 1994;54(2):185–198. doi: 10.1016/0165-1781(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 37.Hutton SB, Crawford TJ, Gibbins H, Cuthbert I, Barnes TR, Kennard C, Joyce EM. Short and long term effects of antipsychotic medication on smooth pursuit eye tracking in schizophrenia. Psychopharmacology (Berl) 2001;157(3):284–291. doi: 10.1007/s002130100803. [DOI] [PubMed] [Google Scholar]
- 38.Küfferle B, Friedmann A, Topitz A, Földes P, Anderer P, Kutzer M, Steinberger K. Smooth pursuit eye movements in schizophrenia: influences of neuroleptic treatment and the question of specificity. Psychopathology. 1990;23(2):106–114. doi: 10.1159/000284646. [DOI] [PubMed] [Google Scholar]
- 39.Sweeney JA, Bauer KS, Keshavan MS, Haas GL, Schooler NR, Kroboth PD. Adverse effects of risperidone on eye movement activity: a comparison of risperidone and haloperidol in antipsychotic-naive schizophrenic patients. Neuropsychopharmacology. 1997;16(3):217–228. doi: 10.1016/S0893-133X(96)00195-9. [DOI] [PubMed] [Google Scholar]
- 40.Reilly JL, Harris MS, Keshavan MS, Sweeney JA. Abnormalities in visually guided saccades suggest corticofugal dysregulation in never-treated schizophrenia. Biol Psychiatry. 2005;57(2):145–154. doi: 10.1016/j.biopsych.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 41.Reilly JL, Harris MS, Keshavan MS, Sweeney JA. Adverse effects of risperidone on spatial working memory in first-episode schizophrenia. Arch Gen Psychiatry. 2006;63(11):1189–1197. doi: 10.1001/archpsyc.63.11.1189. [DOI] [PubMed] [Google Scholar]
- 42.Harris MS, Reilly JL, Keshavan MS, Sweeney JA. Longitudinal studies of antisaccades in antipsychotic-naive first-episode schizophrenia. Psychol Med. 2006;36(4):485–494. doi: 10.1017/S0033291705006756. [DOI] [PubMed] [Google Scholar]
- 43.Kapur S, Zipursky RB, Remington G. Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry. 1999;156(2):286–293. doi: 10.1176/ajp.156.2.286. [DOI] [PubMed] [Google Scholar]
- 44.Spitzer RL, Williams JB, Gibbons M, First MB, editors. Structured Clinical Interview for DSM-III-R (SCID) New York: New York State Psychiatric Institute; 1987. [Google Scholar]
- 45.Ammons RB, Ammons CH. The Quick Test (QT): provisional manual. Psychol Rep. 1962;11:111–161. [Google Scholar]
- 46.Overall JE, Beller SA. The Brief Psychiatric Rating Scale (BPRS) in geropsychiatric research, I: factor structure on an inpatient unit. J Gerontol. 1984;39(2):187–193. doi: 10.1093/geronj/39.2.187. [DOI] [PubMed] [Google Scholar]
- 47.Andreasen NC. Methods for assessing positive and negative symptoms. Mod Probl Pharmacopsychiatry. 1990;24:73–88. doi: 10.1159/000418013. [DOI] [PubMed] [Google Scholar]
- 48.McEvoy JP, Hogarty GE, Steingard S. Optimal dose of neuroleptic in acute schizophrenia: a controlled study of the neuroleptic threshold and higher haloperidol dose. Arch Gen Psychiatry. 1991;48(8):739–745. doi: 10.1001/archpsyc.1991.01810320063009. [DOI] [PubMed] [Google Scholar]
- 49.Sweeney JA, Clementz BA, Haas GL, Escobar MD, Drake K, Frances AJ. Eye tracking dysfunction in schizophrenia: characterization of component eye movement abnormalities, diagnostic specificity, and the role of attention. J Abnorm Psychol. 1994;103(2):222–230. doi: 10.1037//0021-843x.103.2.222. [DOI] [PubMed] [Google Scholar]
- 50.Mather JA, Neufeld RW, Merskey H, Russell NC. Release of saccades in schizophrenics: inattention or inefficiency? Eur Arch Psychiatry Neurol Sci. 1989;239(1):23–26. doi: 10.1007/BF01739739. [DOI] [PubMed] [Google Scholar]
- 51.Trillenberg P, Heide W, Junghanns K, Blankenburg M, Arolt V, Kömpf D. Target anticipation and impairment of smooth pursuit eye movements in schizophrenia. Exp Brain Res. 1998;120(3):316–324. doi: 10.1007/s002210050405. [DOI] [PubMed] [Google Scholar]
- 52.Nagel M, Sprenger A, Nitschke M, Zapf S, Heide W, Binkofski F, Lencer R. Different extraretinal neuronal mechanisms of smooth pursuit eye movements in schizophrenia: an fMRI study. Neuroimage. 2007;34(1):300–309. doi: 10.1016/j.neuroimage.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 53.Clementz BA. Saccades to moving targets in schizophrenia: evidence for normal posterior cortex functioning. Psychophysiology. 1996;33(6):650–654. doi: 10.1111/j.1469-8986.1996.tb02360.x. [DOI] [PubMed] [Google Scholar]
- 54.Lencer R, Trillenberg P, Trillenberg-Krecker K, Junghanns K, Kordon A, Broocks A, Hohagen F, Heide W, Arolt V. Smooth pursuit deficits in schizophrenia, affective disorder and obsessive-compulsive disorder. Psychol Med. 2004;34(3):451–460. doi: 10.1017/s0033291703001314. [DOI] [PubMed] [Google Scholar]
- 55.Broerse A, Crawford TJ, den Boer JA. Differential effects of olanzapine and risperidone on cognition in schizophrenia? a saccadic eye movement study. J Neuropsychiatry Clin Neurosci. 2002;14(4):454–460. doi: 10.1176/jnp.14.4.454. [DOI] [PubMed] [Google Scholar]
- 56.Farber RH, Clementz BA, Swerdlow NR. Characteristics of open- and closed-loop smooth pursuit responses among obsessive-compulsive disorder, schizophrenia, and nonpsychiatric individuals. Psychophysiology. 1997;34(2):157–162. doi: 10.1111/j.1469-8986.1997.tb02126.x. [DOI] [PubMed] [Google Scholar]
- 57.Flechtner KM, Steinacher B, Sauer R, Mackert A. Smooth pursuit eye movements of patients with schizophrenia and affective disorder during clinical treatment. Eur Arch Psychiatry Clin Neurosci. 2002;252(2):49–53. doi: 10.1007/s004060200011. [DOI] [PubMed] [Google Scholar]
- 58.Matsui-Sakata A, Ohtani H, Sawada Y. Pharmacokinetic-pharmacodynamic analysis of antipsychotics-induced extrapyramidal symptoms based on receptor occupancy theory incorporating endogenous dopamine release. Drug Metab Pharmacokinet. 2005;20(3):187–199. doi: 10.2133/dmpk.20.187. [DOI] [PubMed] [Google Scholar]
- 59.Friedman L, Jesberger JA, Meltzer HY. The effect of apomorphine, MK-212 (6-chloro-2-[1-piperazinyl]-pyrazine) and placebo on smooth pursuit gain and corrective saccades in normal subjects. Neuropsychopharmacology. 1994;11(1):49–62. doi: 10.1038/npp.1994.35. [DOI] [PubMed] [Google Scholar]
- 60.Green JF, King DJ, Trimble KM. Antisaccade and smooth pursuit eye movements in healthy subjects receiving sertraline and lorazepam. J Psychopharmacol. 2000;14(1):30–36. doi: 10.1177/026988110001400103. [DOI] [PubMed] [Google Scholar]
- 61.Hepp K, Henn V, Vilis T, Cohen B. Brainstem regions related to saccade generation. Rev Oculomot Res. 1989;3:105–212. [PubMed] [Google Scholar]
- 62.Kazakov VN, Kravtsov P, Krakhotkina ED, Maisky VA. Sources of cortical, hypothalamic and spinal serotonergic projections: topical organization within the nucleus raphe dorsalis. Neuroscience. 1993;56(1):157–164. doi: 10.1016/0306-4522(93)90570-6. [DOI] [PubMed] [Google Scholar]
- 63.Karoumi B, Ventre-Dominey J, Dalery J. Predictive saccade behavior is enhanced in schizophrenia. Cognition. 1998;68(3):B81–B91. doi: 10.1016/s0010-0277(98)00052-3. [DOI] [PubMed] [Google Scholar]
- 64.Karoumi B, Ventre-Dominey J, Vighetto A, Dalery J, d’Amato T. Saccadic eye movements in schizophrenic patients. Psychiatry Res. 1998;77(1):9–19. doi: 10.1016/s0165-1781(97)00126-1. [DOI] [PubMed] [Google Scholar]
- 65.Collins CJ, Barnes GR. Scaling of smooth anticipatory eye velocity in response to sequences of discrete target movements in humans. Exp Brain Res. 2005;167(3):404–413. doi: 10.1007/s00221-005-0044-8. [DOI] [PubMed] [Google Scholar]
- 66.Tregellas JR, Tanabe JL, Miller DE, Ross RG, Olincy A, Freedman R. Neurobiology of smooth pursuit eye movement deficits in schizophrenia: an fMRI study. Am J Psychiatry. 2004;161(2):315–321. doi: 10.1176/appi.ajp.161.2.315. [DOI] [PubMed] [Google Scholar]
- 67.Simó LS, Krisky CM, Sweeney JA. Functional neuroanatomy of anticipatory behavior: dissociation between sensory-driven and memory-driven systems. Cereb Cortex. 2005;15(12):1982–1991. doi: 10.1093/cercor/bhi073. [DOI] [PubMed] [Google Scholar]
- 68.Nitschke MF, Arp T, Stavrou G, Erdmann C, Heide W. The cerebellum in the cerebro-cerebellar network for the control of eye and hand movements: an fMRI study. Prog Brain Res. 2005;148:151–164. doi: 10.1016/S0079-6123(04)48013-3. [DOI] [PubMed] [Google Scholar]
- 69.Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31(2–3):236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- 70.Keefe RS, Silva SG, Perkins DO, Lieberman JA. The effects of atypical antipsychotic drugs on neurocognitive impairment in schizophrenia: a review and meta-analysis. Schizophr Bull. 1999;25(2):201–222. doi: 10.1093/oxfordjournals.schbul.a033374. [DOI] [PubMed] [Google Scholar]
- 71.Stone JM, Davis JM, Leucht S, Pilowsky LS. Cortical dopamine D2/D3 receptors are a common site of action for antipsychotic drugs: an original patient data meta-analysis of the SPECT and PET in vivo receptor imaging literature [published online ahead of print February 26, 2008] Schizophr Bull. 2008 doi: 10.1093/schbul/sbn009. [DOI] [PMC free article] [PubMed] [Google Scholar]