Abstract
Estrogen plays an important role in bone metabolism and only high dose can stimulate osteoblast bone formation. However, the underlying mechanisms have not been elucidated. The epithelial sodium channel (ENaC) is a key pathway for sodium transport in epithelia, vascular endothelium, and other tissues; although the expressions of α and γ ENaC mRNA were found in osteoblasts, the regulation of ENaC by estrogen in osteoblasts has not been studied. Our recent data confirmed the ENaC expression in mouse primary osteoblasts by immunocytofluorescence, RT-PCR, western blot, and patch clamp. Furthermore, we found estrogen (10−5M) increased the expression of α and γ ENaC subunits at both the mRNA and protein levels in osteoblasts. On the other hand, 17βestradiol (20nM) increased inward Na+ currents which were inhibited by amiloride. The estrogen dose used in patch clamp is much lower than those of mRNA and protein analysis, which means single cell ENaC electrophoretic mobility is much more sensitive to estrogen than the mRNA and protein production by estrogen stimulation. Our results suggest that estrogen regulates expression and function of ENaC in osteoblasts may provide a new clue that the mechanism of high dose of estrogen influence osteoblast bone formation via ENaC activity.
Keywords: Epithelial sodium channels, ENaC, osteoblast, estrogen
INTRODUCTION
Epithelial sodium channels (ENaC) are crucial for sensing acidosis, sustaining sodium homeostasis, and transducting mechanical stimuli. These channels were discovered in 1994, and belong to ENaC/DEG supergene family. ENaC are a new type of non-voltage dependent sodium ion channel that can be inhibited by amiloride. They are made up of three homologous subunits (α, β, and γ) (3,5,6). ENaC are large glycosylation molecules found in the apical membrane of epithelial tissues. ENaC have been found in many different tissues including the brain, urinary bladder, colon, respiratory tract, reproductive organs, kidney, and sweat glands (11). Great advances have been made in understanding the ENaC’s molecular regulation in these tissues (1,15,19).
Osteoblasts, as key cells in bone biology, originated from pluripotent mesenchymal stem cells of the bone marrow, is intimately linked to bone formation, repair, and remodeling of osteogenesis. Bone is a living tissue which is constantly under-going renewal and remodeling bone by coupling of osteoclast and osteoblast. More and more evidences show that osteoblasts play a principal role in bone remodeling, because osteoblasts not only take part in new bone formation, but also indirectly mediate osteoclastic bone resorption (13).
Little known is ion channels in skeleton, however, it has been established that skeletal cells such as osteoblasts and chondrocytes express a number of ion channels with different biophysical and pharmacological properties (9,14). There are a lot of studies about calcium channel in bone cells but less about sodium channel (24).
However, the adult human body contains from 90 to 130 g sodium. Roughly half of sodium is known in bone and has high exchange rate with the extracellular fluid (ECF). Calcium, on the other hand, has an exchange rate with ECF of less than 1%. The previous research often overly focused on the function of calcium on osteoblast, but ignored the role of sodium on osteoblasts. The exact role of bone sodium is not known, but there is the research showed that sodium of the ECF bathes the bone forming site during its mineralization (18). This sodium may relate to ENaC of osteoblast which alter the osteogenesis.
Estrogen had since been thought only to affect osteoclasts. Now researches show that estrogen can also stimulate osteoblasts proliferation (23). Turners (22) thought that estrogen can promote bone forming by mainly increasing the number of osteoblasts, which is coincide with its effects on osteoporosis integrally. It’s even viewed that estrogen maybe act on iNOS gene to release NO gradually and then regulate osteoblasts activity (12). Moreover, Estrogen have the effects that regulate the ENaC’s expression and activity in rat lung and kidney(10,21).
Our study is to determine whether estrogen regulate of osteoblast ENaC expression and function with aim to discover the inner relation of ENaC and osteoblasts. Here we found the whole-cell Na+ currents of ENaC in osteoblasts by using patch clamp technique, and 20nM estrogen increased these currents which were inhibited by amiloride. The estrogen dose used in patch clamp is much lower than 10-5 M estrogen in cultured cells, which means single cell ENaC electrophoretic mobility is much more sensitive to estrogen than the mRNA and protein production by estrogen stimulation. In addition, our results showed that on primary osteoblasts estrogen-mediated regulation of α, γ ENaC were increased compared with control group in mRNA and protein level, and the regulation of βENaC is still unclear. These findings indicated that estrogen regulates ENaC activity and expression on primary osteoblasts, which provide another possible pathway to study osteogenesis and further cause of bone relative diseases.
MATERIALS AND METHODS
Cell culture
Osteoblasts were isolated from 1-day-old C57/BL6J mouse skulls. Briefly, skulls (frontal and perietal bones) were dissected from anesthetized mice, and endosteum and periosteum were stripped off. The bone tissues were cut into approximately 1–2 mm2 pieces and digested with trysin (2.5 g/L, 1:250, Gibco) and collagenase A (2.0 g/L, Sigma). The initial digestions including 20 min and 40 min digests were discarded. The third digestion lasted for another 60 min, and the cells were collected and cultured in control medium consisting of DMEM-high glucose with 10% FBS (Hyclone) and 100–100μg/ml penicillin-streptomycin (Gibco) in 35 mm flask. The culture medium was changed every 3 days. After culture for 5–7 d, the cells were divided into three groups, one still in control medium as blank control group and the other two were cultured in osteogenic condition medium. The osteogenic medium composing of DMEM-high glucose, 10% FBS (Hyclone), 100–100 μg/ml penicillin-streptomycin (Gibco), 50 μg/ml L-ascorbic acid-2-phosphate (Sigma), 100 nM dexamethasone (Sigma-Aldrich), and 10 mM β-glycerophosphate (Sigma). After culture for 2–3 d, one group was added E2 (10−5 M) into osteogenic medium as estrogen group and the other as match control group.
Patch clamp analysis
Primary osteoblasts of three groups on coverslips were mounted on the stage of a Leica DMIRB inverted fluorescent microscope (Meyer Instruments Inc) and continuously perfused with an external solution containing (in mM): 100 K gluconate, 40 KCl, 2 MgCl2, 0.5 CaCl2, 4 EGTA/KOH (10 nM free Ca2+), 2K2ATP, and 10 Hepes (pH 7.2). The bath solution contained (in mM):140 NaCl, 1 MgCl2, 1.8 CaCl2, and 10 Hepes (pH 7.2). The whole-cell currents were recorded with a patch-clamp amplifier (Axopatch 200B, Molecular Device). The holding potential was −40 mV. Inward and outward currents were elicited by altering the membrane potential from −100 to +100 mV in 10-mV increments. The recording duration was 500 ms. The amplifier was controlled by Clampex program (pCLAMP 10.1, Molecular Device). Currents were digitized with a digital-to-analog and analog-to-digital converter (DigiData 1320A, Molecular Device). Currents were filtered through an internal four-pole Bessel filter at either 0.5 kHz, and sampled at 1 KHz. Current-voltage (I-V) curves were constructed by measuring the steady-state current values 200ms from the start of voltage pulses with the Clampfit Program (Molecular Device) and OriginPro 8.0 (Microcal).
To test the extent to which whole cell currents were inhibited by amiloride and whether the current is affected by E2, we measured I-V relationships of cells in the whole cell mode and then repeated the measurements after perfusing cells with bath solution containing 1mM amiloride or 20nM E2. The amiloride-sensitive currents were computed by subtracting the currents in the presence of 1mM amiloride from the total currents in the absence or presence of 20nM E2.
Reverse Transcription
PCR—Total RNA was extracted with Trizol reagent (Life Technologies Inc, Gaithersburg, MD, USA) from osteoblasts cultured in 6-well dishes or 24-well cell culture inserts (Corning Costar Corp., Cambridge, MA, USA) with or without incubation of estrogen. ENaC mRNA expression was determined by reverse transcription in accordance with manufacturer’s instructions. To ensure unbiased analysis, semi-quantitative PCR was performed blindly, and the identity of the samples was revealed only after mRNA measurements had been made. The primers used were as follows: A 163-bp product of mouse α-ENaC primers: 5- tctgcgtcgcctccttcttg -3 (sense) and 5- tgagcatctaatacagcatg -3 (antisense); A 116-bp product of mouse γ-ENaC primers: 5- acctcaagcacatgatcttg -3 (sense) and 5- caatgactgaggtcagcaac-3 (antisense); A 112-bp product of mouse 18S ribosome RNA primers: 5-cctggataccgcagctagga-3 (sense) and 5- gcggcgcaatacgaatgcccc -3 (antisense). RT-polymerase chain reaction (RT-PCR) product was electrophoresized on 3% agarose gel containing ethidiumbromide.
Western blot assay for ENaC isoforms
The confluent cells cultured in the same condition as RT-PCR studies were lysed with CelLytic™ MT Cell Lysis Reagent (Sigma-Aldrich). Bicinchoninic acid protein assay reagent (Pierce, Rockford, IL) was used to determine the protein concentration of the samples. Equal amounts (30 μg) of cellular protein were loaded into each well and separated by 10% SDS-PAGE. Proteins were subsequently transferred electrophoretically onto Nitrocellulose membranes (Millipore), blocked for 1h with 5% dry milk, and then probed with anti-ENaC α and anti-ENaC γ antibodies (Santa Cruz, 1:2,000). ENaC subunits were detected by Western blotting using immunopurified goat polyclonal IgG ENaC antibodies. Negative controls were performed by omitting the primary antibody. Blots were also incubated with a β-actin goat polyclonal antibody (Santa Cruz, 1:2,000). Then Blots were incubated with HRP-conjugated rabbit-anti-goat secondary antibody (Santa Cruz, 1:10,000), and visualized by enhanced chemiluminescent detection (Pierce).
Data presentation and statistical analysis
All results are presented as mean±S.E. Paired t-test or One-way ANOVA computation combined with the Bonferroni test was used to analyze the significant difference of means. A probability level of 0.05 was considered significant.
RESULTS
Estrogen effect on whole-cell currents on osteoblasts
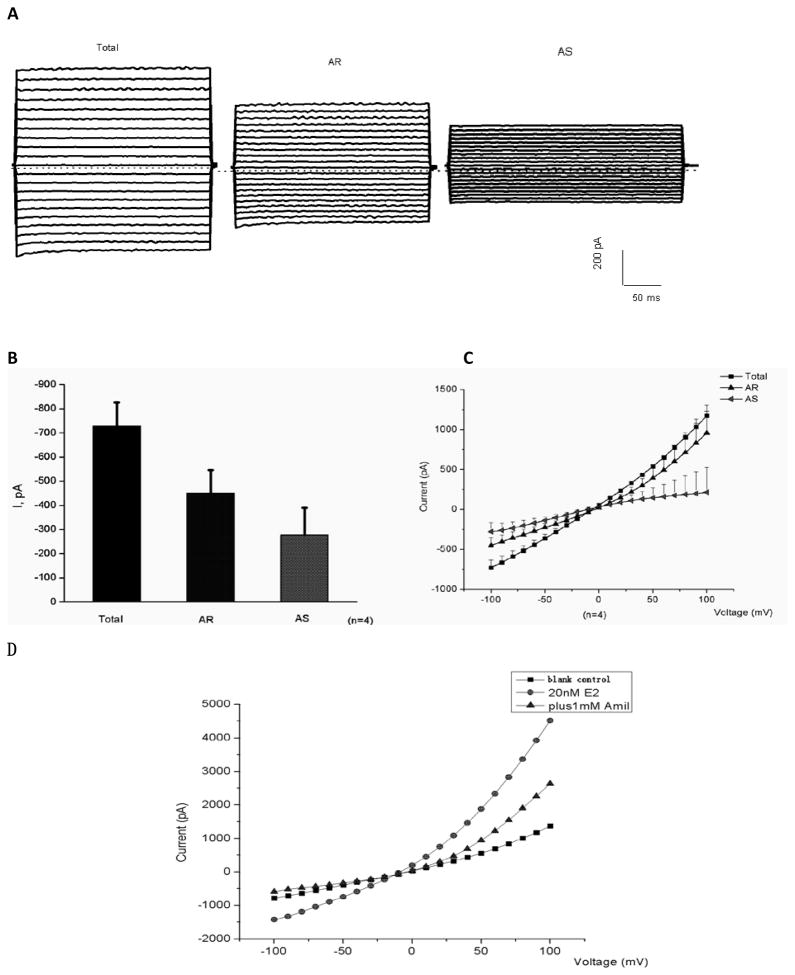
In the whole cell mode, osteoblast cells exhibited inward currents (Fig. 1). Perfusion of the cells with the bath solution containing 1 mM amiloride rapidly reduced the currents (Fig. 1, A). Amiloride-sensitive currents (AS) were obtained by subtracting amiloride-resistant currents (AR) from total current (Total). Mean values of amiloride-sensitive currents measured at −100 mV were shown in Fig. 1, B. The calculated average AS was about 270 PA (n=4). Current-voltage (I-V) relationships of Total, AR, and AS currents were shown as Fig. 1, C. Values of currents were elicited by applying voltage steps between −100 and +100 mV in 10-mV voltage step. Fig 1. D illustrates that the currents being reduced by 1 mM Amiloride are affected increasing by 20 nM E2. Very low concentration of E2 (only 20nM) could regulate ENaC activity and increase AS currents in patch clamp.
Figure 1. Expression of whole-cell amiloride-sensitive currents in primary cultured osteoblasts.
Representative recordings (A) and mean values (B–C) of whole-cell currents in osteoblasts and representative current-voltage (D) after using 20 nM 17 β-estradiol(E2). A: cell was held at a potential of −40 mV. Currents were elicited by applying voltage steps between −100 and +100 mV in 10-mV steps lasting 500 ms every 10 s. Pipette was filled with standard internal solution, and cell was perfused with standard external solution (see MATERIALS AND METHODS for details). Currents were recorded after perfusion of cell with the bath solution containing 1 mM amiloride. Amiloride-sensitive currents (AS) were obtained by subtracting amiloride-resistant currents (AR) from total current (Total). B. Mean values of whole-cell currents in osteoblast cells recorded in −100 mV. C: current-voltage (I-V) relationships of Total, AR, and AS currents. Values are means ± SE; n =4 cells. D. Cell was held at a potential of −40 mV. Currents were elicited by applying voltage steps between −100 and +100 mV in 10-mV steps lasting 500 ms every 10 s. Currents were recorded after perfusion of cell with the bath solution (control), the bath solution containing 20 nM 17 β-estradiol (20 nM E2), the bath solution containing 1 mM amiloride (plus 1mM Amil).
Estrogen effect on ENaC mRNA expression in primary cultured osteoblasts
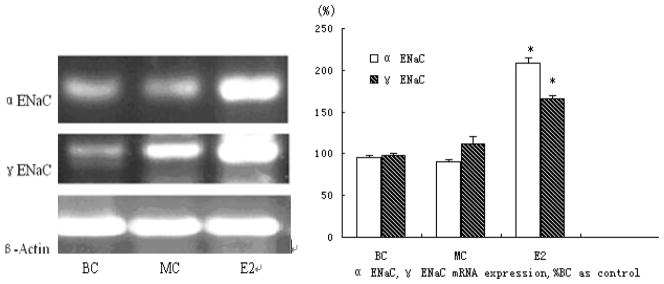
In order to determine whether or not E2 induces ENaC mRNA expression on primary osteoblasts, mouse primary cultured osteoblasts were treated with 10-5M estrogen (E2) for 24 hours. Fig. 2 illustrates the levels of α ENaC mRNA expression within untreated and E2-treated cells compared with the expression of 18S ribosome RNA, used as an internal standard. Incubating for 24 h with E2 induced a 2-fold (208 % ± 3.6) increase in α ENaC mRNA, and an increase (166 % ± 6.2) in γ ENaC mRNA (Fig. 2).
Figure 2. Analysis of ENaC mRNA expression in primary mouse osteoblasts.
Total RNAs were isolated according to manufacture instruction. Messenger RNA levels for ENaC α, γ were determined by RT-PCR. The levels of mRNA were normalized to that of 18S ribosome RNA. Three independent experiments were carried out (* represents P<0.05, ** represents P<0.01). BC, MC and E2 individually represent blank control group match control group and the estrogen (10−5 mol/L) group.
Estrogen effect on ENaC protein expression in primary cultured osteoblasts
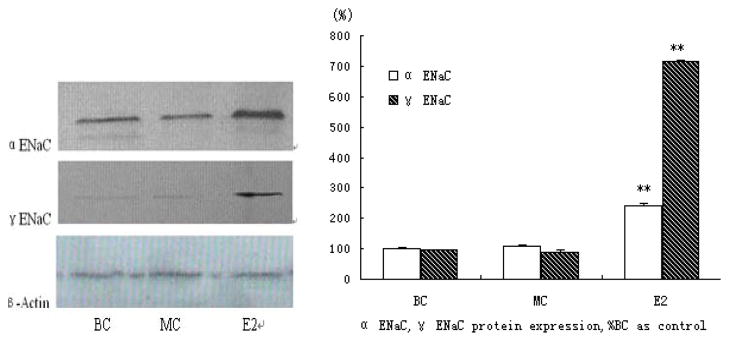
E2 induced ENaC α, γ protein expression by western blot analysis, compared with the expression of β-actin as an internal standard. The expression of α ENaC protein was increased up to 2-fold after 24 hours of treatment and γ ENaC protein had marvelous 7-fold increasing (Fig. 3).
Figure 3. Analysis of ENaC protein expression in primary mice osteoblasts.
Proteins in the same condition as RT-PCR studies were extracted as methods. ENaC α, γ were determined by western-blot using immunopurified anti-goat ENaC antibodies. The levels of proteins were normalized to that of β-actin as an internal standard. No background staining was seen when omitting the primary antibody (data not shown) (* represents P<0.05, ** represents P<0.01).
DISCUSSION
Similar to previous studies (7), the current study clearly showed the existence of amiloride-sensitive epithelial sodium channels (ENaC) in skeletal cells, such as in chondrocytes and osteoblasts. We also observed that the mRNA and the protein level of ENaC α, γ in E2 treatment groups are higher than those in the control groups in the primary cultured osteoblasts. These results indicated that estrogen regulates the expression of ENaC α, γ in cultured osteoblasts. Furthermore, we found that estrogen increased the ENaC activity of osteoblasts, which was represented by the whole-cell amiloride-sensitive currents as observed by using the patch clamp method. The inner amiloride-sensitive currents implied that the inward Na+ currents and the perfusion of the bath solution containing 20 nM E2 greatly increased the recorded total currents, indicating the enhanced effects of E2 on the currents and that its stimulation on osteoblasts is rapid and extremely sensitive. This similar phenomenon also occurs in lung and kidney tissues.
One previous study (21) found that female ovarian hormones up-regulate ENaC α, γ mRNA expression in lung tissue of female rats. Another study (10) found that mRNA levels of ENaC subunits in the kidney were higher in female rats than in male rats; ovariectomy made no differences between the sexes. Moreover, the ovariectomized rats showed that estrogen up-regulates ENaC α mRNA levels in the kidney. The current study is consistent with these previous findings which reveal that estrogen also regulates ENaCs in primary cultured osteoblasts. However, ENaCs are tissue-specific and species- specific in their responses to estrogen. ENaC γ was increased by estrogen stimulation in rat lung tissue and mouse osteoblasts, and not in rat kidney tissue. The expression of β-ENaC In our experiments was not determined may related to these reason, the exact reason need to be explored in future.
Lastly, in our study, the expression of α, γ ENaC, mRNA and protein were increased when conditional medium promoted primary osteoblast differentiation, which indicated that the expressions of ENaCs varied at different stages of their development within the culture, meaning that ENaCs increase during osteoblast maturation. Our result shows the difference of expression between protein and mRNA level. There might be some reasons for it: 1. Protein can not only be regulated on transcription level but also translation level and translocation level. That is why mRNA is not a direct indication of protein level. 2. There is also one possibility though comparatively rare, the antibody epitope may be changed by alternative mRNA splicing.
Based on our findings, estrogen may serve as a new regulatory pathway through which ENaC can regulate osteoblast activity. Estrogen’s function of protection against the progression of bone disease has been well known. For example, estrogen deficiency in women can cause severe and rapid bone loss, which can be prevented or reversed by estrogen replacement. Moreover, the loss of estrogen activity was a leading cause for osteoporosis in men as well (2,16,20).
Classical receptors for estrogens (ERα and ERβ) are present (4) in bone, osteoblasts, osteoclasts and their progenitors, indicating that the effects of sex steroids on bone are at least, in part, directly mediated. However, the level of receptor expression in bone cells is low (at least 10-fold) compared to that in reproductive organs. Moreover, the level of receptor expression in bone cells does not vary by gender, whereas similar levels (1,8,17) of ER have been found in both sexes. In addition to traditional effects, estrogen has traditionally been known for its indirect effects on systemic hormones, which regulate calcium balance, a change of the ratio among RANKL, cytokine-Inducing osteoclastogenesis, and osteoprotegerin (OPG), its decoy-soluble receptor (25).
The findings of our study provide a new perspective on the regulatory roles of estrogen in ENaC expression and the functions of osteoblasts. Since half of the sodium in the body exits in bone (18), we speculate that the elevated level of ENaCs may be due to estrogen increasing the amount of sodium entering into the osteoblasts, which may further influence bone formation and mineralization. Combining the above results, we postulate that ENaC may be involved in bone metabolism. More research needs to be done to further explore this area.
In summary, our study has confirmed the presence of ENaCs in osteoblasts, and has found that an increase in ENaC expression and function induced by estrogen in osteoblasts may relate to bone metabolism. The insight of the current study suggests that ENaCs may be a potential target for developing pharmaceutical intervention for metabolic bone diseases.
Acknowledgments
Special thanks are given to Ke Fang in University of New York at Albany or the support of the revision of the manuscript. This work was supported by the National Natural Science Foundation of China (Grant No. 30971172), the Planned Science and Technology Project of Guangdong Province, China (Grant No. 2010B050700022) and the Planned Science and Technology Project of Dongguan, Guangdong Province, China (Grant No. 201010815207).
Abbreviations
- ENaC
epithelial sodium channel
- RT-PCR
reverse transcription-polymerase chain reaction
References
- 1.Mobasheri Ali, Martín-Vasallo Pablo. Epithelial sodium channels in skeletal cells; a role in mechanotransduction? Cell Biol Int. 1999;23(4):37–240. doi: 10.1006/cbir.1999.0405. [DOI] [PubMed] [Google Scholar]
- 2.Bilezikian JP, Morishima A, Bell J, Grumbach MM. Increased bone mass as a result of estrogen therapy in a man with aromatase deficiency. N Engl J Med. 1998;339:599–603. doi: 10.1056/NEJM199808273390905. [DOI] [PubMed] [Google Scholar]
- 3.Butterworth M, Edinger R, Frizzell R, Johnson J. Regulation of the epithelial sodium channel by membrane trafficking. Am J Physiol Renal Physiol. 2009;296(4):10–24. doi: 10.1152/ajprenal.90248.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bord S, Horner A, Beavan S, Compston J. Estrogen receptors alpha and beta are differentially expressed in developing human bone. J Clin Endocrinol Metab. 2001;86(5):2309–2314. doi: 10.1210/jcem.86.5.7513. [DOI] [PubMed] [Google Scholar]
- 5.Canessa CM, Horisberger JD, Rossier BC. Epithelial sodium channel related to proteins involved in neurodegeneration. Nature. 1993;361:467–470. doi: 10.1038/361467a0. [DOI] [PubMed] [Google Scholar]
- 6.Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na channel is made of three homologous subunits. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 7.Cohen A, Roe F. Review of risk factors for osteoporosis with particular reference to a possible aetiological role of dietary salt. Food Chem Toxicol. 2000;38(2–3):237–253. doi: 10.1016/s0278-6915(99)00145-3. [DOI] [PubMed] [Google Scholar]
- 8.DeCherney A. Physiologic and pharmacologic effects of estrogen and progestins on bone. J Reprod Med. 1993 Dec;38(12 Suppl):1007–1014. [PubMed] [Google Scholar]
- 9.Duncan RL, Akanbi KA, Farach-Carson MC. Calcium signals and calcium channels in osteoblastic cells. Semin Nephrol. 1998;18:178–190. [PubMed] [Google Scholar]
- 10.Gambling L, Dunford S, Wilson C, McArdle H, Baines D. Estrogen and Progesterone regulate α, β, and γ ENaC subunit mRNA levels in female rat kidney. Kidney Int. 2004;65(5):1774–1781. doi: 10.1111/j.1523-1755.2004.00593.x. [DOI] [PubMed] [Google Scholar]
- 11.Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation. Phys Rev. 1997;77:359–396. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- 12.MacIntyre I, Zaidi M, Alam AS, Datta HK, Moonga BS, Lidbury PS, Hecker M, Vane JR. Osteoclast inhibition: an action of nitric oxide not mediated by cyclic GMP. Proc Natl Acad Sci USA. 1991;88(7):2936–2940. doi: 10.1073/pnas.88.7.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manolagas S, Jilka R. Bone marrow cytokines and bone remodeling- Emerging insights into the pathophysiology of osteoporosis. N Engl J Med. 1995;2;332(5):305–11. doi: 10.1056/NEJM199502023320506. [DOI] [PubMed] [Google Scholar]
- 14.Mobasheri A, Golding S, Pagakis SN, Corkey K, Pocock AE, Fermor B, O’BRIEN MJ, Wilkins RJ, Ellory JC, Francis MJ. Expression of cation exchanger NHE and anion exchanger AE isoforms in primary human bone-derived osteoblasts. Cell Biol Int. 1998;22(7–8):551–562. doi: 10.1006/cbir.1998.0299. [DOI] [PubMed] [Google Scholar]
- 15.Ottaviani E, Franchini A, Mandrioli M, Saxena A, Hanukoglu A, Hanukoglu I. Amiloride-sensitive epithelial sodium channel subunits are expressed in human and mussel immunocytes. Dev Comp Immunol. 2002;26(5):395–402. doi: 10.1016/s0145-305x(01)00097-0. [DOI] [PubMed] [Google Scholar]
- 16.Riggs BL, Khosla S, Melton LJ., 3rd A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res. 1998;13:763–773. doi: 10.1359/jbmr.1998.13.5.763. [DOI] [PubMed] [Google Scholar]
- 17.Riggs BL, Khosla S, Melton LJ., 3rd Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23(3):279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 18.Robert P, Heaney MD. Role of Dietary Sodium in Osteoporosis. J Am Coll Nutr. 2006;25(3 Suppl):271S–276S. doi: 10.1080/07315724.2006.10719577. [DOI] [PubMed] [Google Scholar]
- 19.Roudier-Pujol C, Rochat A, Escoubet B, Eugènel E, Barrandon Y, Bonvaletl JP, Farman N. Differential expression of epithelial sodium channel subunit mRNAs in rat skin. J Cell Sci. 1996;109:379–385. doi: 10.1242/jcs.109.2.379. [DOI] [PubMed] [Google Scholar]
- 20.Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 21.Sweezy N, Tcepichev S, Gagnon S. Female gender hormones regulate mRNA levels and function of the rat lung epithelial Na channel. Am J Physiol. 1998;274:C379–C386. doi: 10.1152/ajpcell.1998.274.2.C379. [DOI] [PubMed] [Google Scholar]
- 22.Turner RT, Evans GL, WakIey GK. Mechanism of action of estrogen on cancellous bone balance in tibiae of ovariectomized growing rat: Inhibition of indices of formation and resorption. J Bone Miner Res. 1993;8(3):359–366. doi: 10.1002/jbmr.5650080313. [DOI] [PubMed] [Google Scholar]
- 23.Wang Q, Sun L, Zhang F, Zhang ZY, Weng LL, Zheng H, Liu JS. Effects of 17β-estradiol on cell proliferation, cAMP and cGMP content and iNOS activity in human osteoblast-like osteosarcoma cell line TE85. Chinese Pharmacological Bulletin. 1998;14(4):314–317. [Google Scholar]
- 24.Zahanich I, Graf EM, Heubach JF, Hempel U, Boxberger S, Ravens U. Molecular and functional expression of voltage-operated calcium channels during osteogenic differentiation of human mesenchymal stem cells. J Bone Miner Res. 2005;20(9):1637–1646. doi: 10.1359/JBMR.050521. [DOI] [PubMed] [Google Scholar]
- 25.Zallow A. Direct and indirect estrogen actions on osteoblasts and osteoclasts. Ann N Y Acad Sci. 2006;1068:173–179. doi: 10.1196/annals.1346.019. [DOI] [PubMed] [Google Scholar]