Abstract

One essential requirement for more sensitive gadolinium-based MRI contrast agents is to slow the molecular tumbling of the gadolinium(III) ion, which increases the gadolinium's relaxivity, or the ability to speed up the NMR relaxation of nearby water molecules. One route to this is through conjugation to high-molecular weight polymers such as dendrimers. In this work, amine functionalized TREN-bis(1,2-HOPO)-TAM-ethylamine and TREN-bis(1-Me-3,2-HOPO)-TAM-ethylamine ligands have been synthesized and attached to biocompatible 40 kDa esteramide (EA) and poly-l-lysine based (PLL) dendrimers capable of binding up to eight gadolinium complexes. These conjugates have T1 relaxivities of up to 38.14 ± 0.02 mM-1s-1 per gadolinium at 37 °C, corresponding to relaxivities of up to 228 mM-1s-1 per dendrimer molecule. This relaxivity expressed on a “per Gd” basis is several times that of the small molecule complexes, and an order of magnitude higher than that of current commercial agents. Due to their high performance and low toxicity these macromolecules may constitute an attractive complement to currently available gadolinium(III) based contrast agents.
In the last three decades, magnetic resonance imaging (MRI) has become one of the most prevalent medical imaging modalities used in clinical radiology, with over 27.5 million MRI's performed in 2007. Paramagnetic gadolinium(III) contrast agents are used to enhance signal in about a third of these scans. This is done through the gadolinium's ability to slow the relaxation of water molecules after disturbance by a magnetic field, a parameter known as relaxivity (r1). Concern about gadolinium dosage and release has increased because of nephrogenic systemic fibrosis (NSF), an incurable thickening of tissue and skin seen in patients with late stage renal failure. This condition appears to arise from the tendency for free gadolinium ions to bind to hydroxyapatite in bone tissue and transport across cellular ion channels.1 Increasing the chelate stability, relaxivity and efficiency of contrast agents will allow for smaller doses and lower exposure to free gadolinium ions, improving their performance and lowering the risk for NSF by injection 1/10 the quantity of Gd(III) currently required to obtain signal.
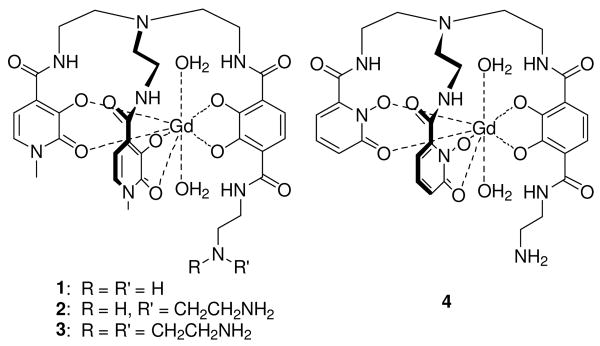
All current commercial contrast agents use octadentate poly(amino) carboxylate ligands as scaffolds to coordinate gadolinium and contain only one inner coordination water molecule (q = 1). Previous research in the Raymond laboratory has developed hexadentate oxygen donor chelators for gadolinium with similar stability to commercial poly(amino)carboxylate contrast agents by utilizing the oxophilicity of gadolinium. Containing either two 1-Me-3,2-hydroxypyridinonate (HOPO) or two 1,2-HOPO rings and an amine functionalized 2,3-dihydroxyterepthalamide (TAM) ring (Figures 1 and 2),2-7 these tris(2-aminoethyl)amine (TREN)-capped ligands have at least two coordinated water molecules, with fast exchange rates, allowing a much higher theoretical relaxivity than the small molecule amine based chelators. These complexes exhibit high T1 relaxivities (10-13 mM-1s-1) and thermodynamic complex stabilities (pGd ∼ 17-18). The 1-Me-3,2-HOPO based ligands used in this study vary by the nature of the amine pendant to the TAM ring. Complex 1 has a short, rigid linker and a known q value of two.2 Complex 2 has a longer, more flexible linkage incorporating a second ethylamine moiety, and complex 3 has a branched linkage with a third ethylamine group. Complex 4, containing the 1,2-HOPO moiety varies from these by its nitrogen substitution within the HOPO rings.
Figure 1.
The Gd-TREN-bis(1-Me-3,2-HOPO)-TAM-ethylamine complexes 1-3 and Gd-TREN-bis(1,2-HOPO)-TAM-ethylamine complex 4.
Figure 2.
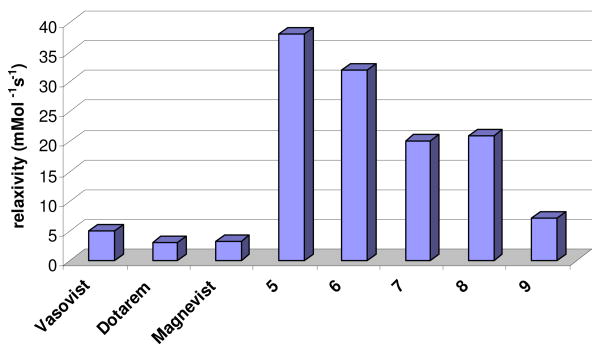
A comparison of the in vitro per gadolinium relaxivities of several clinical Gd(III) contrast agents and the dendrimer contrast agents investigated. All values for conjugates 5-9 were measured at 37 °C at 60 mHz.
MRI contrast agents can be further optimized for higher relaxivity and solubility through conjugation to a biocompatible macromolecule such as a protein,3 polypeptide,8 dendrimer,9-14 or nanoparticle,15,16 which lowers the molecular tumbling time of the gadolinium due to the reduced degrees of freedom of large molecules in solution. We anticipated that the previously reported17 ester-amide (EA) and branched poly-l-lysine (PLL) based dendrimers would be promising, as these degradable dendrimers offer a synthetically straightforward route to high molecular weight conjugates with facile renal clearance and low toxicity. We also anticipated that the densely packed core of the dendrimers would sterically inhibit the motion of the gadolinium complex, further increasing its relaxivity. The EA and PLL dendrimers developed by the Fréchet group have exhibited low toxicity in vivo, favorable degradation profiles (see supplementary information), and can be decorated with up to eight gadolinium complexes per dendrimer.17,18 These dendrimers have also been shown to increase half-life in blood serum and residence time of small molecule drugs, allowing for their improved drug delivery in vivo.19 In addition to enhancing the relaxivity and blood half-life of contrast agents, polymeric carriers are also expected to significantly reduce the toxicity of these agents due to the much slower rates of endocytosis for macromolecules compared to lipophilic small molecule compounds such as non polymeric gadolinium complexes. This should reduce the amount required for injection, therefore reduce the amount of gadolinium entering cells, and thereby facilitate its eventual excretion through the renal system.
We report herein that EA and PLL dendrimers have been successfully conjugated to pre-complexed Gd-TREN-bis-HOPO-TAM-ethylamine based complexes. The conjugates described herein exhibit high relaxivities (r1) of up to 38 mM-1s-1 under the clinically relevant conditions of 37 °C at 60 mHz, which is almost an order of magnitude higher than the relaxivities of many commercial contrast agents (Table 1).
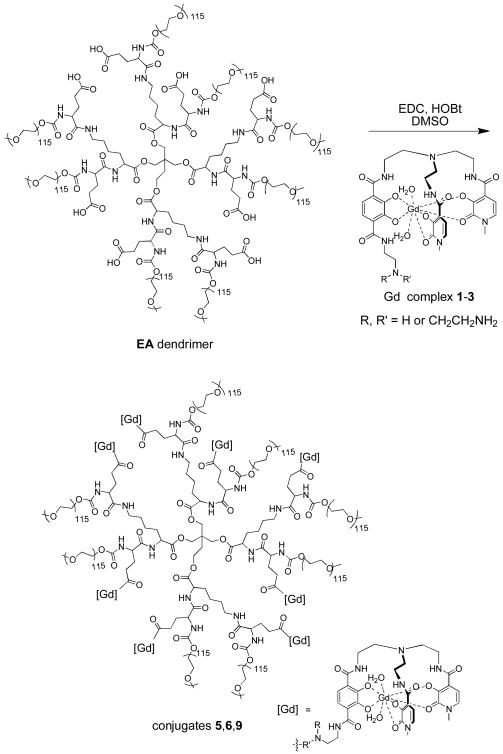
The syntheses of the PLL and EA dendrimers17, complexes 1-32 and complex 420 have been reported previously. Conjugates were obtained by coupling the carboxylic acid functionalities of the dendrimer to the amine moiety of a Gd-TREN-bis-HOPO-TAM complex using carbodiimide as the coupling agent (Scheme 1). Because the gadolinium introduced into the reaction is already tightly bound as a highly stable complex, this pre-complexation of the metal before conjugation reduces the possibility that free gadolinium ions might bind to non-specific coordination sites. Conjugates were purified by precipitation into ether followed by aqueous size exclusion chromatography using PD-10 columns.
Scheme 1.
The conjugation of Gd-TREN-bis(1-Me-3,2-HOPO)-TAM-Ethylamine complexes 1-3 to the EA dendrimer. Conjugates 7 and 8 were synthesized under identical conditions.
When 1 was coupled to the EA dendrimer, (Scheme 1) ICP and SEC measurements suggest an average loading of six gadolinium complexes per dendrimer. Despite extensive efforts, confirmation of this loading and determination of the nature of the gadolinium distribution using MALDI-Tof could not be achieved. This gadolinium-EA dendrimer conjugate, 5, has a relaxivity of 38.14 ± 0.02 mM-1s-1 per gadolinium, or 228 mM-1s-1 per dendrimer, at 37 °C and 60 MHz. This value compares very favorably to other relaxivities measured under clinically relevant conditions, which are typically in the range of 3-5 mM-1s-1 for current commercial agents (Figure 2), and even exceeds the relaxivity of albumin bound Vasovist, which can be in the range of 35-45 mM-1s-1.21
We further investigated 2 as a conjugate with the EA dendrimer (6) due to its high thermodynamic stability. This small molecule complex has a thermodynamic stability value three orders of magnitude larger than that of 1 or 3.2 This increased complex stability should reduce the amount of free gadolinium that can be leached from the conjugate in vivo, which may reduce toxicity concerns. However, due to the longer and more flexible linker, the molecular tumbling time and relaxivity were decreased compared to conjugate 5, giving conjugate 6 a relaxivity of 31.9 ± 0.1 s-1 mM-1 per gadolinium. Although a lower complex loading per dendrimer was observed by ICP and SEC (2.8 complexes/dendrimer), conjugate 6 has the potential to be a safer option due to the higher thermodynamic stability of the gadolinium-chelate binding.
Given the success of the 1-Me-3,2-HOPO chelators, a variation on this moiety, a 1,2-HOPO chelator (4) was evaluated. When conjugated to the EA dendrimer, per gadolinium relaxivity values of 20.2 ± 0.6 mM-1s-1 and an average loading of 8 gadolinium complexes per dendrimer were obtained for conjugate 7. In general the 1,2-HOPO complexes show lower relaxivity than their 1-Me-3,2-HOPO counterparts4,22, and this trend appears to be conserved upon conjugation to the dendrimer. These differences in relaxivities are believed to arise in differences in linker rigidity and the specific water coordination environment surrounding the gadolinium.
We also investigated ligand conjugation to the PLL dendrimer (See supplementary information for structure) using chelate 1 to further test the versatility of this approach. PLL conjugate 8 has a relaxivity of 21.0 ± 0.6 mM-1s-1 per gadolinium and an average loading of 4.5 gadoliniums per dendrimer. While this constitutes a significant increase over the small molecule complex, it is still significantly lower than that obtained with the EA dendrimer. We attribute this decrease to greater internal hydrogen bonding between the inner-sphere water coordination sites of the gadolinium and the more hydrophilic amide based PLL dendrimer core, or to increased tumbling of the gadolinium(III) complexes caused by the less branched and sterically crowded core of this dendrimer. The decreased relaxivity observed with the PLL dendrimer, combined to the more favorable biodegradability of the EA dendrimer, suggests that the EA dendrimer is a superior platform for this application.
Finally, we investigated complex 3 with the EA dendrimer, but found that the extended linker and extra primary amine resulted in a conjugate, 9, with a relatively low relaxivity of 7.19 ± 0.07 s-1 mM-1 and an average loading of 3 gadoliniums per dendrimer. It seems possible that this lower relaxivity for this conjugate may arise from occupation of a gadolinium binding site by one of the free amines of the covalently bound chelate, which would effectively lower the q value of the ligand.
To evaluate the toxicity of these conjugates, cytotoxicity studies were carried out with HeLa cells for 72 h to determine their effect on cell viability using Gd-DTPA, PLL, and EA dendrimers as controls. While most MRI contrast agents are excreted within 24 hours, the macromolecules may be excreted more slowly, and cytotoxicity testing was performed over a period of 72 hours to reflect this increased residence time. Each of the conjugates exhibited no evidence of cytotoxicity at 1.0 mg/mL concentrations of conjugates 5-9 (see Supplementary Information), indicating that these contrast agents have not acquired short term toxicity through their macromolecular conjugation.
Through conjugation to highly biocompatible and readily synthesized dendrimers, the relaxivity of HOPO-based TREN capped Gd(III) complexes was improved over commercial agents without compromising clinical relevance and safety. The conjugates presented here contain tightly bound gadolinium and give relaxivities of up to 38 mM-1s-1 under the clinically relevant conditions of 60 mHz and 37 °C, and were shown to be nontoxic to cells at mM concentrations.
Supplementary Material
Acknowledgments
The authors acknowledge NIH Grant R01 EB 002047, NIH Grant HL069832. We acknowledge Kyle Broaders for assistance with cell studies, Dr. Christopher M. Andolina, and Adam D. Hill.
Footnotes
Supporting Information Available: All conjugation procedures, characterization, cytotoxicity and experimental details; This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Caravan P. Chem Soc Rev. 2006;35:512–523. doi: 10.1039/b510982p. [DOI] [PubMed] [Google Scholar]
- 2.Pierre VC, Botta M, Aime S, Raymond KN. Inorg Chem. 2006;45:8355–8364. doi: 10.1021/ic061262q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Datta A, Raymond KN. Accts Chem Res. 2009;7:938–947. doi: 10.1021/ar800250h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Werner EJ, Avedano S, Botta M, Hay BP, Moore EG, Aime S, Raymond KN. J Am Chem Soc. 2007;129:1870–1871. doi: 10.1021/ja068026z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doble DMJ, Botta M, Wang J, Aime S, Barge A, Raymond KN. J Am Chem Soc. 2001;123:10758–10759. doi: 10.1021/ja011085m. [DOI] [PubMed] [Google Scholar]
- 6.Cohen SM, O'Sullivan B, Raymond KN. Inorg Chem. 2000;39:4339–4346. doi: 10.1021/ic000239g. [DOI] [PubMed] [Google Scholar]
- 7.Raymond KN, Pierre VC. Bioconj Chem. 2005;16:3–8. doi: 10.1021/bc049817y. [DOI] [PubMed] [Google Scholar]
- 8.Mishra R, Su W, Pohmann R, Pfeuffer J, Sauer MG, Ugurbil K, Engelmann J. Bioconj Chem. 2009;20:1860–1868. doi: 10.1021/bc9000454. [DOI] [PubMed] [Google Scholar]
- 9.Pierre VC, Botta M, Raymond KN. J Am Chem Soc. 2006;127:504–505. doi: 10.1021/ja045263y. [DOI] [PubMed] [Google Scholar]
- 10.Ali MM, Woods M, Caravan P, Opina ACL, Spiller M, Fettinger JC, Sherry AD. Chem- Eur J. 2008;14:7250–7258. doi: 10.1002/chem.200800402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiner WC, Konda S, Shadron A, Brechbeil M, Ganswo O. Invest Radiol. 1997;32:748–754. doi: 10.1097/00004424-199712000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, Thorek DLJ, Tsourkas A. Angew Chem Int Ed. 2010;49:346–350. doi: 10.1002/anie.200905133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nwea K, Bernardo M, Regino CAS, Williams M, Brechbiel MW. Bioorg Med Chem. 2010;18:5925–5931. doi: 10.1016/j.bmc.2010.06.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bumb A, Brechbeil MW, Choyke P. Acta Radiol. 2010;51:751–767. doi: 10.3109/02841851.2010.491091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manus LM, Mastarone DJ, Waters EA, Zhang XQ, Schultz-Sikma EA, MacRenaris KW, Ho D, Meade TJ. Nano Lett. 2010;10:484–489. doi: 10.1021/nl903264h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song Y, Xu X, MacRenaris KW, Zhang XQ, Mirkin CA, Meade TJ. Angew Chem Intl Ed. 2009;48:9143–9147. doi: 10.1002/anie.200904666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Poll DG, Kieler-Ferguson HM, Floyd WC, Guillaudeu SJ, Jerger K, Szoka FC, Fréchet JMJ. Bioconj Chem. 2010;21:764–773. doi: 10.1021/bc900553n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox ME, Szoka FC, Fréchet JMJ. Acc Chem Res. 2009;42:1141–1151. doi: 10.1021/ar900035f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee CC, Gillies ER, Fox ME, Guillaudeu SJ, Fréchet JMJ, Dy EE, Szoka FC. Proc Nat Acad Sci. 2006;103:16649–16654. doi: 10.1073/pnas.0607705103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Werner EJ, Kozhukh J, Botta M, Moore EG, Avedano S, Aime S, Raymond KN. Inorg Chem. 2009;48:277–286. doi: 10.1021/ic801730u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lauffer RB, Pharmalee DJ, Dunham SU, Oullet HS, Dolan RP, Witte S, McMurry TJ, Walovitch RC. Radiology. 1998;207:529–538. doi: 10.1148/radiology.207.2.9577506. [DOI] [PubMed] [Google Scholar]
- 22.Xu J, Franklin SJ, Wisenhunt DW, Jr, Raymond KN. J Am Chem Soc. 1995;117:7245–7246. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.