Abstract
In addition to genetic disorders, epigenetic alterations have been shown to be involved in cancer, through misregulation of histone modifications. Miswriting, misreading, and mis-erasing of histone acetylation as well as methylation marks can be actually associated with oncogenesis and tumor proliferation. Historically, methylation of Arg and Lys residues has been considered a stable, irreversible process due to the slow turnover of methyl groups in chromatin. The discovery in recent years of a large number of histone Lys demethylases (KDMs, belonging to either the amino oxidase or the JmjC family) totally changed this point of view and suggested a new role for dynamic histone methylation in biological processes. Since overexpression, alteration, or mutation of a number of KDMs has been found in many types of cancers, such enzymes could represent diagnostic tools as well as epigenetic targets to modulate for obtaining novel therapeutic weapons against cancer. The first little steps in this direction are described here.
Keywords: LSD1, Jumonji-containing enzymes, FAD, 2-oxoglutarate, cancer
In addition to genetic alterations, it is now well established that epigenetic dysfunctions can be involved in the pathogenesis and development of cancer. Epigenetic regulation of gene expression involves two major mechanisms, DNA methylation at the CpG islands of gene promoters and chromatin remodeling. Chromatin folding and its switch from heterochromatin, transcriptionally silent, to euchromatin, transcriptionally active, and vice versa, is finely tuned by three categories of proteins: (1) enzymes that add chemical marks to histone substrates, the so-called writers such as histone acetyltransferases (HATs) and histone methyltransferases (further distinct into protein arginine methyltransferases [PRMTs] and histone lysine methyltransferases [HKMTs]); (2) enzymes that remove such chemical covalent modification from histone substrates—namely, “erasers” such as histone deacetylases (HDACs) and histone lysine demethylases (KDMs, the lysine-specific demethylases [LSDs], and the Jumonji C [JmjC] families; Fig. 1); and (3) proteins that recognize and react to specific modified histone residues at epigenetic code level, the “readers.”1-4
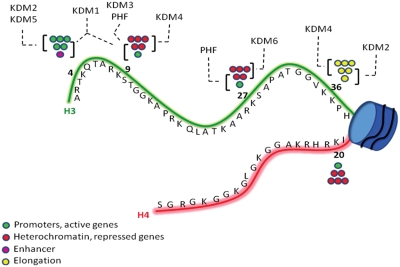
Figure 1.
Schematic representation of H3 and H4 histone tails, with the most important Lys residues involved in mono-, di-, and trimethylation (circles). The most frequent KDM families involved in demethylation of specific methyl markers are shown.
Aberrant expression of writer or eraser enzymes has been implicated in the course of tumor initiation and progression, and indeed 2 HDAC inhibitors, vorinostat (suberoylanilide hydroxamic acid, SAHA) and romidepsin (depsipeptide, FK-228), have been approved by Food and Drug Administration (FDA) for the treatment of refractory cutaneous T cell lymphoma and actually are in clinical trials, alone or in combination, for a variety of both hematological and solid tumors.5
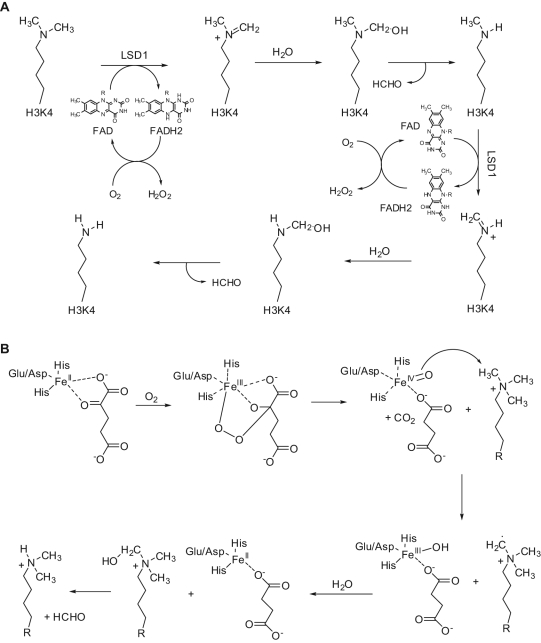
KDMs are the most recently discovered families of erasers. Methylation of lysine residues at histone substrates was long retained an irreversible reaction. In 2004, the first protein showing mammalian KDM activity was reported to be a flavin-containing amino oxidase (AO) that specifically demethylated mono- or dimethylated lysine 4 at histone H3 (H3K4me1 and H3K4me2) and has been named LSD1 (lysine-specific demethylase 1)/KDM1A (Fig. 2A).6,7 More recently, a second flavin-dependent H3K4me1/2 demethylase called LSD2/AOF1/KDM1B has been identified in mammals.8 In 2006, a larger and more versatile family of KDMs structurally different from LSD1 was identified, all containing as a signature motif the JmjC domain and all working through a Fe2+/2-oxoglurate (2-OG) mechanism for catalysis (Fig. 2B).9,10 The reaction goes on generating a superoxide radical by the complex Fe2+/2-OG, which hits the C2 atom of 2-OG, leading to its decarboxylation to succinate and formation of a Fe4+-oxo species. Afterwards, the Fe4+-oxo species abstracts a hydrogen from the N-methyl group of the amine substrate while it is reduced to Fe3+. The last two steps involve the generation of a carbinolamine intermediate on the substrate, which leaves formaldehyde and the amine with one methyl group less (Fig. 2B).
Figure 2.
Catalytic mechanism of LSD1 (A) and JmjC (B) enzymes.
Recently, overexpression or mutation of KDMs has been linked to mis-erased histone methyl modifications and, from a clinical point of view, to many types of cancer (Table 1). Thus, such enzymes could represent new potential diagnostic tools as well as therapeutic targets in oncology, and compounds able to inhibit KDMs could be of considerable interest as novel anticancer agents.
Table 1.
Histone Lysine Demethylases (KDMs): Specificity, Transcriptional Effects, and Potential Links to Cancer
| Cluster | Names | Demethylase Activity | Transcriptional Effects | Cancer Alterations and Possible Mechanisms | Putative Cancer Roles | References |
|---|---|---|---|---|---|---|
| KDM1 | KDM1A; LSD1; BHC110; AOF2 | H3K4me2/1 | Repression | Overexpression in breast, small cell lung, colorectal, prostate, neuroblastoma, and bladder cancer | Putative oncogene | 35-37 |
| H3K9me2/1 | Activation | |||||
| KDM1B; LSD2; AOF1 | H3K4me2/1 | Repression | NR | NR | ||
| KDM2 | KDM2A; JHDM1A; FBXL11 | H3K36me2/1 | Repression | Reduced expression in prostate cancer; suppression of NF-κB-dependent growth of colon cancer cells; effects on chromosomal segregation | Context-dependent pro- and anti-oncogenic functions | 76-84 |
| KDM2B; JHDM1B; FBXL10 | H3K36me2/1 | Repression | Overexpression in lymphomas and adenocarcinomas; downregulation in glioblastoma multiform; mutation in hematological tumors; cell protection against ultraviolet (UV)–induced apoptosis, oxidative stress, and spontaneous mutagenesis | Context-dependent pro- and anti-oncogenic functions | 76-84 | |
| H3K4me3 (?) | Repression | 74, 76 | ||||
| KDM3 | KDM3A; JMJD1A; JHDM2A; TSGA | H3K9me2/1 | Activation | Co-activator of androgen receptor (AR)–mediated transcription; under hypoxia increases a subset of hypoxia-inducible genes enhancing tumor growth | NR | 85-90, 93 |
| KDM3B; JMJD1B; JHDM2B; 5qNCA | H3K9me2/1 | Activation | 5q31 (JMJD1B) frequent deletion in cancer | Candidate tumor suppressor | 91, 92 | |
| KDM3C; JMJD1C; JHDM2C; TRIP8 | NR | Reduced expression in breast cancer | Candidate tumor suppressor | 91, 92 | ||
| KDM4 | KDM4A; JMJD2A; JHDM3A | H3K9me3/2 | Activation | Overexpression in prostate cancer | Putative oncogene | 26, 94-102 |
| H3K36me3/2 | Repression | |||||
| KDM4B; JMJD2B; JHDM3B | H3K9me3/2 | Activation | Overexpression in prostate cancer | Putative oncogene | 26, 94-102 | |
| H3K36me3/2 | Repression | |||||
| KDM4C; JMJD2C; JHDM3C; GASC1 | H3K9me3/2 | Activation | Overexpression/amplification in prostate cancer, esophageal squamous cell carcinoma, desmoplastic medulloblastoma, metastatic lung sarcomatoid carcinoma, mucosa-associated lymphoid tissue (MALT) lymphoma, and breast cancer; increasing genomic instability; transcriptional activation of oncogenes (e.g., NOTCH1, MDM2) and of AR target genes | Putative oncogene | 26, 94-102 | |
| H3K36me3/2 | Repression | |||||
| KDM5 | KDM5A; JARID1A; RBP2 | H3K4me3/2 | Repression | Translocation: NUP98-JARID1A fusion protein; overexpression in gastric cancer; key effector of pRB-mediated cell cycle withdrawal and differentiation; elevated expression in drug-tolerant subpopulation of cancer cells | Conflicting reports and likely context-dependent pro- and anti-oncogenic functions | 103-111 |
| KDM5B; JARID1B; PLU-1 | H3K4me3/2 | Repression | Overexpression in breast, testis, prostate, ovary, and esophageal cancer; silencing of tumor suppressor genes (e.g., BRCA1, CAV1, HOXA5) | Putative oncogene | 112-114 | |
| KDM5C; JARID1C; SMCX | H3K4me3/2 | Repression | Regulation of human papilloma virus (HPV) oncogene expression | NR | 67, 116 | |
| KDM5D; JARID1D; SMCY | H3K4me3/2 | Repression | NR | 67 | ||
| KDM6 | KDM6A; UTX | H3K27me3/2 | Activation | Downregulation; inactivating somatic mutations in multiple myeloma, esophageal squamous cell carcinomas, and renal cell carcinomas; transcriptional activation of pRB binding proteins contributing to the pRB-mediated cell cycle arrest | Candidate tumor suppressor | 117-120, 123, 126, 127 |
| KDM6B; JMJD3 | H3K27me3/2 | Activation | Downregulation in subsets of human cancers; 17p13.1 (JMJD3) frequent deletion in cancer; transcriptional activation of INK4A/ARF region promoting senescence | Candidate tumor suppressor | 117-120, 124, 125, 128 |
NR, not reported.
The Lys-Specific Demethylase LSD1
LSD1/BHC110/AOF2/KDM1A was identified for the first time as a stable component of the BRAF-HDAC transcriptional co-repressor complex containing also the REST co-repressor, CoREST, already known for a repressive role for neuronal genes in nonneuronal cells.11-14 LSD1 consists of an N-terminal SWIRM domain and an AO domain containing 2 sites to bind the methylated substrate and the flavin adenine dinucleotide (FAD) cofactor, respectively. Between these 2 sites in the AO domain, there is the active site of the enzyme, and from them 2 antiparallel α-helices project away as a unique feature forming the Tower domain, crucial for the interaction of LSD1 with CoREST and for the ability to demethylate nucleosomal substrates.15 Indeed, LSD1 alone only demethylates H3K4 in histone substrates lacking associated DNA and requires CoREST to catalyze nucleosomal demethylation.14,16,17 The reaction starts with generation of an iminium cation intermediate from the methylated amino group of Lys, which is in turn hydrolyzed to yield a carbinolamine that spontaneously degrades to give formaldehyde and the demethylated amine (Fig. 2A). Since such mechanism of demethylation requires a protonated nitrogen to initiate the reaction, the demethylation is limited to dimethyl and monomethyl Lys. The FAD cofactor, reduced during the reaction to FADH2, is reoxidized by O2 in a following step that leads to production of H2O2 (Fig. 2A).7,18,19 The X-ray structure of the complex between LSD1 and H3K4me2 has been solved, and the observed tight constraints on the H3 N terminus account for no more than 3 amino acids to be present on the N-terminal side of the methyllysine in the active site of the enzyme, near to the FAD cofactor. This, combined with sequence-specific interactions between the active site and Arg2, Thr3, and Gln5, provides an explanation for the observed specificity of LSD1 toward the H3K4me1 and H3K4me2 substrates.20-22 When bound to androgen receptor (AR), LSD1 seems to change its substrate specificity: under AR signaling, protein kinase C β1 (PKCβ1) is recruited at the receptor and phosphorylates threonine 6 at histone H3 (H3T6).23 Such histone H3 modification blocks the H3K4 demethylating activity of LSD1, which in this case catalyzes the H3K9me1 and H3K9me2 (2 repressive markers) demethylation, playing a role of co-activator.24,25 Such a role seems to be corroborated by the cooperation of LSD1 and JMJD2C, a H3K9me3 demethylase, into a specific demethylase complex on AR target genes, which was found to co-localize with AR in normal prostate as well as in prostate carcinomas.26 Thus, depending on the its binding partners and its substrates, LSD1 seems to be able to have a role as a co-repressor (H3K4 demethylation) or a co-activator (H3K9 demethylation). In addition, LSD1 has been shown to act on nonhistone substrates. LSD1-mediated demethylation of K370me2 in the tumor suppressor p53 prevents its interaction with the co-activator 53BP1 (p53-binding protein 1) and represses its transcriptional activation and apoptosis induction.27 Demethylation of DNMT1 by LSD1 stabilizes the enzyme from protein degrading and is essential for maintaining global DNA methylation in embryonic stem (ES) cells.28,29 Interestingly, many types of cancer show upregulation of both DNMT1 and LSD1, but a real link between the 2 epigenetic enzymes in such pathologies has to be established. The retinoblastoma protein 1 (RB1) regulator myosin phosphatase target subunit 1 (MYPT1) is also demethylated by LSD1 at Lys442, losing in such way its stability and leading to enhancement of RB1 phosphorylation.30
In 2009, the second FAD-dependent demethylase, AOF1/LSD2/KDM1B, was identified in mammals.8 Similar to LSD1, LSD2 contains a conserved SWIRM domain required for its catalytic activity and specifically demethylates H3K4me1 and H3K4me2; nevertheless, its repressing activity seems to be unrelated to the demethylase function.31 Different from LSD1, LSD2 lacks the Tower domain and thus it is not able to bind CoREST; it forms active complexes with euchromatic histone methyltransferases such as G9a and NSD3 as well as cellular factors involved in transcription elongation rather than with HDACs, and it is localized at the gene body level rather than at the promoters.32 With respect to LSD1, LSD2 has a more restricted expression pattern, it being abundant essentially in growing oocytes and being required for de novo DNA methylation of some imprinted genes.33 Thus, considering the involvement of both the 2 flavin-containing amino oxidases LSD1 and LSD2 with DNMTs and de novo DNA methylation (see also above and below), it seems feasible that a real functional link between DNA methylation and histone demethylation would exist. Very recently, LSD2 has been reported to promote H3K9me2 in addition to H3K4me2 demethylation, leading to control of stimulus-induced recruitment of NF-κB to the MDC and IL12B promoters and activation of these inflammatory genes.34
LSD1 in Cancer
Aberrant expression of LSD1 has been shown in many types of cancers. In particular, LSD1 expression is upregulated in bladder, small cell lung, and colorectal clinical cancer tissues when compared with the corresponding nonneoplastic tissues.35 In bladder carcinogenesis, LSD1 is highly overexpressed even in tumors at an early grade, thus suggesting LSD1 to be one of the initiators of the whole process. Although estrogen receptor (ER)–positive breast tumors are efficiently treated with antihormonal therapy, ER-negative breast tumors are usually treated with nonselective cytotoxic drugs, and they generally have a less favorable prognosis. LSD1 expression has been found strongly upregulated in ER-negative breast cancers, so LSD1 could be suggested as a predictive biomarker for aggressive tumor biology and tumor recurrence during therapy.36 Pharmacological (by tranylcypromine, clorgyline) downregulation of LSD1 in ER-negative breast cancer led to growth inhibition, and LSD1 silencing by siRNA resulted in increased expression of the tumor suppressor p21WAF1/CIP1 and in downregulation of the pro-proliferative genes CCNA2 and ERBB2.36 LSD1 expression was also highly upregulated in poorly differentiated neuroblastoma, it being strongly associated with adverse clinical outcome and inversely correlated with differentiation.37 Conversely, LSD1 was not expressed in benign ganglioneuroblastomas/ganglioneuromas or in nonmalignant cells such as stromal tissue or infiltrating leukocytes. Downregulation of LSD1 in vitro with both siRNA and monoamine oxidase (MAO) inhibitors (pargyline, clorgyline, tranylcypromine) in SH-SY5Y cells led to growth inhibition and differentiation with an increase of H3K4 methylation, and tranylcypromine treatment in vivo reduced the growth of neuroblastoma xenograft in nude mice,37 indicating that LSD1 may provide not only a predictive marker for aggressive biology but also a novel therapeutic target for the treatment of aggressive cancer.
On the other side of the coin, LSD1 was shown to be recruited by the Mi-2/nucleosome remodeling and deacetylase (NuRD) complex that in such way possesses at the same time ATPase, deacetylase, and demethylase activities, influencing various pathways comprising transforming growth factor β (TGFβ) signaling, cell growth, and migration and invasion.38 Through a negative correlation with the TGFβ1 level, highly relevant in epithelial-mesenchymal transitions and invasion, LSD1 suppressed breast cancer metastatic potential in vitro and in vivo. Thus, depending on a variety of factors such as the biological context, the type and the grade of the tumor, and the presence of different substrates, LSD1 seems to have either oncogenic or tumor-suppressive functions, and further studies are needed to better manage this point.
Putative Mechanisms for LSD1-Mediated Oncogenesis
Mechanisms by which overexpression of LSD1 leads or contributes to tumor formation could involve its capacity to silence tumor suppressor genes as a transcriptional co-repressor, mainly through H3K4 demethylation. The physical association of LSD1 with HDACs at the co-repressor complex contributes to repress transcription of common sets of genes. This interplaying activity has been confirmed by experiments made on LNCaP prostate cancer cells treated with pan- (vorinostat) and class I selective (HDAC42, entinostat) HDAC inhibitors.39 Such compounds led to increased H3K4 methylation levels and suppression of expression of the JmjC demethylase RBP2 and LSD1 through downregulation of Sp1 expression. Moreover, shRNA-mediated silencing of the class I HDAC isozymes 1, 2, 3, and 8, but not that of the class IIb isozyme HDAC6, mimicked the drug effects on H3K4 methylation and H3K4 demethylases, which could be reversed by ectopic Sp1 expression.39 Further evidence of the role of LSD1 as a transcriptional co-repressor involved in cancer development came from the recruitment, from the CoREST/HDACs/LSD1 complex, of the oncoprotein ZNF217, able to negatively regulate the tumor suppressor gene p15INK4B.40 Similarly, the DNA binding factor SNAIL, which correlates with high tumor grade and nodal metastasis and is also predictive of a poor outcome in patients with breast cancer, interacts through its SNAG domain with the AO domain of LSD1/CoREST to repress E-cadherin in a variety of cancer cell lines and forms the ternary complex SNAIL/LSD1/CoREST in 116 primary breast cancer samples.41,42 Functionally, LSD1 is linked to DNA methylation and subsequent gene silencing. DNMT3L, a noncatalytic paralog of de novo DNMT3A and -3B, specifically interacts with the histone H3 tail only when H3K4 is unmethylated, and thus the formation of a complex LSD1/DNMT3L/DNMT3A is highly reliable.43 Alternatively, LSD1 has been reported to demethylate the Lys residue of DNMT1, thus preserving it from degradation.28 Interestingly, LSD1 and DNMT1 are both upregulated in many types of cancers, but whether the first is responsible for the increased expression of the latter is still unknown. However, further evidence of this DNMT1-LSD1 interplay came from the effect of combination treatment of LSD1 inhibitors and DNMT inhibitors against colon cancer xenograft mice, resulting in a great inhibition of the growth of the tumor.44 Another possibility for explaining the oncogenic potential of LSD1 is its effect on the tumor suppressor p53. p53 is a nonhistone substrate that is demethylated by LSD1 at Lys370. Methylated Lys370 in p53 is important for its binding to the transcriptional co-activator p53-binding protein–1 (PBP-1), and its demethylation led to inhibition of the p53 apoptotic activity, whereas the knockdown of LSD1 increased p21WAF1/CIP1 and MDM2, well-known p53 target genes.27 A further putative oncogenic mechanism of LSD1 resides into its capability to trigger Myc-induced transcription through transient H3K4 demethylation.45 The local DNA oxidation given by the LSD1 catalytic activity and the following production of hydrogen peroxide gave the recruitment of 8-oxoguanine-DNA glycosylase (OGG1) and of the nuclease Ape1 on the E-box chromatin sequence, leading to the assembly of the transcription initiation complex of the oncogene Myc.45
LSD1 Inhibitors
Substrate Analogues
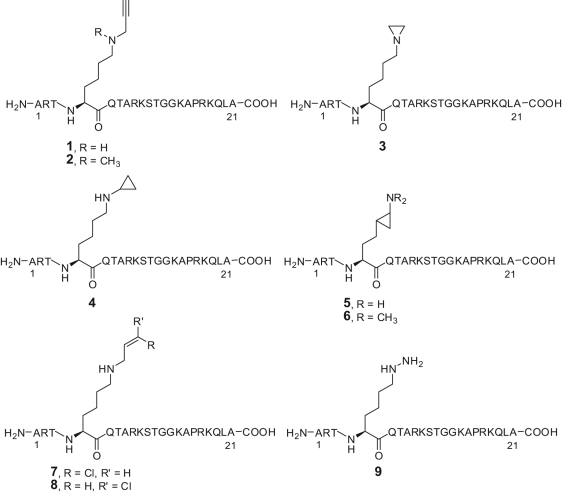
From the knowledge that LSD1 is a FAD-dependent AO that catalyzes the oxidative demethylation of monomethyl- and dimethyl-K4 residue at histone H3, as well as H3-tail peptides requiring at least 15 amino acid residues for efficient reaction, the propargyl- Lys-derivatized peptide 1 was designed as a mechanism-based LSD1 inhibitor (Fig. 3). Actually, 1 exhibited K i = 16.6 and then 0.77 µM against LSD1, showing a clear time- and concentration-dependent inhibition of the enzyme.46,47
Figure 3.
Substrate analogues as LSD1 inhibitors.
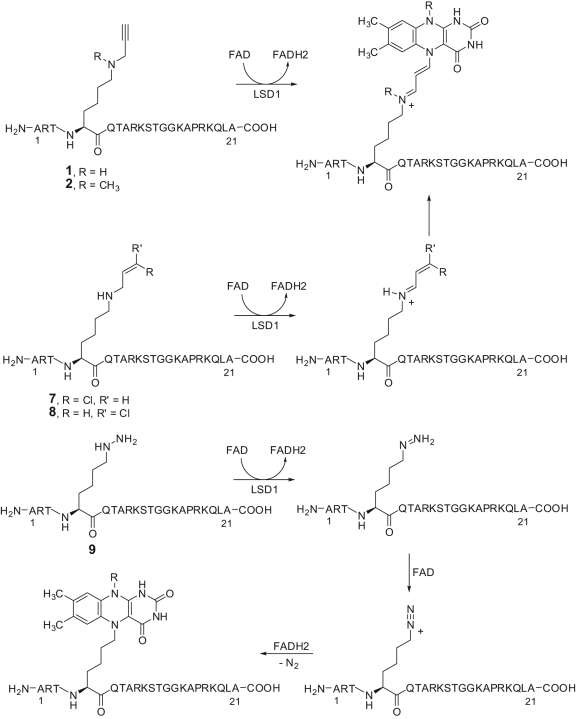
Mechanistic studies made on 1 revealed that its LSD1 inactivation involves amine oxidation followed by Michael addition of the FADH2 N5 to the propargylic imine (Fig. 4).48 The structure of the similar covalent adduct involving FADH2 and 2 (Fig. 3), the N-methyl analogue of 1, was then confirmed by X-ray crystal structure of the LSD1/2 complex.20 In addition to the propargyl residue, other warheads taken from more than 50 years of anti-MAO research story have been applied to the H3-peptide scaffold to explore their ability to inhibit LSD1.47
Figure 4.
Proposed mechanism of inactivation of LSD1 by substrate analogue inhibitors.
Although aziridine and exo- and endo-cyclopropylamine units (compounds 3-6) failed to inactivate LSD1, the cis- and trans-3-chloropropyl groups (compounds 7 and 8) gave to the H3-peptide skeleton similar ability as 1 to inhibit LSD1 (K i = 0.95 and 0.76 µM, respectively), and the hydrazine-Lys-4 H3-21 peptide 9 showed the highest enzyme inhibition, reaching K i = 0.00435 µM (Fig. 3). Also for the chloroallyl- and hydrazine-H3-peptides 7-9, a mechanism of suicide inactivation has been proposed (Fig. 4).47
Polyamine Analogues
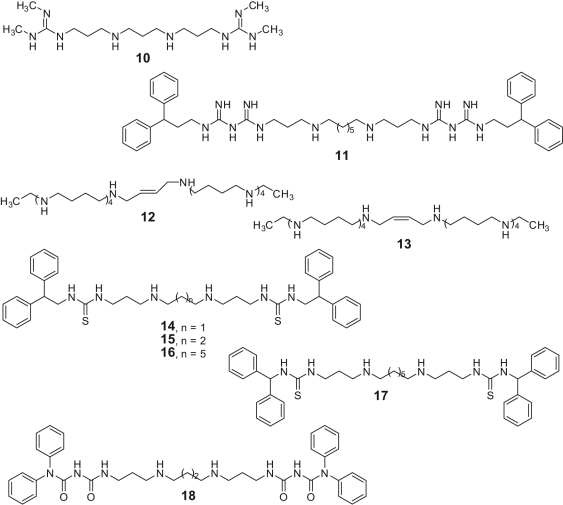
The AO domain of LSD1 shares 20% sequence similarity with those of the FAD-dependent MAO and PAO (polyamine oxidases such as spermine oxidase [SMO] and N1-acetylpolyamine oxidase), with the catalytic domains of LSD1 and SMO showing 60% similarity in amino acid sequences. As compounds bearing guanidine groups were shown to inhibit both SMO and other polyamine oxidases, a pioneer series of (bis)guanidines and (bis)biguanides, previously synthesized as potential antitrypanosomal agents,49 were tested as inhibitors of LSD1 (Fig. 5).50 Among them, compounds 10 and 11 showed high, noncompetitive LSD1 inhibition at <2.5 µM and were able to induce the reexpression of aberrantly silenced tumor suppressor genes in colon cancer cells in vitro, together with an increase of H3K4me2 and acetyl-H3K9 marks and a decrease of the repressive H3K9me1 and H3K9me2 marks. The same effects on recombinant LSD1 as well as human colon cancer HCT116 and RKO cell growth were observed by a series of octamines and decamines (see compounds 12 and 13 as the most potent derivatives), reported by the same research group (Fig. 5).44 In addition, 12 showed striking synergy in combination with the DNMT inhibitor 5-azacytidine against HCT116 tumors in vivo, without significant overall toxicity as indicated by animal weight. The series of polyamine analogues was further expanded with some (bis)urea and (bis)thiourea derivatives, such as compounds 14-18 (Fig. 5).51 Two of the most potent LSD1 inhibitors among them (i.e., 15 and 16) were also able to inhibit the growth of Calu-6 non–small cell lung carcinoma cells in vitro with GI50 = 10.3 and 9.4 µM, respectively.51
Figure 5.
Polyamine analogues as LSD1 inhibitors.
Anti-MAO-Based Small Molecules
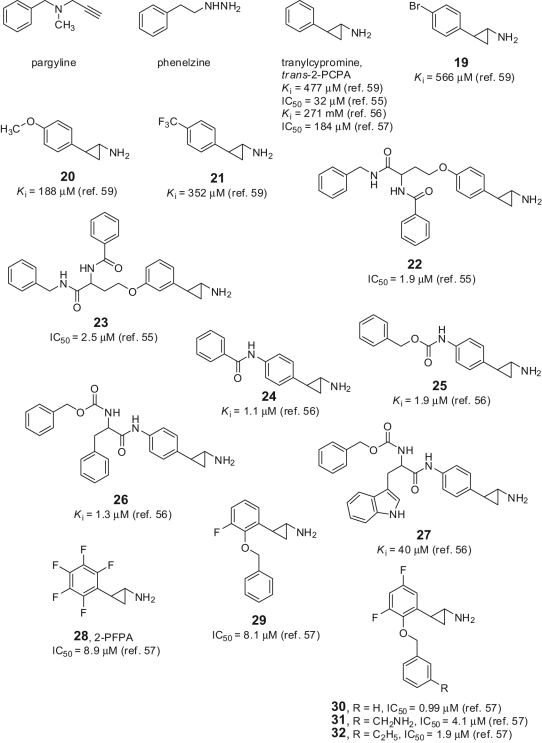
Due to the high sequence similarity between LSD1 and MAO, some known anti-MAO agents were tested against LSD1. Pargyline (Fig. 6), a propargylamine-based anti-MAO initially suggested as a valuable LSD1 inhibitor prototype, failed in further studies to inhibit the Lys demethylase.21,52 Phenelzine (Fig. 6), an anti-MAO agent bearing a hydrazine moiety, was initially reported as a weak LSD1 inhibitor, and when reinvestigated on its inhibiting activity, it resulted in having highly improved potency against LSD1 (K i = 17.6 µM), similar to that displayed versus MAOs.47
Figure 6.
Anti-MAO-based LSD1 inhibitors.
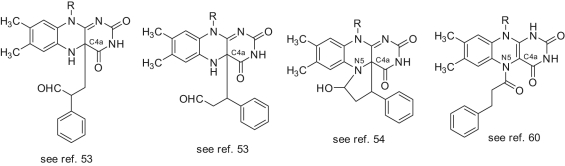
Tranylcypromine (trans-2-phenylcyclopropyl-1-amine, trans-2-PCPA) (Fig. 6) was reported to inhibit LSD1 with a K i value ranging from 477 to 22 µM, depending on the type of assay, the time of addition, the substrate, and the enzyme source used for testing.52-58 Nevertheless, trans-2-PCPA became a useful lead to develop highly potent small-molecule inhibitors of LSD1. Some trans-2-PCPA analogues carrying 4-bromo, 4-methoxy, and 4-trifluoromethoxy substitutions at the benzene ring led to compounds (19-21) with similar or slightly improved activity against LSD1.59 Interestingly, the introduction of a bulky, branched substituent at the 4 position of the benzene ring, able to occupy the wide space present in the LSD1 cavity surrounding the FAD cofactor and the AO domain, furnished tranylcypromine-based compounds such as 22-27 endowed with high potency against LSD1 and scarce or no activity against MAOs.55,56 Compounds 22 and 23 were also reported to be active against a panel of cancer cell lines with growth inhibition 50% (GI50) values ranging from 6.0 to 67 µM (Table 2),55 whereas 26 was able to inhibit cell growth and induce differentiation in murine acute promyelocytic leukemia (APL) blasts as a single agent and in APL-derived NB4 cells in synergy with retinoic acid (RA).56 The perfluorinated trans-2-PCPA analogue 28 (trans-2-pentafluorophenylcyclopropylamine, 2-PFPA), 21-fold more potent than the parent compound against LSD1, is allowed to identify through structure-based drug design new fluorine-containing trans-2-PCPA analogues (29-32) with single-digit- to sub-micromolar IC50 values against LSD1.57 Trans- 2-PCPA and analogues inhibit LSD1, forming a covalent linkage with the FAD cofactor. Different adducts between trans-2-PCPA and FAD have been postulated (Fig. 7).53,54,60
Table 2.
Antiproliferative Activity of trans-2-PCPA, 22, and 23 on a Panel of Cancer Cell Lines
| GI50, µM | |||
|---|---|---|---|
| Cancer cell line | trans-2-PCPA | 22 | 23 |
| HeLa cervix cancer | >500 | 30 | 13 |
| HCT116 colon cancer | >1000 | 67 | 43 |
| PC3 prostate cancer | 600 | 17 | 6 |
| KYSE150 esophagus cancer | 260 | 18 | 11 |
| SH-SY5Y neuroblastoma | 500 | 43 | 27 |
Figure 7.
Proposed structures for the flavin adenine dinucleotide (FAD)–tranylcypromine adducts in the LSD1 complex.
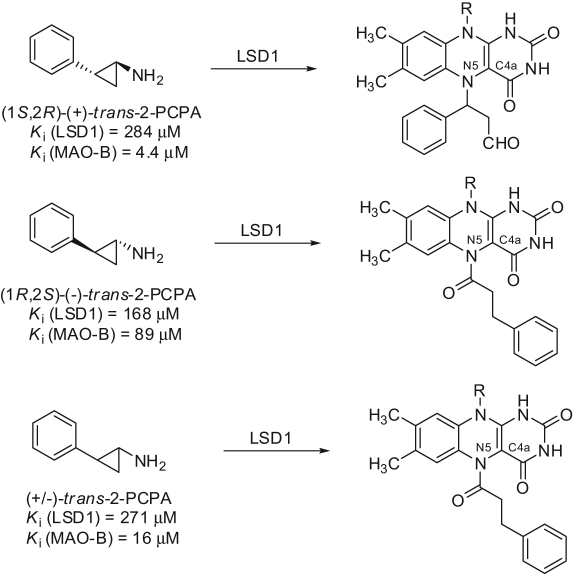
As trans-2-PCPA is the racemic mixture of 2 enantiomers, (+)-trans-2-PCPA and (–)-trans-2-PCPA, we prepared large amounts of trans- and cis-2-PCPA, separated the 2 trans- and 2 cis-enantiomers, and tested them against LSD1 and MAOs, determining their binding to FAD through X-ray crystallography.56 Thus, we found that the 2 trans-enantiomers of 2-PCPA were slightly more potent than the 2 cis congeners against LSD1, with (–)-trans-2-PCPA being 1.7-fold more efficient than (+)-trans-2-PCPA (Fig. 7). Conversely, against MAO-B, (+)-trans-2-PCPA was 20-fold more potent than the corresponding (–) enantiomer, highlighting the crucial role of stereochemistry in MAO-B inhibition by trans-2-PCPA. From X-ray crystal structures, it was clear that (–)-trans-2-PCPA connects its carbonyl carbon in a covalent bond with the flavin N5 atom, positioning its phenyl ring in the core of the substrate-binding pocket, above the flavin ring. Differently, (+)-trans-2-PCPA links the flavin N5 atom through the phenyl-substituted carbon of the inhibitor, pointing the phenyl ring away from the flavin ring (Fig. 8). Due to the higher potency shown by the (–) enantiomer, when the racemic trans-2-PCPA is crystallized with LSD1, the enzyme selects this enantiomer for the binding with FAD, and the N5-carbonyl adduct is obtained, according to the literature.56,60
Figure 8.
Structures of flavin adenine dinucleotide (FAD)–tranylcypromine adducts obtained by the trans-2-PCPA enantiomers.
LSD1 Activators?
Two immunomodulatory drugs, pomalidomide and lenalidomide (Fig. 9), have been reported to induce p21WAF1 expression in Burkitt lymphoma and multiple myeloma cell lines, leading to cell cycle arrest. This p21WAF1 induction is generated by modification of the chromatin structure at the p21 promoter involving a switch from H3K9 methylation (a repressive mark) to H3K9 acetylation (an activation mark of transcription). As LSD1 silencing reduced the observed pomalidomide and lenalidomide-induced p21 upregulation, LSD1 has been suggested to be involved in this effect of p21 induction through demethylation of H3K9, and pomalidomide and lenalidomide could act as direct LSD1 activators or indirectly through activation of a third partner required for LSD1 activation.61
Figure 9.
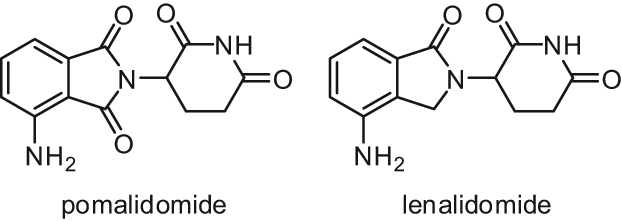
Structures of pomalidomide and lenalidomide.
The Jumonji C Family of Lysine Demethylases
The second and largest class of histone lysine demethylases (KDMs) to be discovered is the Fe+2/2-OG-dependent family of KDMs.9,10,62 These enzymes belong to a much larger enzyme superfamily, the 2-OG oxygenases, whose members (about 70-80 genes in the human genome) catalyse a diverse range of oxidation reactions, using 2-OG, molecular oxygen, and Fe2+ as co-substrates/co-factors (Fig. 2B).63 In humans, the two main reactions catalyzed by 2-OG oxygenases identified so far are protein/nucleotide hydroxylation and N-methyl demethylation.64 In particular, the 2-OG-dependent histone lysine demethylases are members of the Jumonji family of 2-OG oxygenases, which have common structural features (e.g., with respect to 2-OG binding residues) and comprise nonhistone modifying enzymes such as factor inhibiting HIF (FIH).65 The Jumonji protein family is named this way because it contains the conserved Jumonji C (JmjC) catalytic domain that was first identified in the so-called Jumonji protein (JARID2).66 In fact, jumonji means cruciform in Japanese, and the gene was so named because mice with a genetrap inserted in the Jumonji locus develop an abnormal cross-like neural tube.66 Among the 30 JmjC domain-containing proteins identified so far within the human genome, about 20 have been published to demethylate specific lysines in the histone proteins.
The JmjC histone lysine demethylases catalyze the hydroxylation at the carbon of the Nε-methyl group to give an unstable hemiaminal intermediate that rapidly breaks down, leading to the release of formaldehyde and the demethylated lysine residue.67 Unlike LSD1, the hydroxylation-based mechanism of the JmjC KDMs does not require a protonatable lysine ε-amine group, enabling these enzymes to demethylate all 3 lysine methylation states (tri-, di- and monomethylation) at H3K4, H3K9, H3K27, and H3K36, as well as H1K26.67 Six different subfamilies (JMJD1s, JMJD2s, JARID1s, UTX/Y-JMJD3, PHFs, and FBXLs) of JmjC histone demethylases have been identified, which have different histone sequence and methylation state selectivities.67,68 For instance, KDMs of the JmjC domain-containing 2 (JMJD2) subfamily are selective for the demethylation of the tri- and di-Nε-methylation states of specific lysines on histone H3, whereas other subfamilies (e.g., the PHF and FBXL subfamilies) are selective for the di- and mono-Nε-methylated states and do not accept the trimethylated state. Recent crystallographic analyses have shown that variations in the size of the active site region binding the Nε-methyl group are in part responsible for the methylation state selectivity.69 Selectivity for a particular histone sequence seems to be conferred by binding interactions directly with the catalytic domain and, at least for some enzymes, by the presence of other noncatalytic domains that target the catalytic one to particular residues, as recently demonstrated for PHF8.69-71 In fact, this histone demethylase has a plant homeobox domain (PHD), in which binding the Nε-trimethylated form of the K4 of the histone H3 increases the activity of the catalytic domain with regard to demethylation of the Nε-dimethylated H3K9. Mounting evidence (especially works with peptides) suggests that some JmjC demethylases have also nonhistone substrates, a conceivable finding given the rising data on nonhistone proteins that are Nε-methylated.72
Despite the functional characterization of many JmjC histone demethylases being still at a relatively early stage, recent evidence suggests for these enzymes many important biological roles ranging from the regulation of cellular differentiation and development to the control of neuronal function, from senescence process modulation to the control of genome stability and cancer cell proliferation.64,67,68 Jumonji histone demethylases are being targeted for inhibition by small molecules, both with a view to developing “chemical probes” potentially useful in functional assignments and for the eventual development of pharmaceuticals, with the most likely application being cancer.18,73 In fact, the misregulation of JmjC KDMs has significantly being implicated in cancer initiation and progression, and this will be reviewed here by analyzing the involvement of different subfamilies of the JmjC histone lysine demethylases.
The KDM2 Cluster (FBXL Subfamily)
KDM2A/JHDM1A (JmjC domain–containing histone demethylation protein 1A)/FBXL11 (F-box and leucine-rich repeat protein 11) was the first JmjC domain–containing protein shown to be a histone lysine demethylase. Human KDM2A and KDM2B/JHDM1B/FBXL10 have been shown to catalyze H3K36me2/me1 demethylation.74,75 In addition, mammalian FBXL10 has been suggested to act as an H3K4me3 demethylase,76,77 but reports on its activity toward H3K4me3 are conflicting.74,76 Both proteins contain an F-box domain in addition to 2 leucine-rich repeat (LRR) domains.76 Retroviral insertional mutagenesis within the FBXL10/JHDM1B gene has been shown to cause lymphoma in BLM (Bloom syndrome RecQ protein-like-3 DNA helicase)–deficient mice, indicating KDM2B as a candidate tumor suppressor.78 Other studies have proposed FBXL10, along with FBXL11, as a putative proto-oncogene.79 Expression data from human cancers show overexpression of KDM2B in lymphomas and adenocarcinomas79 but reduced expression of KDM2A and KDM2B in prostate cancer and in the most aggressive of the primary brain tumors, the glioblastoma multiform, respectively.76,80 Cell culture studies support the view that KDM2 proteins might have context-dependent pro- and anti-oncogenic functions.
KDM2A can suppress NF-κB-dependent growth of colon cancer cells,81 whereas KDM2B has been shown to inhibit proliferation of tumor cells correlating with repression of rRNA genes.76 By contrast, KDM2B prevents premature senescence in MEFs (mouse embryonic fibroblasts) through a mechanism that likely involves repression of the Ink4b gene.74,77,82 MEFs ectopically expressing KDM2A or KDM2B bypass stress-induced senescence, depending on an intact JmjC domain, and KDM2B cooperates with Ras in oncogenic transformation.74,79 In addition to the proliferative control, KDM2 proteins are important for genomic stability. KDM2B has been demonstrated to protect cells against ultraviolet (UV)–induced apoptosis, oxidative stress, and spontaneous mutagenesis,78,83,84 whereas depletion of FBXL11 was reported to influence chromosomal segregation through effects exerted in the heterochromatic areas of the genome.80 Taken together, mammalian KDM2 demethylases have been suggested to have context-dependent tumor-promoting and tumor-inhibiting functions.
The KDM3 Cluster (JMJD1 Subfamily)
KDM3A/JHDM2A/JMJD1A (Jumonji domain–containing 1a)/TSGA (testis-specific gene A) is a histone demethylase specific for H3K9me2/me1.85 Two JMJD1A homologues exist in human cells: KDM3B/JHDM2B/JMJD1B and JMJD1C/JHDM2C/TRIP8 (thyroid receptor interacting protein 8). JMJD1A features an LXXLL motif that is a signature involved in nuclear receptor interactions.86 The expression of JMJD1A is very significant in testes and has been implicated in demethylation of H3K9me2 of AR target genes.85 Thus, JMJD1A was found to interact with the AR in a ligand-dependent manner. Inhibition of JMJD1A expression in the prostate cancer cell line LnCaP led to an increase in H3K9me2 in a subset of AR target genes, including PSA, NKX3.1, and TMPRSS22, and a decrease in their expression.85 These results show that JMJD1A acts as a co-activator of AR-mediated transcription.
Studies of genetrap and knockout mice have demonstrated important roles for KDM3A in germ cell development and metabolism.87-90 JMJD1A is highly and dynamically expressed during spermatogenesis, and male Kdm3a genetrap mice are infertile, with small testes and a severe reduction in sperm count. This defect has been linked to KDM3A positively regulating expression of genes involved in sperm chromatin condensation and maturation through demethylation of H3K9me2/me1.88,89 Kdm3a knockout mice also exhibit an adult-onset obesity phenotype, and in this context, KDM3A has been implicated in the transcriptional control of metabolic genes in muscle and adipose tissues.87,90 Little is known about the implication of JMJD1s in tumorigenesis. Frequent deletion of 5q31 locating the JMJD1B gene or reduced expression of JMJD1C in various malignancies suggests possible roles of KDM3 proteins in tumor suppression.91,92 Recent studies have shown that JMJD1A is the target of hypoxia-inducible transcription factors, and under hypoxia, JMJD1A increases a subset of hypoxia-inducible genes enhancing tumor growth.93
The KDM4 Cluster (JMJD2 Subfamily)
Although the potential role of JMJD1s in cancer remains controversial, the JMJD2 subfamily members are mainly regarded as oncogenes. Their oncogenic potential could result from demethylating heterochromatic H3K9me3/2, an important mark for formation and maintenance of heterochromatin and somehow of genomic stability. In fact, KDM4 (JMJD2) proteins were the first published demethylases that showed activity toward trimethylated lysines. There are 4 members of the KDM4 cluster in mammalian cells: KDM4A/JHDM3A/JMJD2A, KDM4B/JHDM3B/JMJD2B, KDM4C/JHDM3C/JMJD2C/GASC1 (gene amplified in squamous cell carcinoma 1), and KDM4D/JHDM3D/JMJD2D. The JMJD2 proteins catalyze the demethylation of H3K9me3/me2 and/or H3K36me3/me2, with the substrate specificity differing between subfamily members.94-98 Moreover, the KDM4 proteins have recently been demonstrated to demethylate H1.4K26me3/me2.99
Mounting evidence correlates human KDM4 family members—especially KDM4C—to malignant transformation. Significantly, amplification of the KDM4C locus has been reported for esophageal squamous carcinomas, medulloblastomas, and breast cancers, and amplification of KDM4B is detected in medulloblastomas.100-102 Gene expression analysis further shows that JMJD2A-C is overexpressed in prostate cancer.94 Depletion of JMJD2C from cancer cells, expressing high levels of it, resulted in inhibition of cell growth of several tumor cell lines,26,94,101 whereas overexpression of GASC1 in human nontransformed mammary epithelial cells resulted in phenotypic alterations that are hallmarks of neoplastic transformation, including growth factor–independent proliferation and anchorage-independent growth in soft agar.101 Introduction of the JMJD2C gene in normal breast MCF10A cells could increase higher capacity to generate mammospheres, a phenotype of cancer stem cells, suggesting that JMJD2C acts as a transforming gene.101 However, the genomic targets and cellular functions of the mammalian KDM4 subfamily members are still for the most part uncharacterized. To date, different studies have shown that KDM4C can function as a transcriptional activator by removing the heterochromatic H3K9me3 at promoter regions, which is generally associated with transcriptional repression.26 Analysis of genes altered by overexpression of GASC1 in MCF10A- GASC1 cells revealed NOTCH1 as a target gene of GASC1.101 Induction of NOTCH signaling promotes self-renewal of normal human mammary stem cells. This may support the finding that the phenotype of cancer stem cells was increased in MCF10A-GASC1 cells. The involvement of GASC1 in tumorigenesis has been supported further by a recent report demonstrating the functional interaction between GASC1 and AR in prostate carcinoma.26 It was known that LSD1 and JMJD1A are also required for transcriptional activation of AR responsive genes and for proliferation of prostate cancer cells. Although LSD1 and JMJD1A only demethylate mono- and dimethylated H3K9, GASC1 is especially capable of efficiently demethylating trimethylated H3K9, inducing a robust cooperative stimulation of AR transcriptional activity. Thus, specific modulation of GASC1 activity alone, or in combination with LSD1, may be a promising therapeutic strategy to control AR activity in tissues where AR has a pivotal physiological role.26 Clearly, these observations suggest also critical functions not only in cancer but also during development. In summary, growing evidence suggests important roles for human JMJD2B and JMJD2C in tumorigenesis, indicating these proteins as potential targets for the development of new-generation anticancer agents.
The KDM5 Cluster (JARID1 Subfamily)
The JARID1 subfamily, specific for the demethylation of H3K4me3/2, encompasses 4 enzymes: KDM5A/JARID1A (Jumonji, AT-rich interactive domain 1A)/RBP2 (retinoblastoma-binding protein 2), KDM5B/JARID1B/PLU-1, KDM5C/JARID1C/SMCX (selected mouse cDNA on the X), and KDM5D/JARID1D/SMCY.67 Despite the fact that all members of KDM5 cluster catalyze the demethylation of the same histone mark, they appear to have exclusive functional properties probably because of their different expression profiles and presence in distinct protein complexes. RBP2 is broadly expressed in diverse tissues and has been linked by target gene mapping and functional studies to regulation of differentiation, cell cycle progression, and mitochondrial function.103-107 The KDM5A interaction partners identified so far include RBP-J (recombination signal-binding protein–Jk), Sin3, c-Myc, the tumor suppressor pRB (retinoblastoma protein), and PcG proteins of the PRC2 complex, which harbor H3K27 methyltransferase activity.105,106,108-110 Little is known at present about the role of JARID1A/RBP2 in human cancers, and the few available reports are contradictory. RBP2 has been shown to be a key effector of pRB-mediated cell cycle withdrawal and differentiation by interacting with the tumor suppressor pRB.103 Recently, RBP2 protein was shown to be an integral part of the core Notch-RBP-J repressor complex, contributing to switch off the Notch signaling.109 In contrast to its tumor suppressor function, a recent report showed that it is overexpressed in gastric cancer and that its inhibition leads to cellular senescence of gastric and cervical cancer cells by de-repressing cyclin-dependent kinase inhibitors such as p27, p21, and p16.107 Recently, enhanced Jarid1A/Rbp2 expression was found to contribute to drug tolerance with respect to a receptor tyrosine kinase inhibitor in a non–small cell lung carcinoma (NSCLC) cell system, whereas RBP2 knockdown significantly reduced the number of the drug-tolerant population.111 These drug-tolerant NSCLC cells displayed reduced levels of H3K4me2 and H3K4me3, which is reminiscent of the prognostic value of reduced H3K4me3 in NSCLC. This also indicates that acquired drug resistances are accompanied by epigenetic changes, and combining a chromatin-modifying agent with a single, rationally targeted agent would prevent the development of drug resistance.
Although significant expression of PLU-1 (JARID1B) is limited to testis and only low levels of PLU-1 are seen in other normal adult tissues, its expression increases markedly in pathological conditions such as malignant breast cancer, metastatic prostate cancer, and testis cancer.112,113 Based on its overexpression in cancer, PLU-1 was suggested as a testis cancer antigen. Supporting an oncogenic potential at a cellular level, PLU-1 knockdown slowed down the proliferation of MCF-7 breast cancer cells and tumor growth of mammary carcinoma cells in a syngenic mouse mammary tumor model.114 Like LSD1, PLU-1 was shown to be involved in silencing a series of tumor suppressor genes previously linked to breast cancer, including SFN (14-3-3-σ), BRCA1, CAV1, and HOXA5. Although this is consistent with a transcription repressive role for this protein, JARID1B can also act as a transcriptional co-activator for the androgen receptor.112,113 Involvement in hormone signaling is consistent with high expression levels in testis, ovary, and mammary glands of pregnant females.112
JARID1C/SMCX (KDM5C) has been implicated in X-linked mental retardation and epilepsy.115 However, its role in the pathogenesis of human papillomavirus (HPV)–associated cancers was recently identified by a genome-wide siRNA screen that found, among other genes, JARID1C/SMCX as one of the E2-dependent regulators of HPV oncogene expression.116
The KDM6 Cluster (UTX/Y-JMJD3 Subfamily)
The KDM6 cluster in human cells consists of KDM6A/UTX (ubiquitously transcribed X chromosome tetraticopeptide repeat protein), UTY, and KDM6B/JMJD3. KDM6A and KDM6B are histone demethylases specific for H3K27me3/me2, whereas no activity has been reported for UTY so far.117-120
The tri- and dimethylation of H3K27, which are catalyzed by the polycomb group (PcG) proteins, are important repressive histone marks. The PcG genes have been characterized as oncogenes and are frequently overexpressed or amplified in cancer. Their oncogenic potential is mainly mediated through PcG-mediated H3K27 methylation and epigenetic inactivation of the INK4A-ARF locus.121,122 The INK4A-ARF locus encodes tumor suppressor genes p16INK4A and p14ARF, which are key regulators of cellular senescence.
Both the H3K27 demethylases KDM6A and KDM6B are suggested as tumor suppressor genes by functioning antagonistically to the oncogenic PcG proteins. Although JMJD3 and UTX have the same catalytic activity, they most likely have, as the JARID1 proteins, different biological functions. Overexpression of either leads to cell cycle arrest in fibroblasts, dependent on intact catalytic domains.123-125 Although knockdown of KDM6A confers an immediate growth advantage to fibroblasts,123 depletion of KDM6B enables MEFs to overcome the senescence barrier.124,125 The growth arrest induced by KDM6A has been linked to transcriptional activation of components of the pRB pathway. In fact, a genome-wide chromatin occupancy analysis has shown that many pRB-binding proteins, including HMG-box protein 1 (HBP1), are UTX target genes.123 UTX, by means of preventing H3K27 methylation and silencing HBP1, is able to enforce cell cycle arrest by pRB. Consistently, UTX depletion results in elevated levels of proliferation. UTX, the first reported mutated histone demethylase gene associated with cancer, is a frequent target of somatic mutations in human cancers with high prevalence in multiple myeloma, esophageal squamous cell carcinomas, and renal cell carcinomas.126,127
It has been shown that JMJD3, but not UTX, contributes to the activation of INK4A-ARF by removing H3K27me3 during induction of oncogene- or stress-induced senescence.124,125 KDM6B expression in fibroblasts is induced by oncogenes of the RAS-RAF pathway, suggesting that it could provide an important barrier to tumorigenesis. Accordingly, KDM6B levels are high in melanocytic nevi, which constitute pre-neoplastic lesions, whereas data mining indicates that KDM6B expression is decreased in subsets of human cancers.124,125 Moreover, it appears that a large part of the genetic lesions leading to p53 loss likewise causes the deletion of JMJD3. The JMJD3 gene is located on chromosome 17, in close proximity to the p53 tumor suppressor gene. The allelic loss at 17p13.1, including both p53 and JMJD3, was also significantly correlated with more aggressive tumor behaviour.128 Taken together, genetic lesions and/or decreased expression of JMJD3 might contribute to the development of some human cancers, most likely by epigenetic silencing of the INK4A-ARF tumor suppressor locus.
JmjC Histone Demethylase Inhibitors
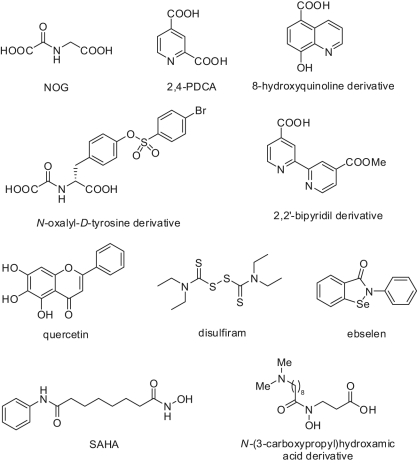
Potent and specific inhibitors of the Jumonji C domain–containing histone lysine demethylases have not been identified yet, and the available structure-activity relationship (SAR) data are limited.129-131 However, the efforts to target the cofactors essential for the activity of this class of enzymes have provided first promising results.129-131 The 2-oxoglutarate analogue, N-oxalylglycine (NOG), can weakly inhibit JMJD2C and the catalytic core of JMJD2A (Fig. 10).132 JMJD2A was also found to be specifically inhibited by disruption of its Zn2+ binding site by Zn-ejecting compounds such as disulfiram and ebselen (Fig. 10).133 Interestingly, Zn-ion ejection seemed to be selective for the JMJD2 subfamily as other KDMs do not contain a Zn-binding site near their catalytic domain.133 In a recent study employing binding analyses by non-denaturing mass spectrometry (MS), dynamic combinatorial chemistry coupled to MS, turnover assays, and crystallography, a set of N-oxalyl-D-tyrosine derivatives was investigated for the inhibition of the JMJD2 (KDM4) subfamily of histone demethylases. Some of the inhibitors were shown to be selective for JMJD2 over the hypoxia-inducible factor prolyl hydroxylase PHD2.130 Other 2-OG mimetics endowed with a promising JMJD2 inhibitory potency are the 2,4-pyridindicarboxylic acid (PDCA), a fragment-size inhibitor with (sub)micromolar activity against JMJD2s and some of its derivatives,129,131 and a 2,2′-bipyridyl derivative containing a 4-carboxylate group (Fig. 10).129 A recent screen of a small library of compounds provided as JmjC KDM inhibitors some flavonoid- and catechol-type molecules, which have the limitation of being promiscuous inhibitors affecting a wide range of enzymatic targets (Fig. 10).134 Quantitative high-throughput screening of a ~236,000-member collection of diverse molecules led to the identification of 8-hydroxy-5-carboxyquinoline as a potent inhibitor of JMJD2 enzymes via chelation of the active-site iron and endowed with activity against JMJD2A in cell-based studies.135 The well-known HDAC inhibitor suberoylanilide hydroxamic acid (SAHA) was shown to be also a micromolar inhibitor of the recombinant enzyme JMJD2E (annotated as a pseudogene) that, for its close analogy in terms of sequence identity to the catalytic domain of the other JMJD2 enzymes, is easily amenable to large-scale inhibition studies (Fig. 10).129 Recently, some hydroxamic acids bearing as a unique feature an N-propionic acid unit have been reported by Hamada and co-workers136 as selective inhibitors of JMJD2A and JMJD2C over PHD1 and PHD2 enzymes (Fig. 10). Two cell-permeable pro-drugs of the best inhibitor of this series displayed a synergistic cell growth inhibition in combination with a tranylcypromine-based LSD1 inhibitor in different cancer cell lines (LNCaP, PC3, and HCT116), suggesting clinical potential for this type of combination in anticancer chemotherapy.136
Figure 10.
Structures of JmjC KDM inhibitors.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403(6765):41-5 [DOI] [PubMed] [Google Scholar]
- 2. Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293(5532):1074-80 [DOI] [PubMed] [Google Scholar]
- 3. Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693-705 [DOI] [PubMed] [Google Scholar]
- 4. Mai A, Altucci L. Epi-drugs to fight cancer: from chemistry to cancer treatment, the road ahead. Int J Biochem Cell Biol. 2009;41(1):199-213 [DOI] [PubMed] [Google Scholar]
- 5. Mai A. Small-molecule chromatin-modifying agents: therapeutic applications. Epigenomics. 2010;2(2):307-24 [DOI] [PubMed] [Google Scholar]
- 6. Shi Y, Lan F, Matson C, et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119(7):941-53 [DOI] [PubMed] [Google Scholar]
- 7. Forneris F, Binda C, Vanoni MA, Mattevi A, Battaglioli E. Histone demethylation catalysed by LSD1 is a flavin-dependent oxidative process. FEBS Lett. 2005;579(10):2203-7 [DOI] [PubMed] [Google Scholar]
- 8. Karytinos A, Forneris F, Profumo A, et al. A novel mammalian flavin-dependent histone demethylase. J Biol Chem. 2009;284(26):17775-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsukada Y, Fang J, Erdjument-Bromage H, et al. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 2006; 439(7078):811-6 [DOI] [PubMed] [Google Scholar]
- 10. Klose RJ, Kallin EM, Zhang Y. JmjC-domain-containing proteins and histone demethylation. Nat Rev Genet. 2006;7(9):715-27 [DOI] [PubMed] [Google Scholar]
- 11. Hakimi MA, Bochar DA, Chenoweth J, Lane WS, Mandel G, Shiekhattar R. A core-BRAF35 complex containing histone deacetylase mediates repression of neuronal-specific genes. Proc Natl Acad Sci U S A. 2002;99(11):7420-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. You A, Tong JK, Grozinger CM, Schreiber SL. CoREST is an integral component of the CoREST–human histone deacetylase complex. Proc Natl Acad Sci U S A. 2001;98(4):1454-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Battaglioli E, Andres ME, Rose DW, et al. REST repression of neuronal genes requires components of the hSWI.SNF complex. J Biol Chem. 2002;277(43):41038-45 [DOI] [PubMed] [Google Scholar]
- 14. Lee MG, Wynder C, Cooch N, Shiekhattar R. An essential role for CoREST in nucleosomal histone 3 lysine 4 demethylation. Nature. 2005; 437(7057):432-5 [DOI] [PubMed] [Google Scholar]
- 15. Forneris F, Binda C, Battaglioli E, Mattevi A. LSD1: oxidative chemistry for multifaceted functions in chromatin regulation. Trends Biochem Sci. 2008;33(4):181-9 [DOI] [PubMed] [Google Scholar]
- 16. Yang M, Gocke CB, Luo X, et al. Structural basis for CoREST-dependent demethylation of nucleosomes by the human LSD1 histone demethylase. Mol Cell. 2006;23(3):377-87 [DOI] [PubMed] [Google Scholar]
- 17. Shi YJ, Matson C, Lan F, Iwase S, Baba T, Shi Y. Regulation of LSD1 histone demethylase activity by its associated factors. Mol Cell. 2005;19(6):857-64 [DOI] [PubMed] [Google Scholar]
- 18. Spannhoff A, Hauser AT, Heinke R, Sippl W, Jung M. The emerging therapeutic potential of histone methyltransferase and demethylase inhibitors. ChemMedChem. 2009;4(10):1568-82 [DOI] [PubMed] [Google Scholar]
- 19. Culhane JC, Cole PA. LSD1 and the chemistry of histone demethylation. Curr Opin Chem Biol. 2007;11(5):561-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang M, Culhane JC, Szewczuk LM, et al. Structural basis of histone demethylation by LSD1 revealed by suicide inactivation. Nat Struct Mol Biol. 2007;14(6):535-9 [DOI] [PubMed] [Google Scholar]
- 21. Forneris F, Binda C, Vanoni MA, Battaglioli E, Mattevi A. Human histone demethylase LSD1 reads the histone code. J Biol Chem. 2005;280(50):41360-5 [DOI] [PubMed] [Google Scholar]
- 22. Forneris F, Binda C, Dall’Aglio A, Fraaije MW, Battaglioli E, Mattevi A. A highly specific mechanism of histone H3-K4 recognition by histone demethylase LSD1. J Biol Chem. 2006;281(46):35289-95 [DOI] [PubMed] [Google Scholar]
- 23. Metzger E, Imhof A, Patel D, et al. Phosphorylation of histone H3T6 by PKCbeta(I) controls demethylation at histone H3K4. Nature. 2011;464(7289):792-6 [DOI] [PubMed] [Google Scholar]
- 24. Metzger E, Wissmann M, Yin N, et al. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature. 2005;437(7057):436-9 [DOI] [PubMed] [Google Scholar]
- 25. Garcia-Bassets I, Kwon YS, Telese F, et al. Histone methylation-dependent mechanisms impose ligand dependency for gene activation by nuclear receptors. Cell. 2007;128(3):505-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wissmann M, Yin N, Muller JM, et al. Cooperative demethylation by JMJD2C and LSD1 promotes androgen receptor-dependent gene expression. Nat Cell Biol. 2007;9(3):347-53 [DOI] [PubMed] [Google Scholar]
- 27. Huang J, Sengupta R, Espejo AB, et al. p53 is regulated by the lysine demethylase LSD1. Nature. 2007;449(7158):105-8 [DOI] [PubMed] [Google Scholar]
- 28. Wang J, Hevi S, Kurash JK, et al. The lysine demethylase LSD1 (KDM1) is required for maintenance of global DNA methylation. Nat Genet. 2009;41(1):125-9 [DOI] [PubMed] [Google Scholar]
- 29. Yuan B, Zhang J, Wang H, et al. 6-Thioguanine reactivates epigenetically silenced genes in acute lymphoblastic leukemia cells by facilitating proteasome-mediated degradation of DNMT1. Cancer Res. 2011;71(5):1904-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cho HS, Suzuki T, Dohmae N, et al. Demethylation of RB regulator MYPT1 by histone demethylase LSD1 promotes cell cycle progression in cancer cells. Cancer Res. 2011;71(3):655-60 [DOI] [PubMed] [Google Scholar]
- 31. Yang Z, Jiang J, Stewart DM, et al. AOF1 is a histone H3K4 demethylase possessing demethylase activity-independent repression function. Cell Res. 2010;20(3):276-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fang R, Barbera AJ, Xu Y, et al. Human LSD2/KDM1b/AOF1 regulates gene transcription by modulating intragenic H3K4me2 methylation. Mol Cell. 2010;39(2):222-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ciccone DN, Su H, Hevi S, et al. KDM1B is a histone H3K4 demethylase required to establish maternal genomic imprints. Nature. 2009;461(7262):415-8 [DOI] [PubMed] [Google Scholar]
- 34. van Essen D, Zhu Y, Saccani S. A feed-forward circuit controlling inducible NF-kappaB target gene activation by promoter histone demethylation. Mol Cell. 2010;39(5):750-60 [DOI] [PubMed] [Google Scholar]
- 35. Hayami S, Kelly JD, Cho HS, et al. Overexpression of LSD1 contributes to human carcinogenesis through chromatin regulation in various cancers. Int J Cancer. 2011;128(3):574-86 [DOI] [PubMed] [Google Scholar]
- 36. Lim S, Janzer A, Becker A, et al. Lysine-specific demethylase 1 (LSD1) is highly expressed in ER-negative breast cancers and a biomarker predicting aggressive biology. Carcinogenesis. 2011;31(3):512-20 [DOI] [PubMed] [Google Scholar]
- 37. Schulte JH, Lim S, Schramm A, et al. Lysine-specific demethylase 1 is strongly expressed in poorly differentiated neuroblastoma: implications for therapy. Cancer Res. 2009;69(5):2065-71 [DOI] [PubMed] [Google Scholar]
- 38. Wang Y, Zhang H, Chen Y, et al. LSD1 is a subunit of the NuRD complex and targets the metastasis programs in breast cancer. Cell. 2009;138(4):660-72 [DOI] [PubMed] [Google Scholar]
- 39. Huang PH, Chen CH, Chou CC, et al. Histone deacetylase inhibitors stimulate histone H3 lysine 4 methylation in part via transcriptional repression of histone H3 lysine 4 demethylases. Mol Pharmacol. 2011;79(1):197-206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thillainadesan G, Isovic M, Loney E, Andrews J, Tini M, Torchia J. Genome analysis identifies the p15ink4b tumor suppressor as a direct target of the ZNF217/CoREST complex. Mol Cell Biol. 2008;28(19):6066-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lin Y, Wu Y, Li J, et al. The SNAG domain of Snail1 functions as a molecular hook for recruiting lysine-specific demethylase 1. EMBO J. 2010;29(11):1803-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lin T, Ponn A, Hu X, Law BK, Lu J. Requirement of the histone demethylase LSD1 in Snai1-mediated transcriptional repression during epithelial-mesenchymal transition. Oncogene. 2010;29(35):4896-904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ooi SK, Qiu C, Bernstein E, et al. DNMT3L connects unmethylated lysine 4 of histone H3 to de novo methylation of DNA. Nature. 2007;448(7154):714-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Huang Y, Stewart TM, Wu Y, et al. Novel oligoamine analogues inhibit lysine-specific demethylase 1 and induce reexpression of epigenetically silenced genes. Clin Cancer Res. 2009;15(23):7217-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Amente S, Bertoni A, Morano A, Lania L, Avvedimento EV, Majello B. LSD1-mediated demethylation of histone H3 lysine 4 triggers Myc-induced transcription. Oncogene. 2010;29(25):3691-702 [DOI] [PubMed] [Google Scholar]
- 46. Culhane JC, Szewczuk LM, Liu X, Da G, Marmorstein R, Cole PA. A mechanism-based inactivator for histone demethylase LSD1. J Am Chem Soc. 2006;128(14):4536-7 [DOI] [PubMed] [Google Scholar]
- 47. Culhane JC, Wang D, Yen PM, Cole PA. Comparative analysis of small molecules and histone substrate analogues as LSD1 lysine demethylase inhibitors. J Am Chem Soc. 2010;132(9):3164-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Szewczuk LM, Culhane JC, Yang M, Majumdar A, Yu H, Cole PA. Mechanistic analysis of a suicide inactivator of histone demethylase LSD1. Biochemistry. 2007;46(23):6892-902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bi X, Lopez C, Bacchi CJ, Rattendi D, Woster PM. Novel alkylpolyaminoguanidines and alkylpolyaminobiguanides with potent antitrypanosomal activity. Bioorg Med Chem Lett. 2006;16(12):3229-32 [DOI] [PubMed] [Google Scholar]
- 50. Huang Y, Greene E, Murray Stewart T, et al. Inhibition of lysine-specific demethylase 1 by polyamine analogues results in reexpression of aberrantly silenced genes. Proc Natl Acad Sci U S A. 2007;104(19):8023-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sharma SK, Wu Y, Steinbergs N, et al. (Bis)urea and (bis)thiourea inhibitors of lysine-specific demethylase 1 as epigenetic modulators. J Med Chem. 2010;53(14):5197-212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lee MG, Wynder C, Schmidt DM, McCafferty DG, Shiekhattar R. Histone H3 lysine 4 demethylation is a target of nonselective antidepressive medications. Chem Biol. 2006;13(6):563-7 [DOI] [PubMed] [Google Scholar]
- 53. Schmidt DM, McCafferty DG. Trans-2-phenylcyclopropylamine is a mechanism-based inactivator of the histone demethylase LSD1. Biochemistry. 2007;46(14):4408-16 [DOI] [PubMed] [Google Scholar]
- 54. Yang M, Culhane JC, Szewczuk LM, et al. Structural basis for the inhibition of the LSD1 histone demethylase by the antidepressant trans-2-phenylcyclopropylamine. Biochemistry. 2007;46(27):8058-65 [DOI] [PubMed] [Google Scholar]
- 55. Ueda R, Suzuki T, Mino K, et al. Identification of cell-active lysine specific demethylase 1–selective inhibitors. J Am Chem Soc. 2009;131(48):17536-7 [DOI] [PubMed] [Google Scholar]
- 56. Binda C, Valente S, Romanenghi M, et al. Biochemical, structural, and biological evaluation of tranylcypromine derivatives as inhibitors of histone demethylases LSD1 and LSD2. J Am Chem Soc. 2010;132(19):6827-33 [DOI] [PubMed] [Google Scholar]
- 57. Mimasu S, Umezawa N, Sato S, Higuchi T, Umehara T, Yokoyama S. Structurally designed trans-2-phenylcyclopropylamine derivatives potently inhibit histone demethylase LSD1/KDM1. Biochemistry. 2010;49(30):6494-503 [DOI] [PubMed] [Google Scholar]
- 58. Benelkebir H, Hodgkinson C, Duriez PJ, et al. Enantioselective synthesis of tranylcypromine analogues as lysine demethylase (LSD1) inhibitors. Bioorg Med Chem. 2011;19(12):3709-16 [DOI] [PubMed] [Google Scholar]
- 59. Gooden DM, Schmidt DM, Pollock JA, Kabadi AM, McCafferty DG. Facile synthesis of substituted trans-2-arylcyclopropylamine inhibitors of the human histone demethylase LSD1 and monoamine oxidases A and B. Bioorg Med Chem Lett. 2008;18(10):3047-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mimasu S, Sengoku T, Fukuzawa S, Umehara T, Yokoyama S. Crystal structure of histone demethylase LSD1 and tranylcypromine at 2.25 A. Biochem Biophys Res Commun. 2008;366(1):15-22 [DOI] [PubMed] [Google Scholar]
- 61. Escoubet-Lozach L, Lin IL, Jensen-Pergakes K, et al. Pomalidomide and lenalidomide induce p21 WAF-1 expression in both lymphoma and multiple myeloma through a LSD1-mediated epigenetic mechanism. Cancer Res. 2009;69(18):7347-56 [DOI] [PubMed] [Google Scholar]
- 62. Klose RJ, Zhang Y. Regulation of histone methylation by demethylimination and demethylation. Nat Rev Mol Cell Biol. 2007;8(4):307-18 [DOI] [PubMed] [Google Scholar]
- 63. Loenarz C, Schofield CJ. Expanding chemical biology of 2-oxoglutarate oxygenases. Nat Chem Biol. 2008;4(3):152-6 [DOI] [PubMed] [Google Scholar]
- 64. Loenarz C, Schofield CJ. Physiological and biochemical aspects of hydroxylations and demethylations catalyzed by human 2-oxoglutarate oxygenases. Trends Biochem Sci. 2011;36(1):7-18 [DOI] [PubMed] [Google Scholar]
- 65. Hewitson KS, McNeill LA, Riordan MV, et al. Hypoxia-inducible factor (HIF) asparagine hydroxylase is identical to factor inhibiting HIF (FIH) and is related to the cupin structural family. J Biol Chem. 2002;277(29):26351-5 [DOI] [PubMed] [Google Scholar]
- 66. Takeuchi T, Yamazaki Y, Katoh-Fukui Y, et al. Gene trap capture of a novel mouse gene, jumonji, required for neural tube formation. Genes Dev. 1995;9(10):1211-22 [DOI] [PubMed] [Google Scholar]
- 67. Cloos PA, Christensen J, Agger K, Helin K. Erasing the methyl mark: histone demethylases at the center of cellular differentiation and disease. Genes Dev. 2008;22(9):1115-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pedersen MT, Helin K. Histone demethylases in development and disease. Trends Cell Biol. 2011;20(11):662-71 [DOI] [PubMed] [Google Scholar]
- 69. McDonough MA, Loenarz C, Chowdhury R, Clifton IJ, Schofield CJ. Structural studies on human 2-oxoglutarate dependent oxygenases. Curr Opin Struct Biol. 2010;20(6):659-72 [DOI] [PubMed] [Google Scholar]
- 70. Yue WW, Hozjan V, Ge W, et al. Crystal structure of the PHF8 Jumonji domain, an Nepsilon-methyl lysine demethylase. FEBS Lett. 2010; 584(4):825-30 [DOI] [PubMed] [Google Scholar]
- 71. Horton JR, Upadhyay AK, Qi HH, Zhang X, Shi Y, Cheng X. Enzymatic and structural insights for substrate specificity of a family of Jumonji histone lysine demethylases. Nat Struct Mol Biol. 2010;17(1):38-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ponnaluri VK, Vavilala DT, Putty S, Gutheil WG, Mukherji M. Identification of non-histone substrates for JMJD2A-C histone demethylases. Biochem Biophys Res Commun. 2009;390(2):280-4 [DOI] [PubMed] [Google Scholar]
- 73. Varier RA, Timmers HT. Histone lysine methylation and demethylation pathways in cancer. Biochim Biophys Acta. 2011;1815(1):75-89 [DOI] [PubMed] [Google Scholar]
- 74. He J, Kallin EM, Tsukada Y, Zhang Y. The H3K36 demethylase Jhdm1b/Kdm2b regulates cell proliferation and senescence through p15(Ink4b). Nat Struct Mol Biol. 2008;15(11):1169-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lagarou A, Mohd-Sarip A, Moshkin YM, et al. dKDM2 couples histone H2A ubiquitylation to histone H3 demethylation during Polycomb group silencing. Genes Dev. 2008;22(20):2799-810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Frescas D, Guardavaccaro D, Bassermann F, Koyama-Nasu R, Pagano M. JHDM1B/FBXL10 is a nucleolar protein that represses transcription of ribosomal RNA genes. Nature. 2007;450(7167):309-13 [DOI] [PubMed] [Google Scholar]
- 77. Tzatsos A, Pfau R, Kampranis SC, Tsichlis PN. Ndy1/KDM2B immortalizes mouse embryonic fibroblasts by repressing the Ink4a/Arf locus. Proc Natl Acad Sci U S A. 2009;106(8):2641-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Suzuki T, Minehata K, Akagi K, Jenkins NA, Copeland NG. Tumor suppressor gene identification using retroviral insertional mutagenesis in Blm-deficient mice. EMBO J. 2006;25(14):3422-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pfau R, Tzatsos A, Kampranis SC, Serebrennikova OB, Bear SE, Tsichlis PN. Members of a family of JmjC domain-containing oncoproteins immortalize embryonic fibroblasts via a JmjC domain-dependent process. Proc Natl Acad Sci U S A. 2008;105(6):1907-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Frescas D, Guardavaccaro D, Kuchay SM, et al. KDM2A represses transcription of centromeric satellite repeats and maintains the heterochromatic state. Cell Cycle. 2008;7(22):3539-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lu T, Jackson MW, Wang B, et al. Regulation of NF-kappaB by NSD1/FBXL11-dependent reversible lysine methylation of p65. Proc Natl Acad Sci U S A. 2010;107(1):46-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gearhart MD, Corcoran CM, Wamstad JA, Bardwell VJ. Polycomb group and SCF ubiquitin ligases are found in a novel BCOR complex that is recruited to BCL6 targets. Mol Cell Biol. 2006;26(18):6880-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Koyama-Nasu R, David G, Tanese N. The F-box protein Fbl10 is a novel transcriptional repressor of c-Jun. Nat Cell Biol. 2007;9(9):1074-80 [DOI] [PubMed] [Google Scholar]
- 84. Polytarchou C, Pfau R, Hatziapostolou M, Tsichlis PN. The JmjC domain histone demethylase Ndy1 regulates redox homeostasis and protects cells from oxidative stress. Mol Cell Biol. 2008;28(24):7451-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yamane K, Toumazou C, Tsukada Y, et al. JHDM2A, a JmjC-containing H3K9 demethylase, facilitates transcription activation by androgen receptor. Cell. 2006;125(3):483-95 [DOI] [PubMed] [Google Scholar]
- 86. Heery DM, Kalkhoven E, Hoare S, Parker MG. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature. 1997;387(6634):733-6 [DOI] [PubMed] [Google Scholar]
- 87. Inagaki T, Tachibana M, Magoori K, et al. Obesity and metabolic syndrome in histone demethylase JHDM2a-deficient mice. Genes Cells. 2009;14(8):991-1001 [DOI] [PubMed] [Google Scholar]
- 88. Liu Z, Zhou S, Liao L, Chen X, Meistrich M, Xu J. Jmjd1a demethylase-regulated histone modification is essential for cAMP-response element modulator-regulated gene expression and spermatogenesis. J Biol Chem. 2010;285(4):2758-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Okada Y, Scott G, Ray MK, Mishina Y, Zhang Y. Histone demethylase JHDM2A is critical for Tnp1 and Prm1 transcription and spermatogenesis. Nature. 2007;450(7166):119-23 [DOI] [PubMed] [Google Scholar]
- 90. Tateishi K, Okada Y, Kallin EM, Zhang Y. Role of Jhdm2a in regulating metabolic gene expression and obesity resistance. Nature. 2009;458(7239):757-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wolf SS, Patchev VK, Obendorf M. A novel variant of the putative demethylase gene, s-JMJD1C, is a coactivator of the AR. Arch Biochem Biophys. 2007;460(1):56-66 [DOI] [PubMed] [Google Scholar]
- 92. Hu Z, Gomes I, Horrigan SK, et al. A novel nuclear protein, 5qNCA (LOC51780) is a candidate for the myeloid leukemia tumor suppressor gene on chromosome 5 band q31. Oncogene. 2001;20(47):6946-54 [DOI] [PubMed] [Google Scholar]
- 93. Krieg AJ, Rankin EB, Chan D, Razorenova O, Fernandez S, Giaccia AJ. Regulation of the histone demethylase JMJD1A by hypoxia-inducible factor 1 alpha enhances hypoxic gene expression and tumor growth. Mol Cell Biol. 2010;30(1):344-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Cloos PA, Christensen J, Agger K, et al. The putative oncogene GASC1 demethylates tri- and dimethylated lysine 9 on histone H3. Nature. 2006;442(7100):307-11 [DOI] [PubMed] [Google Scholar]
- 95. Fodor BD, Kubicek S, Yonezawa M, et al. Jmjd2b antagonizes H3K9 trimethylation at pericentric heterochromatin in mammalian cells. Genes Dev. 2006;20(12):1557-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Klose RJ, Yamane K, Bae Y, et al. The transcriptional repressor JHDM3A demethylates trimethyl histone H3 lysine 9 and lysine 36. Nature. 2006;442(7100):312-6 [DOI] [PubMed] [Google Scholar]
- 97. Lin CH, Li B, Swanson S, et al. Heterochromatin protein 1a stimulates histone H3 lysine 36 demethylation by the Drosophila KDM4A demethylase. Mol Cell. 2008;32(5):696-706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Whetstine JR, Nottke A, Lan F, et al. Reversal of histone lysine trimethylation by the JMJD2 family of histone demethylases. Cell. 2006;125(3):467-81 [DOI] [PubMed] [Google Scholar]
- 99. Trojer P, Zhang J, Yonezawa M, et al. Dynamic histone H1 isotype 4 methylation and demethylation by histone lysine methyltransferase G9a/KMT1C and the Jumonji domain–containing JMJD2/KDM4 proteins. J Biol Chem. 2009;284(13):8395-405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ehrbrecht A, Muller U, Wolter M, et al. Comprehensive genomic analysis of desmoplastic medulloblastomas: identification of novel amplified genes and separate evaluation of the different histological components. J Pathol. 2006;208(4):554-63 [DOI] [PubMed] [Google Scholar]
- 101. Liu G, Bollig-Fischer A, Kreike B, et al. Genomic amplification and oncogenic properties of the GASC1 histone demethylase gene in breast cancer. Oncogene. 2009;28(50):4491-500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Northcott PA, Nakahara Y, Wu X, et al. Multiple recurrent genetic events converge on control of histone lysine methylation in medulloblastoma. Nat Genet. 2009;41(4):465-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Benevolenskaya EV, Murray HL, Branton P, Young RA, Kaelin WG. Binding of pRB to the PHD protein RBP2 promotes cellular differentiation. Mol Cell. 2005;18(6):623-35 [DOI] [PubMed] [Google Scholar]
- 104. Lopez-Bigas N, Kisiel TA, Dewaal DC, et al. Genome-wide analysis of the H3K4 histone demethylase RBP2 reveals a transcriptional program controlling differentiation. Mol Cell. 2008;31(4):520-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Pasini D, Hansen KH, Christensen J, Agger K, Cloos PA, Helin K. Coordinated regulation of transcriptional repression by the RBP2 H3K4 demethylase and Polycomb-Repressive Complex 2. Genes Dev. 2008;22(10):1345-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. van Oevelen C, Wang J, Asp P, et al. A role for mammalian Sin3 in permanent gene silencing. Mol Cell. 2008;32(3):359-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zeng J, Ge Z, Wang L, et al. The histone demethylase RBP2 is overexpressed in gastric cancer and its inhibition triggers senescence of cancer cells. Gastroenterology. 2010;138(3):981-92 [DOI] [PubMed] [Google Scholar]
- 108. Secombe J, Li L, Carlos L, Eisenman RN. The Trithorax group protein Lid is a trimethyl histone H3K4 demethylase required for dMyc-induced cell growth. Genes Dev. 2007;21(5):537-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Liefke R, Oswald F, Alvarado C, et al. Histone demethylase KDM5A is an integral part of the core Notch-RBP-J repressor complex. Genes Dev. 2010;24(6):590-601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Defeo-Jones D, Huang PS, Jones RE, et al. Cloning of cDNAs for cellular proteins that bind to the retinoblastoma gene product. Nature. 1991;352(6332):251-4 [DOI] [PubMed] [Google Scholar]
- 111. Sharma SV, Lee DY, Li B, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141(1):69-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Barrett A, Madsen B, Copier J, et al. PLU-1 nuclear protein, which is upregulated in breast cancer, shows restricted expression in normal human adult tissues: a new cancer/testis antigen? Int J Cancer. 2002;101(6):581-8 [DOI] [PubMed] [Google Scholar]
- 113. Xiang Y, Zhu Z, Han G, et al. JARID1B is a histone H3 lysine 4 demethylase up-regulated in prostate cancer. Proc Natl Acad Sci U S A. 2007;104(49):19226-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Yamane K, Tateishi K, Klose RJ, et al. PLU-1 is an H3K4 demethylase involved in transcriptional repression and breast cancer cell proliferation. Mol Cell. 2007;25(6):801-12 [DOI] [PubMed] [Google Scholar]
- 115. Tahiliani M, Mei P, Fang R, et al. The histone H3K4 demethylase SMCX links REST target genes to X-linked mental retardation. Nature. 2007;447(7144):601-5 [DOI] [PubMed] [Google Scholar]
- 116. Smith JA, White EA, Sowa ME, et al. Genome-wide siRNA screen identifies SMCX, EP400, and Brd4 as E2-dependent regulators of human papillomavirus oncogene expression. Proc Natl Acad Sci U S A. 2010;107(8):3752-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Agger K, Cloos PA, Christensen J, et al. UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature. 2007;449(7163):731-4 [DOI] [PubMed] [Google Scholar]
- 118. De Santa F, Totaro MG, Prosperini E, Notarbartolo S, Testa G, Natoli G. The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell. 2007;130(6):1083-94 [DOI] [PubMed] [Google Scholar]
- 119. Lan F, Bayliss PE, Rinn JL, et al. A histone H3 lysine 27 demethylase regulates animal posterior development. Nature. 2007;449(7163):689-94 [DOI] [PubMed] [Google Scholar]
- 120. Lee MG, Villa R, Trojer P, et al. Demethylation of H3K27 regulates polycomb recruitment and H2A ubiquitination. Science. 2007;318(5849):447-50 [DOI] [PubMed] [Google Scholar]
- 121. Kotake Y, Cao R, Viatour P, Sage J, Zhang Y, Xiong Y. pRB family proteins are required for H3K27 trimethylation and Polycomb repression complexes binding to and silencing p16INK4alpha tumor suppressor gene. Genes Dev. 2007;21(1):49-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Bracken AP, Kleine-Kohlbrecher D, Dietrich N, et al. The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells. Genes Dev. 2007;21(5):525-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Wang JK, Tsai MC, Poulin G, et al. The histone demethylase UTX enables RB-dependent cell fate control. Genes Dev. 2010;24(4):327-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Agger K, Cloos PA, Rudkjaer L, et al. The H3K27me3 demethylase JMJD3 contributes to the activation of the INK4A-ARF locus in response to oncogene- and stress-induced senescence. Genes Dev. 2009;23(10):1171-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Barradas M, Anderton E, Acosta JC, et al. Histone demethylase JMJD3 contributes to epigenetic control of INK4a/ARF by oncogenic RAS. Genes Dev. 2009;23(10):1177-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Dalgliesh GL, Furge K, Greenman C, et al. Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature. 2010;463(7279):360-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. van Haaften G, Dalgliesh GL, Davies H, et al. Somatic mutations of the histone H3K27 demethylase gene UTX in human cancer. Nat Genet. 2009;41(5):521-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Nigro ND, Vaitkevicius VK, Considine B., Jr Dynamic management of squamous cell cancer of the anal canal. Invest New Drugs. 1989;7(1):83-9 [DOI] [PubMed] [Google Scholar]
- 129. Rose NR, Ng SS, Mecinovic J, et al. Inhibitor scaffolds for 2-oxoglutarate-dependent histone lysine demethylases. J Med Chem. 2008;51(22):7053-6 [DOI] [PubMed] [Google Scholar]
- 130. Rose NR, Woon EC, Kingham GL, et al. Selective inhibitors of the JMJD2 histone demethylases: combined nondenaturing mass spectrometric screening and crystallographic approaches. J Med Chem. 2010;53(4):1810-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Thalhammer A, Mecinovic J, Loenarz C, et al. Inhibition of the histone demethylase JMJD2E by 3-substituted pyridine 2,4-dicarboxylates. Org Biomol Chem. 2011;9(1):127-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Chen Z, Zang J, Kappler J, et al. Structural basis of the recognition of a methylated histone tail by JMJD2A. Proc Natl Acad Sci U S A. 2007;104(26):10818-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Sekirnik R, Rose NR, Thalhammer A, Seden PT, Mecinovic J, Schofield CJ. Inhibition of the histone lysine demethylase JMJD2A by ejection of structural Zn(II). Chem Commun (Camb). 2009;(42):6376-8 [DOI] [PubMed] [Google Scholar]
- 134. Sakurai M, Rose NR, Schultz L, et al. A miniaturized screen for inhibitors of Jumonji histone demethylases. Mol Biosyst. 2010;6(2):357-364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. King ON, Li XS, Sakurai M, et al. Quantitative high-throughput screening identifies 8-hydroxyquinolines as cell-active histone demethylase inhibitors. PLoS One. 2010;5(11):e15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Hamada S, Suzuki T, Mino K, et al. Design, synthesis, enzyme-inhibitory activity, and effect on human cancer cells of a novel series of Jumonji domain–containing protein 2 histone demethylase inhibitors. J Med Chem. 2010;53(15):5629-38 [DOI] [PubMed] [Google Scholar]