Abstract
BACKGROUND
We sought here to improve the toxicity of conventional concurrent chemoradiation therapy for stage III non-small cell lung cancer (NSCLC) by using proton-beam therapy to escalate the radiation dose to the tumor. We report early results of a phase II study of high-dose proton therapy and concurrent chemotherapy in terms of toxicity, failure patterns, and survival.
METHODS
Forty-four patients with stage III NSCLC were treated with 74 Gy(RBE) proton therapy with weekly carboplatin (AUC 2) and paclitaxel (50 mg/m2). Disease was staged with positron emission tomography/computed tomography (PET/CT) and treatments simulated with 4-dimensional CT to account for tumor motion. Protons were delivered as passively scattered beams, and treatment simulation was repeated during the treatment process to determine the need for adaptive re-planning.
RESULTS
Median follow-up time was 19.7 months (range, 6.1–44.4 months) and median overall survival time was 29.4 months. . No patient experienced grade 4 or 5 proton-related adverse events. The most common nonhematologic grade 3 toxicities were dermatitis (n=5), esophagitis (n=5), and pneumonitis (n=1). Nine patients (20.5%) experienced local disease recurrence but only four (9.1%) had isolated local failure. Four patients (9.1%) had regional lymph node recurrence but only one (2.3%) had isolated regional recurrence. Nineteen patients (43.2%) developed distant metastasis. The overall survival and progression-free survival rates were 86% and 63% at 1 year.
CONCLUSIONS
Concurrent high-dose proton and chemotherapy is well tolerated, and the median survival time of 29.4 months is encouraging for unresectable stage III NSCLC.
Keywords: Proton therapy, concurrent chemotherapy, non-small cell lung cancer, toxicity, patterns of failure, survival
INTRODUCTION
Lung cancer remains the most common cause of death from cancer in the United States.1 About 75% of patients present with locally advanced disease, for which the current standard therapy is concurrent chemotherapy and radiation therapy.2–4 However, the toxicity associated with concurrent chemoradiation can be significant, and local-regional failure rates remain high at 40%–50%.2–4 Several studies have shown potential benefits in terms of both local control and survival from the use of 3-dimensional conformal radiation therapy (3D-CRT) with radiation doses of up to 74 Gy with concurrent weekly carboplatin and paclitaxel chemotherapy,5–7 but 74 Gy is the maximum tolerated dose when the radiation is delivered as photons (x-rays).7
Proton beams, unlike photon beams, consist of charged particles that have a well-defined range of penetration into tissue.8,9 Tissues beyond this range are not irradiated. Thus, proton beam therapy is ideal when normal tissue sparing is a priority, for example, in lung cancer given the proximity of the esophagus, heart, and spinal cord.9
We conducted a phase II prospective study of proton therapy to a dose of 74 Gy(RBE) at 2 Gy(RBE)/fraction given concurrently with weekly carboplatin-paclitaxel chemotherapy for patients with inoperable stage III non-small cell lung cancer (NSCLC). This is the first report of this approach for stage III NSCLC.
PATIENTS AND METHODS
Patients, Treatment, and Study Design
This phase II prospective study (clinicaltrials.gov identifier NCT00495170) was approved by the institutional review board of The University of Texas MD Anderson Cancer Center, and all patients provided written informed consent to participate. Forty-four patients with unresectable or medically inoperable, histologically or cytologically confirmed stage III NSCLC (according to the 2002 American Joint Committee on Cancer staging system) were enrolled and evaluated from 2006 to 2009. Disease in all cases was staged with magnetic resonance imaging (MRI) or computed tomography (CT) of the brain, CT of the chest, and positron emission tomography (PET) within 3 months before enrollment. Other eligibility criteria included having good performance status (Karnofsky scores of 70–100) and weight loss of no more than 10% during the 6 months before diagnosis.
Treatment Simulation and Target Volume Delineation
Proton therapy was delivered with a variable energy synchrotron. All patients underwent treatment simulation with 4-dimensional CT to account for tumor motion. The internal gross tumor volume (iGTV) was defined as the envelope of motion of the GTV on a reconstructed maximum intensity projection image and verified across all phases of the 4-dimensional CT dataset.10 An 8-mm isotropic expansion of the iGTV was added and edited to cover possible microextensions of the tumor, and the resulting volume was defined as the internal clinical target volume (iCTV=iGTV+8 mm). Elective lymph nodes were not irradiated intentionally.
Radiation Doses
The total radiation dose to the tumor target was 74 Gy(RBE), given in once-daily 2-Gy(RBE) fractions, 5 days per week. Treatment plans were designed in accordance with the following dose-volume constraints:
| Spinal cord | 0% to receive ≥45 Gy(RBE) |
| Normal lung | ≤35% to receive 20 Gy(RBE) |
| Mean dose to entire lung ≤ 20 Gy(RBE) | |
| Heart | ≤30% to receive 40 Gy(RBE) |
| Esophagus | ≤50% to receive 50 Gy(RBE) |
| ≤40% to receive 70 Gy(RBE) | |
| 0% to receive ≥80 Gy(RBE) |
Passive Scattering Proton Therapy Planning and Adaptive Proton Delivery
The iGTV, with the maximum intensity projection density from the set of 3D CT scans used to derive the 4D CT, was used to design compensators and apertures to account for tumor motion, and the treatment plan was calculated by using the average of the phase of the 4D CT. 8–11 Another set of 4D CT scans was obtained during week 3 or 4 of treatment (or as clinically indicated as assessed by the treating physician) to document tumor shrinkage or other anatomic or motion-based changes. If the changes derived from the new dose distribution could not meet the minimum target dose requirement of ≥ 95% of the prescribed dose, or if they exceeded normal tissue dose constraints, a new treatment plan was designed for the remainder of the treatments.
Chemotherapy
All patients received concurrent carboplatin and paclitaxel chemotherapy as weekly intravenous infusions during proton therapy. Paclitaxel was administered at 50 mg/m2, and carboplatin was given at 2 area-under-the-curve (AUC) units. Neoadjuvant (induction) chemotherapy was allowed, and adjuvant (consolidation) chemotherapy with carboplatin and paclitaxel at systemic doses was also allowed.
Evaluation and Follow-Up
Patients were evaluated at least weekly during treatment, at 6 weeks after completing the proton therapy, and then every 3 months for 2 years and every 6 months thereafter. Adverse events were noted and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3. Follow-up visits included an interval medical history and physical examination, hematologic studies, and CT scans. Follow-up PET scans were required during the first 2–6 months after treatment or as needed if clinically indicated.
Local control at the primary tumor site was evaluated by serial thoracic CT scans with contrast. If CT scans showed evidence of recurrent disease, then PET or PET/CT was required and biopsy recommended to confirm recurrence. Unconfirmed recurrent disease was to be followed up with CT or PET. The timing of the recurrence was scored as the time at which the first image (PET or CT) showed abnormalities. The survival/recurrence time was calculated from the date of protocol enrollment to last follow-up.
Statistical Analysis
The primary end point was the median overall survival time after proton therapy administered with concurrent chemotherapy. We hypothesized that the median overall survival time would be increased from a baseline of 16 months to 28 months. Using the normal approximation, we calculated that 44 patients would need to be enrolled to have an 80% chance of demonstrating improvement using a one-sided test with significance level of 0.05. Survival was analyzed by using the Kaplan-Meier method and the survival curves compared with log-rank tests (SPSS v.16.0, SPSS Inc., Chicago).
RESULTS
Characteristics of the 44 enrolled patients are listed in Table 1. At a median follow-up time of 19.7 months (range, 6.1–44.4 months) for all patients (23.3 months [range, 10.3–44.4 months] for living patients), the median overall survival time was 29.4 months (mean ± standard deviation, 28.5 ± 12.4 months). The overall survival and progression-free survival rates at 1 year were 86% and 63%; Kaplan-Meier estimates at 2 years and 3 years are shown in Figure 1. Patterns of failure are listed in Table 2. Nine patients (20.5%) experienced recurrence within the treated area, but only 4 of those patients (9.1%) had isolated local failure. Another 4 patients (9.1%) experienced first recurrence in regional lymph nodes but only 1 of those patients (2.3%) developed isolated regional recurrence. The dominant pattern of failure was distant metastasis (19 patients, 43.2%).
Table 1.
Clinical Characteristics
| Characteristics | Patients, no. (%) |
|---|---|
| Sex | |
| Male | 31 (70.5) |
| Female | 13 (29.5) |
| Age, years | |
| ≤ 70 | 26 (59.1) |
| > 70 | 18 (40.9) |
| AJCC Disease Stage | |
| IIIA | 21 (47.7) |
| IIIB | 23 (52.3) |
| Tumor Size and Node Status | |
| T1–2 | 22 (50) |
| T3–4 | 22 (50) |
| N0–1 | 10 (22.7) |
| N2–3 | 34 (77.3) |
| Tumor Histology | |
| Squamous | 25 (56.8) |
| Adenocarcinoma | 15 (34.1) |
| NSC NOS | 4 (9.1) |
| Gross Tumor Volume, cm3 | |
| Median (range) | 101.3 (4.1–753.2) |
| Karnofsky Performance Score | |
| Median (range) | 90 (70–100) |
| Status | |
| Alive | 22 (50) |
| Dead | 22 (50) |
| Follow-up time, days | |
| Median (range) | 592 (184–1333) |
| Chemotherapy | |
| Neoadjuvant chemotherapy | 11 (25) |
| Median no. cycles [range] | 3 [1–7] |
| Weekly chemotherapy | 44 (100) |
| Median no. cycles [range] | 7 [3–9] |
| Adjuvant chemotherapy | 8 (18.2) |
| Median no. cycles [range] | 2.5 [1–4] |
Abbreviations: AJCC, American Joint Committee on Cancer; NSC NOS, non-small-cell lung cancer, not otherwise specified.
Fig 1.
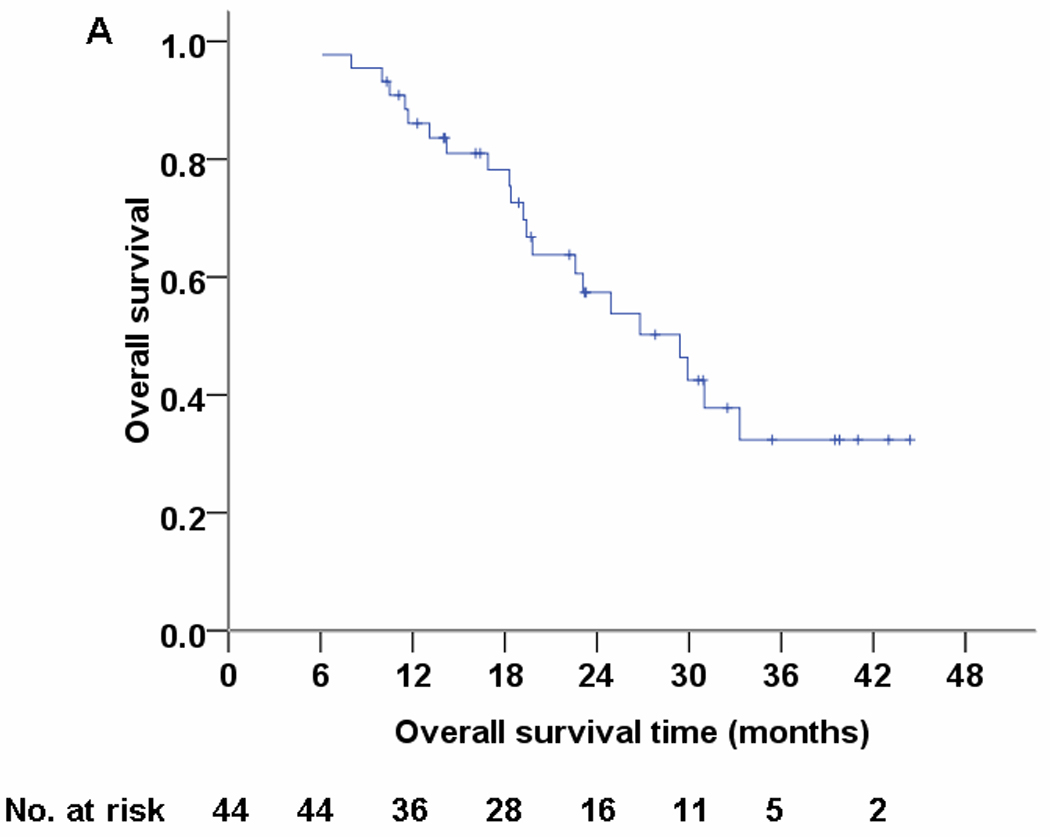
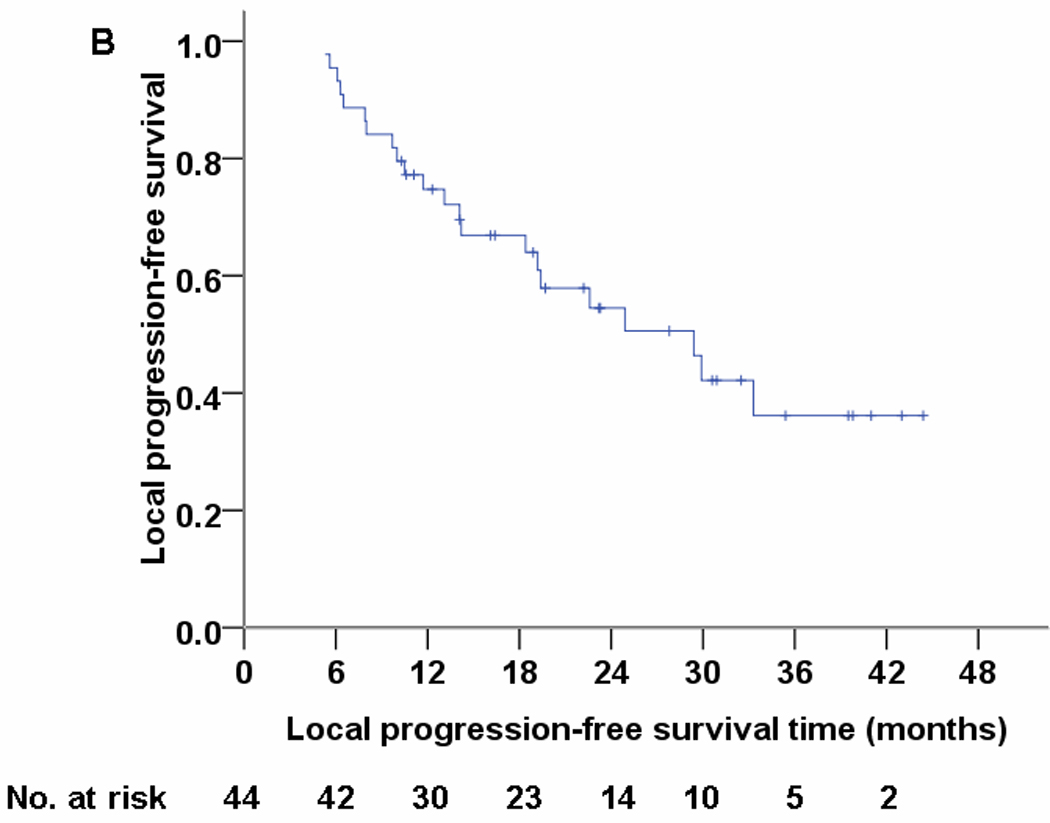
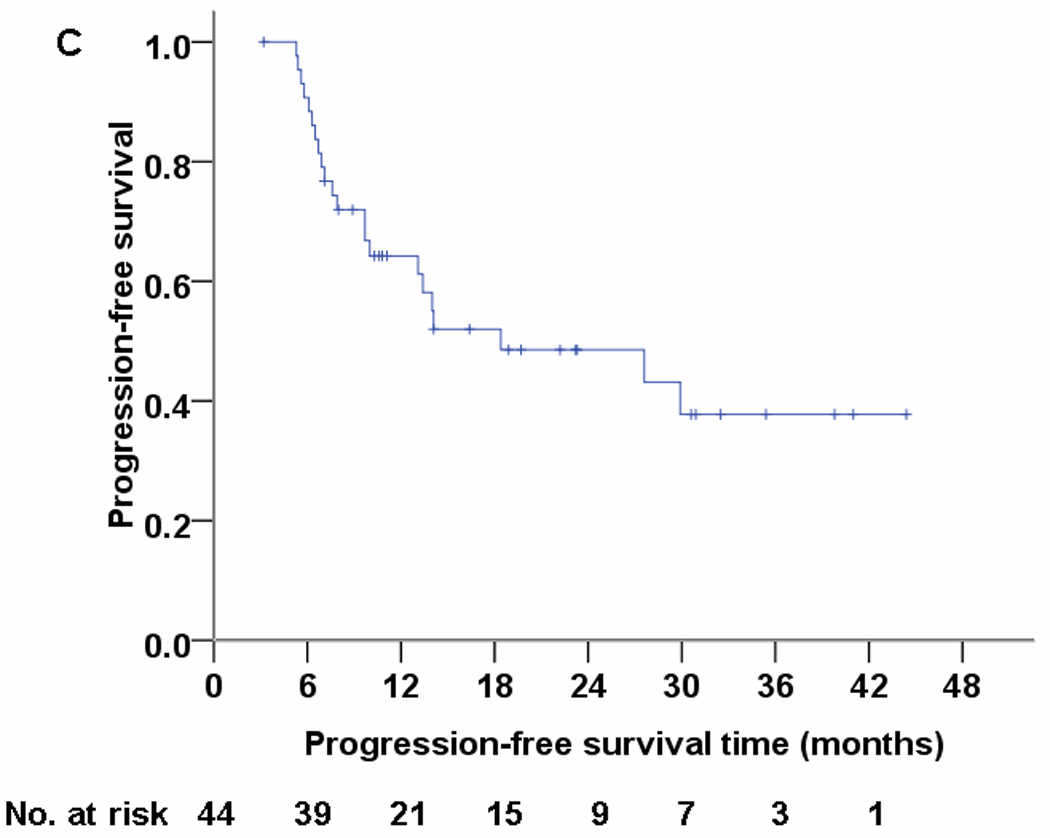
Overall survival (panel A), local progression-free survival (panel B), and progression-free survival (panel C) for 44 patients treated with concurrent proton-beam therapy and chemotherapy.
Table 2.
Failure Patterns after Concurrent Proton Beam Therapy and Chemotherapy
| Site of First Failure | No of Patients (%) |
|---|---|
| Local | 4 (9.1%) |
| Distant | 14 (31.8%) |
| Regional | 1 (2.3%) |
| Local + distant | 3 (6.8%) |
| Regional + distant | 1 (2.3%) |
| Regional + local | 1 (2.3%) |
| Distant + local + regional | 1 (2.3%) |
The toxicity profile is shown in Table 3. No patient experienced grade 5 toxicity; 5 patients (11.4%) developed chemotherapy-related grade 4 toxicity. The most common grade 3 adverse effects related to proton therapy were dermatitis and esophagitis, each experienced by 5 patients (11.4%); 1 patient (2.3%) developed grade 3 pneumonitis and 1 patient had a pulmonary/pleural fistula. Median values of lung V20 (i.e., the volume of lung exposed to ≤20 Gy), V10, and V5 were 25.7% (range, 9.5%–36.4%), 29.8% (range, 13.4%–42.8%), and 32.8% (range, 14.2%–48.3%). Because of the unique physical characteristics of the proton beam, the median absolute increase of lung volume from V5 to V20 was only 7.1%. The median mean dose to the entire lung was 15 Gy (range, 5.5–22.4 Gy); the median V30 for the heart was 6.9% (range, 0.0%–41.6%); the median V50 for the esophagus was 20.4% (range, 0.0%–51.2%); the mean dose to the esophagus was 21.2 Gy; and the median maximum dose to the spinal cord was 33.9 Gy (range, 18.3–42.3 Gy).
Table 3.
Acute and Chronic Toxic Effects after Concurrent Proton Beam Therapy and Chemotherapy
| Toxicity Grade, Radiation-Related |
Toxicity Grade, Chemotherapy-Related |
Toxicity Grade, Radiation+Chemo-Related | Toxicity Grade, Possibly Radiation- or Chemo-Related |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | |
| GASTROINTESTINAL | ||||||||||||
| ANOREXIA | 0 | 0 | 0 | 2 (4.5) | 0 | 0 | 1 (2.3) | 0 | 0 | 2 (4.5) | 0 | 0 |
| NAUSEA | 0 | 0 | 0 | 4 (9.1) | 1 (2.3) | 0 | 4 (9.1) | 0 | 0 | 0 | 0 | 0 |
| VOMITING | 0 | 0 | 0 | 1 (2.3) | 0 | 0 | 0 | 0 | 0 | 1 (2.3) | 0 | 0 |
| ESOPHAGITIS | 0 | 0 | 0 | 0 | 0 | 0 | 14 (31.8) | 5 (11.4) | 0 | 0 | 0 | 0 |
| DYSPHAGIA | 0 | 0 | 0 | 0 | 0 | 0 | 4 (9.1) | 0 | 0 | 0 | 0 | 0 |
| DIARRHEA | 0 | 0 | 0 | 5 (11.4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| DEHYDRATION | 0 | 0 | 0 | 0 | 0 | 0 | 3 (6.8) | 3 (6.8) | 0 | 0 | 0 | 0 |
| LUNG | ||||||||||||
| DYSPNEA | 0 | 0 | 0 | 1 (2.3) | 2 (4.5) | 0 | 1 (2.3) | 0 | 0 | 4 (9.1) | 1 (2.3) | 0 |
| COUGH | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (6.8) | 1 (2.3) | 0 |
| PNEUMONITIS | 2 (4.5) | 1 (2.3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| HEMOPTYSIS | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2.3) | 0 | 0 |
| ATELECTASIS | 3 (6.8) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PULMONARY/PLEURAL FISTULA | 0 | 1 (2.3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| BONE MARROW | ||||||||||||
| LEUKOCYTES | 0 | 0 | 0 | 16 (36.4) | 5 (11.4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| NEUTROPHILS | 0 | 0 | 0 | 7 (15.9) | 2 (4.5) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| HEMOGLOBIN | 0 | 0 | 0 | 7 (15.9) | 3 (6.8) | 3 (6.8) | 0 | 0 | 0 | 0 | 0 | 0 |
| THROMBOCYTOPENIA | 0 | 0 | 0 | 2 (4.5) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| OTHERS | ||||||||||||
| FEVER | 0 | 0 | 0 | 5 (11.4) | 1 (2.3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PAIN | 2 (4.5) | 0 | 0 | 3 (6.8) | 1 (2.3) | 0 | 0 | 0 | 0 | 1 (2.3) | 1 (2.3) | 0 |
| WEIGHT LOSS | 0 | 0 | 0 | 0 | 0 | 0 | 2 (4.5) | 0 | 0 | 1 (2.3) | 0 | 0 |
| HYPERPIGMENTATION | 2 (4.5) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| DERMATITIS | 19 (43.2) | 5 (11.4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| FATIGUE | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2.3) | 1 (2.3) | 0 | 10 (22.7) | 5 (11.4) | 0 |
Values are numbers of patients experiencing that grade of toxicity (%)
To improve target coverage and spare critical structures, all patients had repeated 4D CT treatment simulations during treatment (typically after 3 weeks of proton therapy), and 11 patients (25%) underwent adaptive re-planning/therapy.
DISCUSSION
We found that proton therapy to 74 Gy(RBE), given in combination with carboplatin-plus-paclitaxel chemotherapy, produced a median survival time of 29.4 months with improved nonhematologic acute toxicity (grade 3: esophagitis 11.4%, pneumonitis 2.3%; no grade ≥4 toxicity) and late toxicity (pulmonary-pleural fistula 2.3%) compared with other studies.2,7 In RTOG 0117,7 in which 74 Gy was delivered with concurrent carboplatin and paclitaxel, the median overall survival time for patients with stage III disease was 21.6 months. For all patients enrolled (stage I–III), grade 3–4 acute nonhematologic toxicity rate was 57%, grade 3–4 late radiation toxicity rate was 20%, grade 5 acute toxicity rate was 4%, and grade 5 late toxicity rate was 2%. However, RTOG 0117 did not use a 4D CT-based iGTV approach or heterogeneity corrections. The toxicity rates in the current study are also better than our institutional experience with the highly conformal delivery of intensity-modulated (photon) radiation therapy to 63 Gy with concurrent chemotherapy for stage III NSCLC (44% grade ≥3 esophagitis and 9% grade ≥3 pneumonitis).12
Our toxicity profile was notable for the rarity of severe (grade ≥3) side effects, especially esophagitis and pneumonitis, relative to photon irradiation, either at lower doses (60–63 Gy) or at the equivalent dose of 74 Gy. The lesser toxicity associated with concurrent proton and chemotherapy could have resulted from reduced exposure of normal tissues, particularly the critical structures surrounding the target volume, to high doses. In addition to this reduction in high-dose exposure, the substantial reduction in low-dose exposure, including the lower integral dose9,13 to the non-target tissues, could have had significant influence in improving normal tissue tolerance.
To the best of our knowledge, this is the first publication on concurrent chemotherapy and proton therapy for stage III NSCLC; only two prior published reports have described the use of proton therapy alone for stage III NSCLC. Shioyama14 described 9 patients with stage III NSCLC treated with proton therapy alone in which the 2-year cause-specific survival rate was 70%. Nakayama15 studied 35 patients with stage II/III NSCLC treated with proton therapy alone to a median dose of 78.3 Gy (range, 67.1–91.3 Gy); 11.4% of the patients in that study developed local recurrence, 37.1% regional recurrence, and 20% distant metastasis. The 2-year overall survival rate was 58.9%, and no patients experienced grade ≥3 toxicity. This local recurrence rate seems similar to that of the current study (20.5%); the apparently higher regional recurrence rate (37.1% vs. 9.1% in our study) could have been due to the lack of chemotherapy or PET/CT for disease staging in that study. The higher rate of distant metastasis in our study (43.2%) could have been due to our patients undergoing scheduled PET/CT scans during their follow-up visits, which increases the likelihood of detecting distant metastatic disease. Disease recurrence that occurs as distant metastatic disease has an aggressive phenotype, highlighting the need for additional novel or more aggressive chemotherapy regimens, perhaps with molecular targeted therapy, in the future. Two other studies of proton therapy with chemotherapy for stage III NSCLC are ongoing at Loma Linda University Medical Center and the University of Florida.16
Some concern has been expressed that rates of elective lymph node failure could be higher with proton therapy than with x-ray–based therapy because of the reduced scattering dose to adjacent regional lymph nodes from proton therapy; this scatter is thought to help prevent elective nodal failure when only involved fields are irradiated.17 However, only 1 patient in our study (2.3%) developed isolated nodal failure, indicating that prophylactic elective nodal irradiation is not indicated with our current staging work-up and current proton dose; rather, total local failure (20.5%) and distant metastasis (43.2%) were the predominant patterns of recurrence. The rate of regional lymph node recurrence in our study (9.1%) was not unexpected given the size and location of the lesions. The 20.5% total local failure rate indicates that there is room for improvement. Possible reasons for local failure include the following: (1) a dose of 74 Gy(RBE) may not be enough to eliminate some radiation-resistant cancer cells; (2) inaccuracies in target delineation or tight radiotherapy margins and inadequate target coverage caused by complicated anatomy (e.g., tumors curving around the esophagus, spinal cord, or brachial plexus) could have resulted in “marginal misses”; and (3) the 4D simulation and planning techniques may not have fully addressed tumor motion within fractions11,18 or between fractions, or anatomic changes resulting from the treatment or the disease process.19
Our study did have some shortcomings, chiefly the heterogeneity of the patient population and the relatively short follow-up time. Neither induction nor adjuvant chemotherapy was standardized, and the small tumor volumes (median GTV, 101 cm3) complicates comparison of these findings with others in the literature. The mean lung dose (14.3 Gy), lung V20 (24.9%), and mean esophageal dose (21.2 Gy) in our study were similar to those of RTOG 0117; however, the lesser toxicity, especially pneumonitis and esophagitis, in our study suggests that differences in other dosimetric variables such as low dose exposure may also be informative with regard to predicting toxicity.
Collectively, these findings suggest that passively scattered proton therapy has the potential for delivering high radiation doses in combination with concurrent chemotherapy and can achieve promising survival with tolerable toxicity for patients with stage III lung cancer. Further improvements in conformality for cases involving complicated anatomy, such as tumors that curve around sensitive critical structures, can be achieved by intensity-modulated proton therapy,20 which is delivered with scanning beams rather than scattered beams and can simultaneously optimize the intensities and energies of all scanning beams by using an objective function that accounts for target coverage as well as normal tissue constraints. This technique allows further dose escalation and could lead to improved local control and survival even for patients with more extensive disease. However, the uncertainties associated with tumor and organ motion during the 7-week treatment period remain the main concern for proton therapy, particularly intensity-modulated proton therapy.11,18–20
CONCLUSIONS
Our current study shows that high-dose proton therapy with concurrent chemotherapy is well tolerated and the 29.4-month median survival time and 20.5% total local failure rate are encouraging for inoperable stage III NSCLC. These findings will be useful for a planned prospective phase II randomized study comparing proton therapy vs. intensity-modulated (photon) radiation therapy, both to 74 Gy with concurrent chemotherapy, for stage III NSCLC (clinicaltrials.gov identifier NCT00495040).
Acknowledgments
Supported in part by National Cancer Institute grants P01CA021239 and CA16672.
We thank the Thoracic Radiation Oncology and Proton Therapy Center teams at MD Anderson Cancer Center for their help and support and Ms. Christine Wogan of the Division of Radiation Oncology at MD Anderson for editorial assistance.
Footnotes
Financial disclosures: None of the authors have financial relationships to disclose
Portions of this work were presented at the 2009 annual meeting of the American Society of Therapeutic Radiation Oncology and published in abstract form (Int J Radiat Oncol Biol Phys 75:S446, 2009)
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Curran W, Scott C, Langer C, et al. Long term benefit is observed in a phase III comparison of sequential vs concurrent chemo-radiation for patients with unresectable NSCLC: RTOG 9410 (abstract) Proc Am Soc Clin Oncol. 2003:621a. [Google Scholar]
- 3.Furuse K, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999;17:2692–2699. doi: 10.1200/JCO.1999.17.9.2692. [DOI] [PubMed] [Google Scholar]
- 4.Fournel P, Robinet G, Thomas P, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d'Oncologie Thoracique-Groupe Francais de Pneumo-Cancerologie NPC 95-01 study. J Clin Oncol. 2005;23:5910–5917. doi: 10.1200/JCO.2005.03.070. [DOI] [PubMed] [Google Scholar]
- 5.Socinski M, Rosenman J, Halle J, et al. Dose-escalating conformal thoracic radiation therapy with induction and concurrent carboplatin/paclitaxel in unresectable stage IIIA/B non-small-cell lung carcinoma. Cancer. 2001;92:1213–1223. doi: 10.1002/1097-0142(20010901)92:5<1213::aid-cncr1440>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Schild S, McGinnis W, Graham D, et al. Results of a phase I trial of concurrent chemotherapy and escalating doses of radiation for unresectable non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;65:1106–1111. doi: 10.1016/j.ijrobp.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 7.Bradley JD, Bae K, Graham MV, et al. Primary analysis of the phase II component of a phase I/II dose intensification study using three-dimensional conformal radiation therapy and concurrent chemotherapy for patients with inoperable non-small-cell lung cancer: RTOG 0117. J Clin Oncol. 2010;28:2475–2480. doi: 10.1200/JCO.2009.27.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moyers MF, Miller DW, Bush DA, et al. Methodologies and tools for proton beam design for lung tumors. Int J Radiat Oncol Biol Phys. 2001;49:1429–1438. doi: 10.1016/s0360-3016(00)01555-8. [DOI] [PubMed] [Google Scholar]
- 9.Chang J, Zhang X, Wang X, et al. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in stage I or stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;65:1087–1096. doi: 10.1016/j.ijrobp.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 10.Chang JY, Dong L, Liu H, et al. Image-guided radiation therapy for non-small cell lung cancer. J Thorac Oncol. 2008;3:177–186. doi: 10.1097/JTO.0b013e3181622bdd. [DOI] [PubMed] [Google Scholar]
- 11.Kang Y, Zhang X, Chang JY, et al. 4D Proton treatment planning strategy for mobile lung tumors. Int J Radiat Oncol Biol Phys. 2007;67:906–914. doi: 10.1016/j.ijrobp.2006.10.045. [DOI] [PubMed] [Google Scholar]
- 12.Sejpal S, Komaki R, Tsao A, et al. Early findings on the toxicity of proton beam therapy given with concurrent chemotherapy for non-small cell lung cancer. Cancer. 2011 doi: 10.1002/cncr.25848. in press. [DOI] [PubMed] [Google Scholar]
- 13.Chang JY, Komaki R, Wen HY, et al. Toxicity and patterns of failure of adaptive/ablative proton therapy for early stage medically inoperable non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2011 doi: 10.1016/j.ijrobp.2010.04.049. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shioyama Y, Tokuuye K, Okumura T, et al. Clinical evaluation of proton radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2003;56:7–13. doi: 10.1016/s0360-3016(02)04416-4. [DOI] [PubMed] [Google Scholar]
- 15.Nakayama H, Satoh H, Sugahara S, et al. Proton Beam Therapy of Stage II and III Non-Small-Cell Lung Cancer. Int J Radiat Oncol Biol Phys. 2010 Sep 30; doi: 10.1016/j.ijrobp.2010.06.024. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Bush D. Proton radiation therapy for lung cancer: is there enough evidence? Oncology. 2010;24:1052–1057. [PubMed] [Google Scholar]
- 17.Kepka L, Maciejewski B, Withers RH. Does incidental irradiation with doses below 50 Gy effectively reduce isolated nodal failures in non-small-cell lung cancer: dose-response relationship. Int J Radiat Oncol Biol Phys. 2009;73:1391–1396. doi: 10.1016/j.ijrobp.2008.07.070. [DOI] [PubMed] [Google Scholar]
- 18.Engelsman M, Rietzel E, Kooy HM. Four-dimensional proton treatment planning for lung tumors. Int J Radiat Oncol Biol Phys. 2006;64:1589–1595. doi: 10.1016/j.ijrobp.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 19.Hui Z, Zhang X, Starkschall G, et al. Effects of interfractional motion and anatomic changes on proton therapy dose distribution in lung cancer. Int J Radiat Oncol Biol Phys. 2008;72(5):1385–1395. doi: 10.1016/j.ijrobp.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Li Y, Pan X, et al. Intensity-modulated proton therapy reduces normal tissue doses compared with` intensity-modulated radiation therapy or passive scattering proton therapy and enables individualized radical radiotherapy for extensive stage IIIB-non-small cell lung cancer: a virtual clinical study. Int J Radiat Oncol Biol Phys. 2010;77:357–366. doi: 10.1016/j.ijrobp.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]


