Abstract
Pulsed application of focused ultrasound (FUS) to the regional brain tissue alters the state of tissue excitability, and thus provides the means for non-invasive functional neuromodulation. We report that the application of transcranial FUS to the thalamus of anesthetized rats reduced the time to emergence of voluntary movement from intraperitoneal ketamine-xylazine anesthesia. Low intensity FUS was applied to the thalamus of anesthetized animals. The times required for the animals to show distinct physiological/behavioral changes were measured and compared to those times required in a control session without sonication. The sonication significantly reduced the time to show pinch response and voluntary movement. The modulatory effects of FUS on anesthesia suggest potential therapeutic applications for disorders of consciousness such as minimally consciousness states.
Keywords: Anesthesia, Ultrasound, FUS, Neuromodulation, Brain, Function
Introduction
Since the pioneering works examining the biological effects of ultrasound in the early 1950s and 1960s [1–2], much effort has been made to establish the safe and effective applications of ultrasound for various therapeutic uses. As a new modality that enables non-invasive functional neuromodulation, ultrasound is gaining momentum in neuro-therapeutics, especially in targeting brain disorders [3]. Transcranial application of ultrasound, with formation of an acoustic focus in region-specific brain tissue, has been achieved using multi-arrayed transducers surrounding the head [4–5]. The fundamental frequency used for transcranial ultrasound is typically under 1 MHz [6]-lower than the frequency used in diagnostic ultrasound imaging (typically 2–3 MHz). The highly localized delivery of acoustic energy (approximately the size of a rice grain) to the regional brain areas has been demonstrated in humans for thermal ablation of tumor [7] functional neurosurgery for pain [3].
Application of pulsed-mode ultrasound modulates the excitability of the neural tissue both ex vivo [8] and in vivo [9]. We have recently demonstrated that pulsed focused ultrasound (FUS) to selected brain areas of anesthetized animals (rabbits and rats) modulated (either elicited or suppressed) its excitability [10–11]. During the study, we observed the unusual shortening of anesthetic duration in the animals subjected to the sonication. We hypothesized the FUS altered the function of neural substrates affected by the anesthetic, and thereby decreased the duration of the anesthesia. This motivated us to examine whether or not the FUS can indeed decrease the emergence time from anesthesia in reproducible manner.
Pulsed FUS was delivered to the thalamus of the rats subjected to a general anesthesia of intraperitoneal ketamine (NMDA receptor antagonist) and xylazine (α2 adrenergic receptor agonist). The thalamus was targeted since ketamine, at an anesthetic dose, is known to depress activity in the neocortico-thalamic axis and central nuclei of the thalamus [12–14]. To assess the effects of FUS on emergence from anesthesia, we measured the time points when animals began to show select physiological/behavioral features on emergence: respiratory rates, whisker movement, responses to external stimulations, and initiation of voluntary movement.
Method and Materials
Overview of the Procedures
All experiments were conducted under institutional review and approval by Harvard Medical Area Standing Committee on Animals. Two out of a total of 17 Sprague Dawley rats were used to survey the timing of typical physiological responses emerging from anesthesia. All other animals underwent two sessions of anesthesia, separated by 6.2±2.4 days to allow for recovery from anesthesia. In one of the two sessions the animal was subjected to the sonication condition denoted `FUS'; the session without sonication was denoted `Control' condition. The sequence of the conditions was randomized and counter-balanced across animals. The weight of the animals was also balanced across the conditions. Two animals with atypical hyperventilation (n=1) and incomplete anesthetic induction (n=1) were excluded from further testing and analysis.
Focused ultrasound sonication setup
An air-backed, spherical segment ultrasound transducer operating at a frequency of 650 KHz was used. This fundamental frequency is within the 440 to 700 KHz frequency range known for the optimal transmission through the ex vivo human skull [15–16]. The transducer had a diameter of 6 cm and radius-of-curvature (focal depth) of 7 cm. The method for generating the pulsed FUS, hardware specs and acoustic power calibration, are described elsewhere [17]. The acoustic focus, mapped using a hydrophone (Onda, CA) mounted to a triple-axis stage scanner (Velmex, NY), was cigar-shaped and measured 3.5 mm in diameter and 6.2 mm in length at full-width-half-maximum of the acoustic pressure field. For estimating intensity, the pressure amplitude measured by the hydrophone was corrected for attenuation through the rodent skull in situ [18]. Isppa (spatial-peak-pulse-averaged intensity) was calculated by a pulse intensity integral, which was estimated from the integral of the square of instantaneous pressure waveforms divided by the acoustic impedance of the media [19].
For sonication parameters, a tone burst duration (TBD) of 0.5 ms and pulse repetition frequency (PRF) of 100Hz were used. These pulsing parameters were used previously to provide suppressive effects on cortical excitability, albeit with a lower acoustic intensity, 3.3 W/cm2 Isppa [10–11]. A preliminary test on three animals (data not shown) showed reduction in time to emergence, but with a higher intensity, Isppa of 6 W/cm2, corresponding to a spatial peak temporal average intensity, Ispta = 300mW/cm2. The peak negative pressure to the brain tissue was approximately 490 kPa, with associated mechanical index (MI) of 0.61.
Anesthesia Induction and Sonication
All animals were anesthetized with an intraperitoneal ketamine/xylazine mixture of 80:10 mg/kg, which was administered by the same experimenter throughout the experiment to minimize bias. The onset time of anesthesia was marked by unresponsiveness to pinching of the hind paw. The animal was then positioned on a temperature-regulated heated pad (T-pump, Gaymar, NY) and placed in prone position under the sonication apparatus (Sonomo, Korea; Fig. 1). The ultrasound transducer faced downward, directing the FUS beam through a plastic bag containing degassed-water placed over the scalp of the animal. Ultrasound gel was applied between the scalp and the bag. Optical stereotactic guidance described in our previous work [17] established the sonication focus (spatial peak intensity) at the thalamus. The craniometric approximation was used to locate the thalamus based on the distance from the eye and ear canal (2–3 mm caudal to the bregma and 7 mm deep from the skull surface) [20]. Sonication began 40 minutes after the onset of anesthesia and was maintained for 20 minutes. For consistency, the control session was conducted under the same experimental setting as the sonication session.
Figure 1.
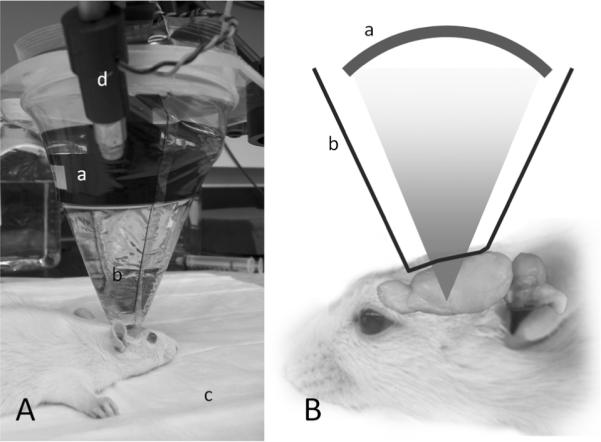
(A) Experimental setup. The animal's head is coupled to an (a) ultrasound transducer by a (b) bag containing degassed water. The (c) heat pad regulated the temperature while the (d) optical imaging system provided the stereotactic guidance to the thalamus. (B) Illustration of the sonication setup (not drawn to scale).
The animal's rectal temperature and respiratory rate were measured every five minutes. The time it took the animal to display the select physiological phenomena of increased respiratory rate (10% above baseline under anesthesia), rapid and irregular respiration and periodic movement of whiskers, were recorded for each animal. The animal's responsiveness to an air puff to the eyes (delivered via a rubber bulb) and hind-paw pinching, as well as the initiation of voluntary movements of the head, forelimbs or hind-paws were monitored. When voluntary movement was detected, the animal was moved back to the cage for full recovery from the anesthetic.
Time to each transition was noted. A repeated measures ANOVA was performed to examine differences in anesthetic emergence time with and without sonication. The animals were kept alive after the second session and their food uptake, defecation, and movement behavior was assessed for abnormality on days 1, 3, 7 and 14 before sacrifice.
Results
The animals' body weight, temperature, and baseline respiratory rate were similar (Table 1); and all showed normal behavior and weight gain (approximately 50%) after sonication.
Table 1.
The weight, respiratory rate, and body temperature of the rats measured from control and FUS session. All these values were indifferent between the two conditions.
| Control session | FUS Session | |
|---|---|---|
| Weight | 303.2 ± 34.4 g | 304.2 ± 33.4 g |
|
| ||
| Respiratory rate | 65.5 ± 3.8 min−1 | 66.4 ± 2.9 min−1 |
|
| ||
| Body temperature | 36.1 ± 0.5 °C | 35.9 ± 0.3 °C |
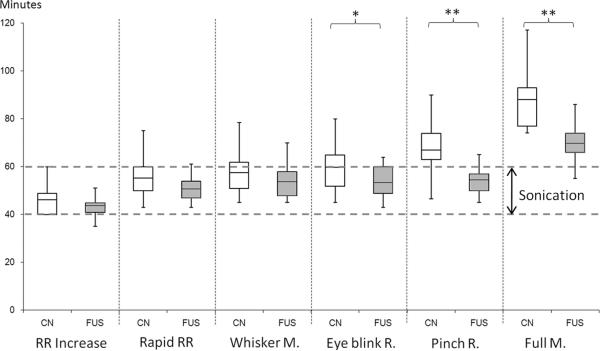
Figure 2 shows the box plot of the measured physiological parameters comparing the two conditions. The intra-peritoneal injection of anesthetic produced distinct physiological responses. The changes in the respiratory rate (abbreviated as `RR') were similar between FUS and Control conditions (F(1,12)=1.03 for increase in RR, F(1,12)=2.24 for rapid irregular RR; both p>0.1). The onset of whisker movement also showed the same trend across the two conditions (i.e. F(1,12)=1.27; p=0.28). However, the time to the initiation of responses to the air puff (noted as `Eye Blink R.') started to show a larger difference between the conditions (F(1,12)=4.33; p=0.06). Remarkably, the sonicated rats responded to hind leg pinch about 55 min after onset of anesthesia, whereas the control condition rats did not respond until 67 min (F(1,12)=12.5; p=0.004). Furthermore, the rats began to move their limbs much earlier under sonication (69.8 ± 9 min) compared to the control condition (88.2 ± 12.9 min; F(1,12)=18.5; p=0.001).
Figure 2.
A comparison box plot indicating the range, first/third quarter percentile, and the mean of the time to reach the measured physiological parameters after the onset of effective anesthesia. FUS was applied from 40 to 60 min after the onset of anesthesia (indicated with dashed lines). White boxes: control condition (denoted by `CN') without sonication, gray boxes: FUS sonication (noted as `FUS'). Acronyms for the parameter; RR increase: increase in respiratory rate, Rapid RR: rapid and irregular respiration, Whisker M: movement of the whisker, Eye blink R: response to the air puff to the eyes, Pinch R: response to the hind-paw pinching, Full M: the onset of the voluntary movement. *: marginal significance (p=0.06), **: significance (p<0.005)
Discussion
Pulsed ultrasound sonication of the rat thalamus significantly reduced time to emergence from anesthesia (as much as 20 min) as measured by the time to voluntary movement. Although an acoustic intensity (Isppa) of 3.3 W/cm2 had suppressive effects on brain function in previous studies [10–11], it is notable that this intensity failed to decrease the duration of the anesthetic state; while a higher intensity of 6 W/cm2 (in Isppa, corresponding Ispta = 300mW/cm2) significantly decrease anesthetic duration. Because pulse parameters TBD and PRF were unchanged, this suggests that the acoustic intensity is important in determining the direction of the neural modulation. It is conceivable that with identical pulse parameters, increasing the acoustic intensity could shift the direction of neuromodulation from suppression to stimulation. Further study is required to elucidate how the various sonication parameters affect neuromodulation.
The prevailing hypothesis as to how pulsed, FUS effects neuromodulation is that the pressure transmitted to the tissue creates a time-varying, nanometer-scale deformation of the neural membrane, possibly modulating the function of ion-channels and mechanoreceptors embedded within the membrane, thereby affecting cellular excitability [21–22]. Cascading events might affect neurotransmitter release, uptake, and a general modification of neural circuits.
As to the mechanism by which sonication reduces emergence time from the anesthetic, several hypotheses can be proposed. First, FUS may modulate levels of neurotransmitters which antagonize xylazine's action on α2 receptors. Our previous studies have already shown that FUS directed to the thalamus affects extracellular serotonin and dopamine levels [17], and neurotransmitter-mediated alteration in anesthesia remains a viable mechanism. Second, the thalamo-cortical connectivity which is dissociated by the action of ketamine, may be reestablished by FUS stimulation of the thalamus, analogous to the way electrical stimulation of the thalamus by deep brain stimulation (DBS) shows promise in improving chronic disorders of consciousness [23–25].
In terms of safety, the acoustic intensity and MI used in the present study were sufficiently below the maximum limits for ultrasound imaging in the FDA guidelines (i.e., MI=1.9 and Ispta = 720mW/cm2 for most of the soft tissue imaging [10]), and this conforms with the normal behavior we observed in all animals after sonication.
The possibility that FUS can modulate anesthetic effects may have implications for treatment of chronic disorders of consciousness, e.g. minimally-consciousness states or vegetative states caused by trauma or stroke, and for studying the mechanisms of consciousness and general anesthesia. Already DBS and dopaminergic or gabanergic drug therapy have shown promising results on these disorders [23–25]. Transcranial magnetic stimulation (TMS) may also provide potential non-invasive alternatives to the therapy [25]; however, it does not provide adequate stimulation for small regions of the brain that are located deep in the brain, such as thalamus. FUS, with its advantages in exquisite control on depth and focal size which have been demonstrated through transcranial application in humans [3], may thus provide unprecedented opportunity for studying and treating disorders of consciousness.
The anesthetics used in the present study belong to a subset of agents that affect consciousness. Therefore, to generalize the effects of FUS on anesthesia and consciousness, further studies involving different classes of anesthetics will be necessary. In addition, the scoring techniques for the evaluation of anesthetic states were somewhat arbitrary. For example, a threshold for responses to pinch and for voluntary movements of whiskers and limbs may vary slightly depending on the evaluator. Adoption of functional neuroimaging techniques to assess the transition in metabolic states during FUS stimulation, as well as the use of less subjective and non-blinded assessment of the anesthetic states, would also help to provide the key to unlocking our understanding of consciousness and its disorders.
Conclusions
Pulsed ultrasound focused at the thalamus significantly decreased time to emergence from ketamine/xylazine anesthesia in rats. Although the mechanism behind this observation is still unknown, the neuromodulatory potential of this non-invasive and spatially-specific tool warrants further investigation.
Acknowledgements
This work was supported by Center for Integration of Medicine and Innovation (CIMIT) and NIH (NINDS NS074124 to Yoo) and the National Research Foundation grant funded by the Korea government (MEST; 2010-0027294 to Park).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Fry FJ, Ades HW, Fry WJ. Production of reversible changes in the central nervous system by ultrasound. Science. 1958;127:83–84. doi: 10.1126/science.127.3289.83. [DOI] [PubMed] [Google Scholar]
- [2].Lele PP. A simple method for production of trackless focal lesions with focused ultrasound: physical factors. J Physiol. 1962;160:494–512. doi: 10.1113/jphysiol.1962.sp006862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Martin E, Jeanmonod D, Morel A, Zadicario E, Werner B. High-intensity focused ultrasound for noninvasive functional neurosurgery. Ann Neurol. 2009;66:858–861. doi: 10.1002/ana.21801. [DOI] [PubMed] [Google Scholar]
- [4].Clement GT, White PJ, King RL, McDannold N, Hynynen K. A magnetic resonance imaging-compatible, large-scale array for trans-skull ultrasound surgery and therapy. J Ultrasound Med. 2005;24:1117–1125. doi: 10.7863/jum.2005.24.8.1117. [DOI] [PubMed] [Google Scholar]
- [5].Aubry JF, Tanter M, Pernot M, Thomas JL, Fink M. Experimental demonstration of noninvasive transskull adaptive focusing based on prior computed tomography scans. J Acoust Soc Am. 2003;113:84–93. doi: 10.1121/1.1529663. [DOI] [PubMed] [Google Scholar]
- [6].Pernot M, Aubry JF, Tanter M, Thomas JL, Fink M. High power transcranial beam steering for ultrasonic brain therapy. Phys Med Biol. 2003;48:2577–2589. doi: 10.1109/TMI.2010.2076829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].McDannold N, Clement GT, Black P, Jolesz F, Hynynen K. Transcranial magnetic resonance imaging- guided focused ultrasound surgery of brain tumors: initial findings in 3 patients. Neurosurgery. 2010;66:323–332. doi: 10.1227/01.NEU.0000360379.95800.2F. discussion 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bachtold MR, Rinaldi PC, Jones JP, Reines F, Price LR. Focused ultrasound modifications of neural circuit activity in a mammalian brain. Ultrasound Med Biol. 1998;24:557–565. doi: 10.1016/s0301-5629(98)00014-3. [DOI] [PubMed] [Google Scholar]
- [9].Tufail Y, Matyushov A, Baldwin N, Tauchmann ML, Georges J, Yoshihiro A, et al. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron. 2010;66:681–694. doi: 10.1016/j.neuron.2010.05.008. [DOI] [PubMed] [Google Scholar]
- [10].Yoo SS, Bystritsky A, Lee JH, Zhang Y, Fischer K, Min BK, et al. Focused ultrasound modulates region-specific brain activity. Neuroimage. 2011;56:1267–1275. doi: 10.1016/j.neuroimage.2011.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Min BK, Bystritsky A, Jung KI, Fischer K, Zhang Y, Maeng LS, et al. Focused ultrasound-mediated suppression of chemically-induced acute epileptic EEG activity. BMC Neurosci. 2011;12:23. doi: 10.1186/1471-2202-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vollenweider FX, Geyer MA. A systems model of altered consciousness: integrating natural and drug-induced psychoses. Brain Res Bull. 2001;56:495–507. doi: 10.1016/s0361-9230(01)00646-3. [DOI] [PubMed] [Google Scholar]
- [13].Denda S, Shimoji K, Tomita M, Baba H, Yamakura T, Masaki H, et al. Central nuclei and spinal pathways in feedback inhibitory spinal cord potentials in ketamine-anaesthetized rats. Br J Anaesth. 1996;76:258–265. doi: 10.1093/bja/76.2.258. [DOI] [PubMed] [Google Scholar]
- [14].Porro CA, Cavazzuti M, Giuliani D, Vellani V, Lui F, Baraldi P. Effects of ketamine anesthesia on central nociceptive processing in the rat: a 2-deoxyglucose study. Neuroscience. 2004;125:485–494. doi: 10.1016/j.neuroscience.2004.01.039. [DOI] [PubMed] [Google Scholar]
- [15].White PJ. Transcranial focused ultrasound surgery. Top Magn Reson Imaging. 2006;17:165–172. doi: 10.1097/RMR.0b013e31803774a3. [DOI] [PubMed] [Google Scholar]
- [16].White PJ, Clement GT, Hynynen K. Longitudinal and shear mode ultrasound propagation in human skull bone. Ultrasound Med Biol. 2006;32:1085–1096. doi: 10.1016/j.ultrasmedbio.2006.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Min BK, Yang PS, Bohlke M, Park SS, Vago DR, Maher TJ, et al. Focused Ultrasound Modulates the Level of Cortical Neurotransmitters: Potential as a New Functional Brain Mapping Technique. Int J Imaging Syst Technol. 2011;21:232–240. [Google Scholar]
- [18].Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc Natl Acad Sci U S A. 2006;103:11719–11723. doi: 10.1073/pnas.0604318103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].NEMA . Acoustic Output Measurement Standard for Diagnostic Ultrasound Equipment. National Electrical Manufactures Association; Washinton, D.C.: 2004. [Google Scholar]
- [20].Paxinos G, Watson C, Pennisi M, Topple A. Bregma, lambda and the interaural midpoint in stereotaxic surgery with rats of different sex, strain and weight. J Neurosci Methods. 1985;13:139–143. doi: 10.1016/0165-0270(85)90026-3. [DOI] [PubMed] [Google Scholar]
- [21].Krasovitski B, Frenkel V, Shoham S, Kimmel E. Intramembrane cavitation as a unifying mechanism for ultrasound-induced bioeffects. Proc Natl Acad Sci U S A. 2011;108:3258–3263. doi: 10.1073/pnas.1015771108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tyler WJ. Noninvasive neuromodulation with ultrasound? A continuum mechanics hypothesis. Neuroscientist. 2011;17:25–36. doi: 10.1177/1073858409348066. [DOI] [PubMed] [Google Scholar]
- [23].Schiff ND, Giacino JT, Kalmar K, Victor JD, Baker K, Gerber M, et al. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007;448:600–603. doi: 10.1038/nature06041. [DOI] [PubMed] [Google Scholar]
- [24].Schiff ND. Recovery of consciousness after brain injury: a mesocircuit hypothesis. Trends Neurosci. 2010;33:1–9. doi: 10.1016/j.tins.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Oliveira L, Fregni F. Pharmacological and electrical stimulation in chronic disorders of consciousness: new insights and future directions. Brain Inj. 2011;25:315–327. doi: 10.3109/02699052.2011.556103. [DOI] [PubMed] [Google Scholar]