Abstract
BACKGROUND
α-Dystroglycan (DG) carries glycan chains that bind to laminin and thus function in homeostasis of not only skeletal muscle but also of various epithelial cells. Loss of glycosylation has been suggested to play important roles in tumor development, particularly in detachment and migration of carcinoma cells. We previously reported that glycosylation of α-DG, but not levels of α-DG core protein itself, is reduced in prostate carcinoma. In this study, we investigate the association between reduction of laminin-binding glycans on α-DG and the degree of tumor cell differentiation and/or infiltrative properties, as assessed by the Gleason grading system.
METHODS
Immunohistochemical analysis of 146 biopsy specimens of prostate adenocarcinoma with various Gleason scores was carried out employing IIH6 and 6C1 antibodies, which recognize laminin-binding glycans on α-DG and α-DG core proteins, respectively. Double immunofluorescence staining was performed to evaluate colocalization of α-DG and laminin, and to determine which types of epithelial cells express laminin-binding glycans on α-DG.
RESULTS
Reduction of α-DG glycosylation, rather than loss of α-DG core protein, was correlated with higher Gleason patterns. Reduction was most conspicuous at the interface between carcinoma cells and the basement membrane. In addition, in non-neoplastic prostate glands, laminin-binding glycans were expressed predominantly on the basolateral surface of basal cells.
CONCLUSIONS
Reduced expression of laminin-binding glycans on α-DG may contribute to formation of highly infiltrative behavior of prostate carcinoma cells. Substantial reduction of laminin-binding glycans in carcinoma tissue could be partly ascribed to disappearance of pre-existing basal cells.
Keywords: prostatic neoplasms, dystroglycans, glycosylation
INTRODUCTION
Prostate cancer is the fifth most common clinically manifest cancer in the world and the second most common in men [1], and is currently recognized as one of the major medical problems facing the male population. Although prostate cancer as a whole has a better prognosis compared to cancers of other organs [1], systemic disease reflects a poor prognosis in individual patients [2]. In today’s clinical practice: (i) Gleason score, (ii) clinical stage, and (iii) prostate-specific antigen (PSA) serum levels at diagnosis are widely accepted risk factors that predict the likelihood of tumor progression [3].
The Gleason grading system [4], which is based on microscopic tumor architecture, consists of 5 histological patterns ranging from well differentiated/less infiltrative (pattern 1) to poorly differentiated/highly infiltrative (pattern 5) features. A number from 1 to 5 is assigned to the most prevalent pattern (primary pattern) and also to the second most prevalent pattern (secondary pattern). The Gleason score, which is the sum of these two numbers, thus has a value between 2 and 10. In current practice, the vast majority of prostate cancers have a Gleason score ≥6 [5]. Hence, tumors composed of patterns 3, 4, and/or 5 are considered clinically significant [6].
Dystroglycan (DG) is an adhesion molecule expressed in skeletal muscle as well as in a wide variety of tissues, including prostate epithelial cells [7]. DG is composed of α- and β-DG subunits; extracellular α-DG protein is not attached directly to the cell membrane but is anchored to it via the transmembrane protein β-DG [8]. β-DG binds to the cytoplasmic proteins dystrophin and utrophin, which, in turn, bind to the actin cytoskeleton and several adaptor molecules functioning in cellular signaling [9]. α-DG protein is highly and heterogenously glycosylated with O-mannosyl glycans, mucin-type O-glycans, and N-glycans; such glycosylation plays a major role in DG function and is essential to form contacts with its binding partners, particularly laminin on the basement membrane (BM) [8, 10–13]. Loss of α-DG glycosylation has been suggested to function in the process of tumor invasion and metastasis through detachment of carcinoma cells from the BM and subsequent migration [13–17].
We recently reported a tumor suppressor function for laminin-binding glycans on α-DG [18]. In aggressive prostate carcinoma cell lines, such as LNCaP and PC3 cells, laminin-binding glycans on α-DG were dramatically decreased, while levels of α- and β-DG core proteins were maintained. A decrease of laminin-binding glycans on α-DG and consequent increase in cell migration were associated with decreased expression of β3-N-acetylglucosaminyltransferase 1 (B3GNT1). B3GNT1 was found to be required for laminin-binding glycan synthesis through formation of a complex with acetylglucosaminyltransferase-like protein (LARGE). In this study, we performed immunohistochemical analysis of human prostate tissues and demonstrated that glycosylation of α-DG, but not expression of α-DG core protein itself, is reduced in prostate carcinoma cells, while non-neoplastic prostate epithelial cells express α-DG decorated with laminin-binding glycans.
Sgambato et al. [15] have demonstrated that low-α-DG staining by VIA4-1 antibody, which recognizes the laminin-binding glyco-epitope, is associated with high-grade prostate carcinoma defined as Gleason score >7. However, it has not been determined whether the reduction in laminin-binding glycans on α-DG correlates with the degree of tumor cell differentiation and/ or infiltrative properties, as assessed by dominant histological patterns (Gleason primary pattern) rather than Gleason score.
Here, we show that reduced glycosylation of α-DG core proteins tends to occur in prostate carcinoma showing highly infiltrative histological patterns, preferentially at the interface between carcinoma cells and the BM. We propose that reduced expression of laminin-binding glycans on α-DG may contribute to infiltrative behavior of prostate carcinoma cells.
MATERIALS AND METHODS
Human Tissue Samples
Formalin-fixed paraffin-embedded (FFPE) tissue blocks of biopsy specimens of prostate carcinoma with various Gleason scores ranging from 6 to 10 (n = 146) were retrieved from the pathological archive at Nagano Municipal Hospital. The present study focused on the common or acinar variant of adenocarcinoma, and special variants such as ductal adenocarcinoma and small cell carcinoma were not included. The specimens analyzed consisted of 34 (23.3%) of Gleason score 6, 45 (30.1%) of Gleason score 7, 35 (24.0%) of Gleason score 8, 29 (20.0%) of Gleason score 9, and 3 (2.1%) of Gleason score 10. The Gleason score of each specimen was re-evaluated and confirmed by two different pathologists (H.S. and M.K.) based on the current Gleason grading system [19]. As noted, the Gleason score is the sum of two numbers assigned for primary and secondary patterns, hence identical Gleason scores do not necessarily indicate the same histological tumor architectures. As for the primary pattern, the samples consisted of 56 (38.4%) of Gleason pattern 3, 78 (53.4%) of Gleason pattern 4, and 12 (8.2%) of Gleason pattern 5.
For double immunofluorescence staining, fresh prostate tissues were obtained at Shinshu University Hospital from radical prostatectomy specimens, with written informed consent in accordance with the Helsinki Declaration. The analysis of human prostate tissues was approved by the Ethics Committee of Shinshu University School of Medicine.
Antibodies
The following antibodies served as primary antibodies: 6C1 (mouse IgM) directed to the extracellular domain near the C-terminus of the α-DG core protein (Millipore, Billerica, MA), IIH6 (mouse IgM) directed to the carbohydrate moiety of α-DG laminin-binding glycans (Millipore), 34βE12 (mouse IgG) directed to high-molecular weight cytokeratin (Dako, Glostrup, Denmark), and rabbit polyclonal anti-laminin antibody (Dako).
Immunohistochemistry
Immunostaining for IIH6 and 6C1 on FFPE tissue sections was performed using the EnVision+ system (Dako) as described previously [20]. Briefly, after deparaffinization and rehydration, tissue sections were boiled in 10 mM Tris–HCl buffer (pH 8.0) containing 1 mM EDTA for 25 min in a microwave oven for antigen retrieval. After endogenous peroxidase activity was quenched in methanol containing 0.3% hydrogen peroxide for 30 min, sections were blocked with 1% bovine serum albumin (BSA; Sigma–Aldrich, St. Louis, MO) in Tris-buffered saline (TBS) for 15 min. After incubation with either IIH6 or 6C1 (each diluted 1:100) at 4°C overnight, sections were incubated with goat anti-mouse immunoglobulin- and horseradish peroxidase (HRP)-conjugated polymers for 30 min. The color reaction was developed in TBS containing 0.2% 3,3′-diaminobenzidine (Dojindo, Kumamoto, Japan) and 0.02% hydrogen peroxide. Sections were briefly counterstained with hematoxylin. Negative controls, which showed no specific staining, were obtained by replacing primary antibodies with species-and class-matched immunoglobulins.
Evaluation of Immunostained Sections
Immunostained sections were observed under a BX-51 microscope (Olympus, Tokyo, Japan). Two pathologists (H.S. and M.K.) examined 6C1- or IIH6-stained sections within the Gleason primary pattern and evaluated two properties using 4 grades each:(i) signal intensity ranging from 0 to 3 (0, negative; 1, weak; 2, moderate; and 3, strong), and (ii) percentage of positive cells (0, 0%; 1, <25%; 2, 25–50%; and 3, >50%).
Double Immunofluorescence Staining
Fresh prostate tissues were embedded in Tissue-Tek OCT compound (Sakura Finetek, Tokyo, Japan) and frozen at −80°C. Frozen tissues were sectioned at 6 μm and fixed with acetone for 5 min, followed by air-drying. After blocking in 1% BSA in TBS, sections were incubated with a cocktail of primary antibodies, consisting of either 6C1 (diluted 1:10) and anti-laminin (diluted 1:100), IIH6 (diluted 1:10) and anti-laminin (diluted 1:100), or IIH6 (diluted 1:10) and 34βE12 (diluted 1:100), for 30 min. After washing, sections were incubated for 30 min with a cocktail of secondary antibodies differentially labeled with Alexa Fluor 488 and Alexa Fluor 555 (Invitrogen, Carlsbad, CA). Sections were mounted using Vectashield mounting medium with DAPI (Vector Laboratories, Burlingame, CA) and viewed under an AX-80 fluorescence microscope (Olympus).
Statistical Analysis
Differences between groups were statistically analyzed by the Kruskal–Wallis test with Dunn’s post hoc test, and correlations between Gleason pattern and either (i) signal intensity or (ii) percentage of positive cells in 6C1- and IIH6-stained tissues were analyzed by Spearman’s rank correlation coefficient, using InStat 3 software (GraphPad Software, San Diego, CA). P-values <0.05 were considered significant.
RESULTS
Reduced α-DG Glycosylation in Prostate Carcinoma Cells
We previously showed that α-DG glycosylation rather than expression of α-DG core protein is substantially reduced in prostate carcinoma cells [18]. To confirm this finding in a much larger population, we carried out immunohistochemical analysis of 143 biopsy specimens of prostate adenocarcinoma with various Gleason scores. As shown in Figure 1, positive staining for 6C1, which recognizes α-DG core proteins, was seen in non-neoplastic prostate epithelial cells seen in normal and hyperplastic glands, as well as in most carcinoma cells (Fig. 1, middle column). Only a small number of carcinoma samples (n = 6) showed little or no staining. On the other hand, IIH6, which recognizes a carbohydrate moiety of laminin-binding glycans on α-DG, stained predominantly the basolateral aspect of non-neoplastic epithelial cells, whereas most carcinoma cell samples showed less intense staining. In some regions of carcinoma samples, IIH6 staining was completely absent (Fig. 1, right column). Overall, expression of IIH6-positive laminin-binding glycans on α-DG was decreased in carcinoma cells in 124 of 146 (84.9%) specimens, and reductions in IIH6 staining were more evident than were reductions in 6C1 staining. These results confirm our previous finding that reduction of functional α-DG in prostate carcinoma is largely due to decreased α-DG glycosylation rather than loss of α-DG core proteins.
Fig. 1.
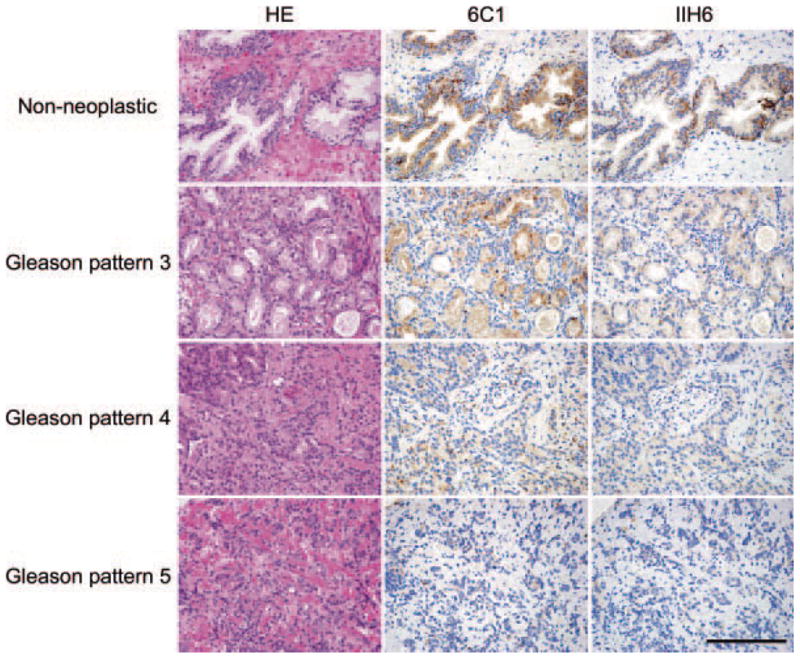
Expression of α-dystroglycan (α-DG) core proteins (6C1) and laminin-binding glycanson α-DG(IIH6) in non-neoplastic prostate glands and prostate adenocarcinomas exhibiting Gleason pattern 3 (discrete glandular units), Gleason pattern 4 (cribriform glands with an irregular border), and Gleason pattern 5 (no glandular differentiation, composed of cords, or single cells) [19]. Note that carcinomas are progressively more poorly differentiated and/or highly infiltrative compared with samples shown in upper panels. HE, hematoxylin and eosin, Bar = 100 μm.
Decreases in α-DG Glycosylation Are Correlated With Higher Gleason Patterns
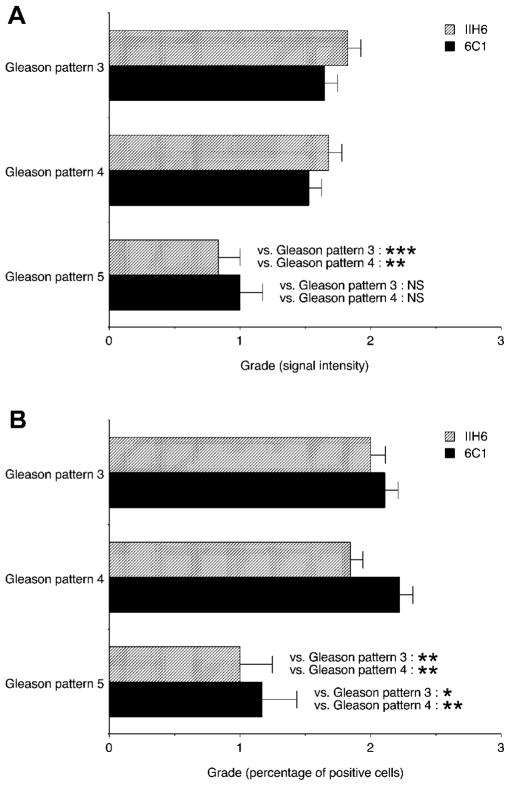
To examine whether the extent of α-DG glycosylation reduction is associated with tumor cell differentiation and/or infiltrative properties, we evaluated (i) signal intensity and (ii) the percentage of positive cells within the Gleason primary pattern in 6C1- and IIH6-stained specimens. In terms of signal intensity, 6C1-positivity of α-DG core proteins did not differ significantly among Gleason patterns, while expression of IIH6-positive laminin-binding glycans on α-DG was reduced in Gleason pattern 5, with high statistical significance (P < 0.001 vs. Gleason pattern 3, P < 0.01 vs. Gleason pattern 4; Fig. 2A, see also Fig. 1). On the other hand, percentages of cells positive for 6C1 or IIH6 were significantly reduced in Gleason pattern 5 (6C1: P < 0.05 vs. Gleason pattern 3, P < 0.01 vs. Gleason pattern 4; IIH6: P < 0.001 vs. Gleason pattern 3, P < 0.01 vs. Gleason pattern 4; Fig. 2B, see also Fig. 1). Spearman’s rank correlation coefficient revealed that in IIH6-stained cells, both parameters were inversely correlated with Gleason pattern (intensity: Spearman’s ρ = − 0.2321, P = 0.0048; percentage of positive cells: Spearman’s ρ = − 0.2133, P = 0.0097); however, in 6C1-stained cells, neither parameter was significantly correlated with Gleason patterns. These results collectively indicate that α-DG glycosylation, rather than expression of α-DG core protein, is reduced in tissues with higher Gleason patterns, suggesting that reduction in the level of laminin-binding glycans on α-DG may contribute to formation of highly infiltrative histological patterns, particularly in Gleason pattern 5.
Fig. 2.
Expression of α-dystroglycan (α-DG) protein stained with 6C1(black boxes) and laminin-binding glycans on α-DG stained with IIH6 (stippled boxes) in prostate adenocarcinoma with different Gleason primary patterns, as assessed by signal intensity (A) and the percentage of positive cells (B). Data are presented as means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant.
Reduced α-DG Glycosylation Occurs at the Carcinoma Cell/BM Interface
The above findings prompted us to ask whether the reduced α-DG glycosylation occurs at the interface between carcinoma cells and the BM where laminin is found. To do so, we performed double immunofluorescence staining of prostate specimens containing both carcinoma and non-neoplastic glands for either IIH6 or 6C1 together with an anti-laminin antibody. As shown in Fig. 3, IIH6 signals were observed in a line along the basolateral surface of non-neoplastic glandular epithelial cells, and those signals colocalized with laminin staining along the BM (Fig. 3, upper panels, arrows). However, IIH6 staining patterns were substantially reduced in carcinoma tissues and did not colocalize with laminin staining (Fig. 3 upper panels, arrowheads). 6C1 staining showed a similar pattern; however, 6C1 signals were also detected at the apical surface and in the cytoplasm of non-neoplastic epithelial cells. These findings indicate that laminin-binding glycans on α-DG observed in non-neoplastic glands are reduced in prostate carcinoma predominantly at the interface with the BM, and that reduction in levels or alterations in localization of α-DG core proteins may contribute in part to reduced IIH6 signals.
Fig. 3.
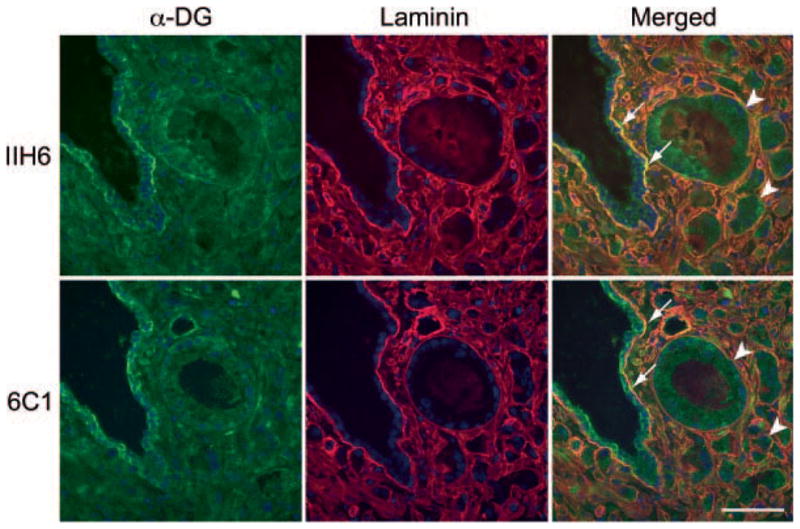
Double immunofluorescence staining of prostate tissues containing both non-neoplastic (arrows) and carcinoma (arrowheads) tissues. Green and red signals indicate positive staining for laminin-binding glycans on α-dystroglycan (α-DG; upper panels, IIH6) or α-DG core protein (lower panels, 6C1) and laminin (both upper and lower panels), respectively. Yellow signals (Merged) show colocalization of both antigens. Bar = 50 μm.
Laminin-Binding Glycanson α-DG Are Expressed Predominantly on Basal Cells in Non-Neoplastic Prostate Glands
Normal prostate glands are composed primarily of two types of epithelial cells: luminal and basal cells [21]. To determine which cell type predominantly expresses laminin-binding glycans on α-DG, we undertook double immunofluorescence staining for IIH6 and for 34βE12, which preferentially stains basal cells. As shown in Figure 4, linear IIH6 staining signals were detected at the interface between prostate epithelial cells and the BM. Those signals colocalized with the basal side of basal cells, as identified by 34βE12 staining. 34βE12-negative luminal cells, as recognized by nuclear DAPI staining, occasionally showed weak IIH6 signals in the cytoplasm. These results indicate that most IIH6-positive laminin-binding glycans on α-DG localize at the basal surface of basal cells in contact with the BM.
Fig. 4.
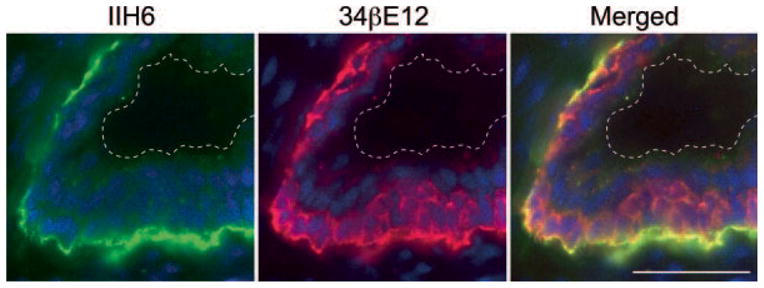
Double immunofluorescence staining of non-neoplastic prostate glands. Green and red signals represent IIH6-positive laminin-binding glycans on α-dystroglycan (α-DG) and 34βE12-positive high-molecular weight cytokeratin, which is preferentially expressed in prostate basal cells, respectively. Yellow (Merged) images illustrate colocalization of two antigens. Note the presence of luminal cells between basal cells and the luminal surface, as indicated by the dotted lines. Bar = 50 μm.
DISCUSSION
The present study demonstrates that reduction of α-DG glycosylation, rather than in levels of α-DG core protein itself, in prostate carcinoma cells is associated with highly infiltrative histological patterns, as assessed by the Gleason grading system. A previous report by Sgambato et al. [15] demonstrated that high-grade prostate carcinoma tends to show reduced α-DG expression. These authors evaluated α-DG expression in samples between high-grade (Gleason score >7) and low-grade (Gleason score ≤7) prostate carcinomas using the VIA4-1 antibody, which recognizes laminin-binding glycans on α-DG. Since the Gleason score is the sum of two numbers assigned for primary and secondary patterns, identical scores do not reflect identical histological tumor architectures, for example, a Gleason score 8 can be either 3 + 5, 4 + 4, or 5 + 3. We therefore evaluated expression of α-DG core proteins and laminin-binding glycans on α-DG separately among Gleason patterns rather than Gleason scores.
We show here that reduction in IIH6-positive laminin-binding glycans on α-DG, which is most pronounced at the interface between basal side of the carcinoma cell membrane and the laminin-expressing BM, is positively correlated with the infiltrative character of prostate adenocarcinoma, as assessed by the Gleason pattern. This finding is consistent with our previous findings demonstrating that PC3 cells expressing substantial levels of IIH6-positive glycans (PC3-H) are much less invasive than those expressing minimal amounts of IIH6-positive glycans (PC3-L), as assessed by a Boyden chamber assay and by the observation that PC3-H cells migrate more slowly than do PC3-L cells in a wound-healing assay [18]. These findings, taken together, suggest that prostate carcinoma cells with reduced laminin-binding glycans on α-DG detach from the BM, enabling invasion and migration of carcinoma cells characteristic of Gleason pattern 5.
In routine histopathological diagnosis, one of the most important hallmarks of prostate adenocarcinoma is the disappearance of basal cell layers. Immunohistochemical detection of basal cells, employing the 34βE12 antibody against high-molecular weight cytokeratin [22], is therefore a valuable diagnostic tool to distinguish prostate carcinoma from various benign mimickers [23]. Here, we also showed that in non-neoplastic glands IIH6-positive laminin-binding glycans on α-DG are expressed predominantly on the basal surface of basal cells, which eventually disappear in carcinoma. Thus, it is possible that the substantial reduction in IIH6-positive laminin-binding glycans in adenocarcinoma tissue can be partly ascribed to loss of this cell population.
CONCLUSIONS
In conclusion, the present study demonstrates that in prostate carcinoma cells reduced α-DG glycosylation, rather than reduced levels of α-DG core protein, is associated with highly infiltrative histological patterns, as assessed by Gleason primary patterns. We also found that IIH6-positive laminin-binding glycans on α-DG, expressed predominantly on the basal side of basal cells, colocalize with laminin in the BM in non-neoplastic prostate tissue, and that this association is absent in prostate adenocarcinoma. Reduced expression of laminin-binding glycans on α-DG may contribute to formation of highly infiltrative behavior of prostate carcinoma cells. Whether reduced expression of laminin-binding glycans on α-DG predicts poor prognosis awaits further studies.
Acknowledgments
We thank Dr. Xingfeng Bao for useful discussion, Ms. Tomoko Nishizawa for technical assistance, and Dr. Elise Lamar for critical reading of the study. Part of the work was presented as a poster at the Annual Meeting of the Society for Glycobiology, held in St. Petersburg, Florida, November 7–10, 2010.
Grant sponsor: The Japan Society for the Promotion of Science; Grant numbers: C-22590311; B-21390104; Grant sponsor: The Ministry of Education, Culture, Sports, Science and Technology of Japan; Grant number: B-22790343; Grant sponsor: The National Institutes of Health; Grant number: RO1 CA48737, PO1 CA71932.
Footnotes
Disclosure statement: The authors have nothing to disclose.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Kamidono S, Ohshima S, Hirao Y, Suzuki K, Arai Y, Fujimoto H, Egawa S, Akaza H, Hara I, Hinotsu S, Kakehi Y, Hasegawa T. Evidence-based clinical practice guidelines for prostate cancer (summary—JUA 2006 edition) Int J Urol. 2008;15:1–18. doi: 10.1111/j.1442-2042.2007.01959.x. [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich A, Aus G, Bolla M, Joniau S, Matveev VB, Schmid HP, Zattoni F. EAU guidelines on prostate cancer. Eur Urol. 2008;53:68–80. doi: 10.1016/j.eururo.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol. 1974;111:58–64. doi: 10.1016/s0022-5347(17)59889-4. [DOI] [PubMed] [Google Scholar]
- 5.Epstein JI. Gleason score 2–4 adenocarcinoma of the prostate on needle biopsy: A diagnosis that should not be made. Am J Surg Pathol. 2000;24:477–478. doi: 10.1097/00000478-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 6.True L, Coleman I, Hawley S, Huang CY, Gifford D, Coleman R, Beer TM, Gelmann E, Datta M, Mostaghel E, Knudsen B, Lange P, Vessella R, Lin D, Hood L, Nelson PS. A molecular correlate to the Gleason grading system for prostate adenocarcinoma. Proc Natl Acad Sci USA. 2006;103:10991–10996. doi: 10.1073/pnas.0603678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durbeej M, Henry MD, Ferletta M, Campbell KP, Ekblom P. Distribution of dystroglycan in normal adult mouse tissues. J Histochem Cytochem. 1998;46:449–457. doi: 10.1177/002215549804600404. [DOI] [PubMed] [Google Scholar]
- 8.Ervasti JM, Campbell KP. Membrane organization of the dystrophin–glycoprotein complex. Cell. 1991;66:1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- 9.Barresi R, Campbell KP. Dystroglycan: From biosynthesis to pathogenesis of human disease. J Cell Sci. 2006;119:199–207. doi: 10.1242/jcs.02814. [DOI] [PubMed] [Google Scholar]
- 10.Chiba A, Matsumura K, Yamada H, Inazu T, Shimizu T, Kusunoki S, Kanazawa I, Kobata A, Endo T. Structures of sialylated O-linked oligosaccharides of bovine peripheral nerve α-dystroglycan. The role of a novel O-mannosyl-type oligosaccharide in the binding of α-dystroglycan with laminin. J Biol Chem. 1997;272:2156–2162. doi: 10.1074/jbc.272.4.2156. [DOI] [PubMed] [Google Scholar]
- 11.Manya H, Chiba A, Yoshida A, Wang X, Chiba Y, Jigami Y, Margolis RU, Endo T. Demonstration of mammalian O-mannosyltransferase activity: Coexpression of POMT1 and POMT2 required for enzymatic activity. Proc Natl Acad Sci USA. 2004;101:500–505. doi: 10.1073/pnas.0307228101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanagawa M, Saito F, Kunz S, Yoshida-Moriguchi T, Barresi R, Kobayashi YM, Muschler J, Dumanski JP, Michele DE, Oldstone MB, Campbell KP. Molecular recognition by LARGE is essential for expression of functional dystroglycan. Cell. 2004;117:953–964. doi: 10.1016/j.cell.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 13.de Bernabe DB, Inamori K, Yoshida-Moriguchi T, Weydert CJ, Harper HA, Willer T, Henry MD, Campbell KP. Loss of α-dystroglycan laminin binding in epithelium-derived cancers is caused by silencing of LARGE. J Biol Chem. 2009;284:11279–11284. doi: 10.1074/jbc.C900007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sgambato A, Migaldi M, Montanari M, Camerini A, Brancaccio A, Rossi G, Cangiano R, Losasso C, Capelli G, Trentini GP, Cittadini A. Dystroglycan expression is frequently reduced in human breast and colon cancers and is associated with tumor progression. Am J Pathol. 2003;162:849–860. doi: 10.1016/S0002-9440(10)63881-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sgambato A, De Paola B, Migaldi M, Di Salvatore M, Rettino A, Rossi G, Faraglia B, Boninsegna A, Maiorana A, Cittadini A. Dystroglycan expression is reduced during prostate tumorigenesis and is regulated by androgens in prostate cancer cells. J Cell Physiol. 2007;213:528–539. doi: 10.1002/jcp.21130. [DOI] [PubMed] [Google Scholar]
- 16.Muschler J, Levy D, Boudreau R, Henry M, Campbell K, Bissell MJ. A role for dystroglycan in epithelial polarization: Loss of function in breast tumor cells. Cancer Res. 2002;62:7102–7109. [PubMed] [Google Scholar]
- 17.Singh J, Itahana Y, Knight-Krajewski S, Kanagawa M, Campbell KP, Bissell MJ, Muschler J. Proteolytic enzymes and altered glycosylation modulate dystroglycan function in carcinoma cells. Cancer Res. 2004;64:6152–6159. doi: 10.1158/0008-5472.CAN-04-1638. [DOI] [PubMed] [Google Scholar]
- 18.Bao X, Kobayashi M, Hatakeyama S, Angata K, Gullberg D, Nakayama J, Fukuda MN, Fukuda M. Tumor suppressor function of laminin-binding α-dystroglycan requires a distinct β3-N-acetylglucosaminyltransferase. Proc Natl Acad Sci USA. 2009;106:12109–12114. doi: 10.1073/pnas.0904515106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Epstein JI, Allsbrook WC, Amin MB, Egevad LL. ISUP Grading Committee. The 2005 International Society of Urologycal Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;29:1228–1242. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi M, Mitoma J, Nakamura N, Katsuyama T, Nakayama J, Fukuda M. Induction of peripheral lymph node addressin in human gastric mucosa infected by Helicobacter pylori. Proc Natl Acad Sci USA. 2004;101:17807–17812. doi: 10.1073/pnas.0407503101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verhagen AP, Aalders TW, Ramaekers FC, Debruyne FM, Schalken JA. Differential expression of keratins in the basal and luminal compartments of rat prostatic epithelium during degeneration and regeneration. Prostate. 1988;13:25–38. doi: 10.1002/pros.2990130104. [DOI] [PubMed] [Google Scholar]
- 22.O’Malley FP, Grignon DJ, Shum DT. Usefulness of immunoperoxidase staining with high-molecular-weight cytokeratin in the differential diagnosis of small-acinar lesions of the prostate gland. Virchows Arch A Pathol Anat Histopathol. 1990;417:191–196. doi: 10.1007/BF01600133. [DOI] [PubMed] [Google Scholar]
- 23.van Leenders GJ, Aalders TW, Hulsbergenvan de Kaa CA, Ruiter DJ, Schalken JA. Expression of basal cell keratins in human prostate cancer metastases and cell lines. J Pathol. 2001;195:563–570. doi: 10.1002/path.993. [DOI] [PubMed] [Google Scholar]