Abstract
The current study assessed whether activation of the novel estrogen receptor GPR30 ameliorates salt-dependent renal damage in intact mRen2.Lewis (mRen2) females. Hemizygous mRen2 were maintained on either a normal salt (NS, 0.5% Na) or high salt (HS, 4% Na) diet for 10 wks (5 to 15 wks of age), and HS animals were treated with the GPR30 agonist G-1 or vehicle for two weeks. Systolic blood pressure markedly increased with HS [149 ± 3 to 219 ± 5 mmHg, P<0.01], but G-1 did not influence pressure [P=0.42]. G-1 and estradiol induced relaxation of pre-constricted mesenteric vessels from NS mRen2, but both responses were attenuated in the HS group. Despite the lack of an effect on blood pressure, G-1 decreased renal hypertrophy, proteinuria, urinary 8-isoprostane excretion, and tubular 4-HNE staining. HS significantly increased GPR30 mRNA [1.01 ± 0.04 vs. 1.59 ± 0.13; P<0.01] and protein [0.60 ± 0.31 vs. 3.99 ± 0.75; P<0.01] in the renal cortex. GPR30 was highly expressed in the brush border of proximal tubules and co-localized with megalin. Finally, megalin expression was reduced by HS and restored with G-1. We conclude that GPR30-mediated beneficial effects in salt-sensitive mRen2 females occurred independent of changes in systolic blood pressure. The failure of G-1 to influence pressure may reflect a salt-induced impairment in GPR30-mediated vasorelaxation. The renoprotective actions of GPR30 may involve attenuation of tubular oxidative stress and activation of megalin-mediated protein reabsorption.
Keywords: GPR30, estrogen receptors, oxidative stress, megalin, dietary sodium
INTRODUCTION
In contrast to the classical estrogen receptors alpha (ERα) and beta (ERβ), GPR30 is a membrane-bound G protein-coupled estrogen receptor that induces rapid signaling events.1,2 Synthesis of the selective GPR30 agonist G-1 and antagonist G15 has stimulated studies to elaborate the role of this membrane receptor in the myriad and complex actions of estrogen.3,4 Ongoing studies in our laboratory utilize the congenic mRen2.Lewis (mRen2) hypertensive rat to elucidate the role of estrogen in cardiovascular disease.5 We previously showed in ovariectomized mRen2 females that chronic treatment with the GPR30 agonist G-1 markedly reduces blood pressure but does not influence total body or uterine weight, suggesting activation of a receptor distinct from ERα and ERβ.6 We subsequently showed that estradiol and G-1 dilate isolated mesenteric vessels from the female mRen2 to a similar extent, and both responses are blocked by the GPR30 antagonist G15.7
In addition to estrogen-sensitivity, mRen2 females exhibit a marked salt-dependent increase in blood pressure.8 In comparison to a normal salt (NS) diet (0.5% sodium), high salt (HS; 4% sodium) significantly increases blood pressure, proteinuria, and albuminuria, decreases creatinine clearance, and induces renal and cardiac hypertrophy.5,8 Maintenance of the normotensive Lewis female, the background strain for the mRen2, on a HS diet does not increase blood pressure or proteinuria, suggesting an activated renin-angiotensin-aldosterone system (RAAS) is essential to the development of salt-sensitivity in the mRen2 female. The mRen2 strain is a unique congenic model in that HS profoundly elevates blood pressure in females via an Ang II-dependent mechanism, but males do not respond to salt with an increase in pressure.8–10 Estrogen depletion exacerbates the salt-dependent increase in blood pressure, as well as the extent of proteinuria and albuminuria in this model.8,10 Furthermore, we previously showed that G-1 administration in intact HS mRen2 females improves diastolic function and attenuates myocyte hypertrophy without influencing blood pressure.11 Thus, the mRen2 strain presents a unique opportunity to establish the protective effects of GPR30 in a salt-sensitive female. The current study addressed the hypothesis that chronic GPR30 activation ameliorates salt-sensitive renal damage in the absence of blood pressure effects.
METHODS
Please see http://hyper.ahajournals.org for all procedures and methods.
RESULTS
Effects of chronic G-1 on systolic blood pressure and vascular reactivity
Consistent with previous studies, intact mRen2 females fed HS developed severe hypertension in comparison to females on a NS diet (Figure 1A).8,9 By 13 weeks of age, HS females exhibited a 70 mmHg increase in systolic blood pressure (219 ± 5 mmHg) as compared to the NS group (149 ± 3 mmHg, P<0.001). Administration of the GPR30 agonist G-1 for two weeks did not significantly alter systolic blood pressure (vehicle: 224 ± 8 vs. G-1: 216 ± 4 mmHg, P=0.42). In mesenteric vessels, HS blunted vasorelaxation to both G-1 and estradiol by ~60% (P<0.01, Figure 1B); however, the response to acetylcholine (10−6 M) was not altered by salt (NS: 14±2.8% HS: 13±3.9%, P=0.85; data not shown). Pretreatment of HS vessels with the nitric oxide synthase inhibitor L-NAME further reduced the response to G-1 (P<0.05).
Figure 1.
A, In comparison to mRen2 females fed normal salt (NS), high salt (HS) significantly increased systolic blood pressure (*P<0.05). Pressure was not different in HS mRen2 treated (Tx) with vehicle (veh) or G-1 (P>0.05, n=9). B, Relaxation to G-1 and estradiol (E2) in isolated mesenteric vessels from NS and HS females pre-constricted with phenylephrine (PE). Some HS vessels were pretreated with 100 μM L-NAME *P<0.05, **P<0.01.
Effects of chronic G-1 on renal indices
G-1 significantly decreased renal hypertrophy and increased creatinine clearance (Table 1). G-1 did not alter urinary excretion of sodium, potassium, norepinephrine or epinephrine. As previously shown, ten weeks of HS significantly increased both proteinuria and albuminuria (Figure 2).8,9 Proteinuria was significantly reduced following G-1, and albuminuria in G-1 treated animals was not different from NS controls.
Table 1.
Physiological parameters in high salt (HS) mRen2 females treated with vehicle (veh) or G-1.
| Measurement | HS+veh (n=9) | HS+G-1 (n=9) |
|---|---|---|
| Body Weight (g) | 199 ± 4 | 207 ± 5 |
| Kidney/body weight ratio (mg/g) | 5.1 ± 0.1 | 4.7 ± 0.1 * |
| Urine volume (ml/day) | 48 ± 5 | 50 ± 5 |
| Creatinine clearance (ml/min) | 1.0 ± 0.1 | 1.7 ± 0.2 * |
| Clearance/kidney weight ratio (ml/min/mg/g) | 0.21 ± 0.02 | 0.36 ± 0.05 * |
| Urinary Sodium (mEq/day) | 17.1 ± 1.7 | 18.2 ± 1.8 |
| Urinary Potassium (mEq/day) | 2.48 ± 0.2 | 2.67 ± 0.3 |
| Urinary Norepinephrine (ng/day) | 932 ± 156 | 1054 ± 156 |
| Urinary Epinephrine (ng/day) | 102 ± 24 | 152 ± 27 |
P<0.05 vs. HS+veh.
Figure 2.
A, Proteinuria was significantly increased with high salt (HS) and attenuated by G-1 (*P<0.05, **P<0.001, n=7–9). B, Albuminuria was significantly increased by HS, while the HS+G-1 group was not different from NS controls (*P<0.05, n=5–9).
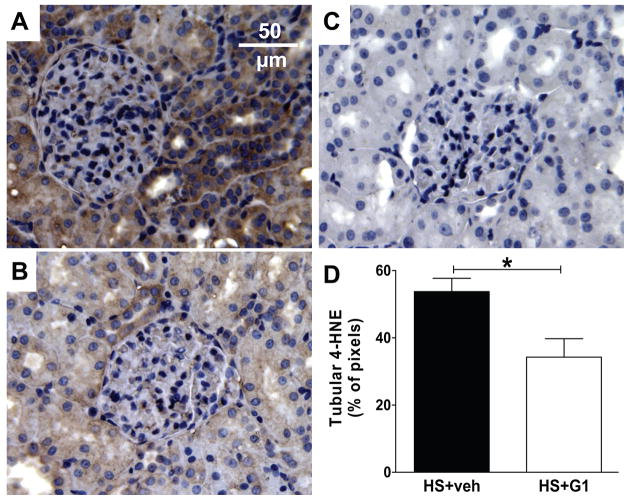
HS mRen2 displayed significant staining for the lipid peroxidation product 4-hydroxynonenal (4-HNE) in the renal cortex (Figure 3). The majority of staining was localized to the tubulointerstitial area (~50% of pixels) with less staining in glomeruli (~7%). G-1 treatment significantly reduced staining in the whole renal cortex (P<0.05), more specifically in tubules (P<0.05) but not in glomeruli (P=0.94). Medullary 4-HNE staining was not altered (data not shown). Finally, urinary excretion of the reactive oxygen species (ROS) metabolite 8-isoprostane F2α was significantly reduced in the G-1 group and correlated with proteinuria in HS females (Figure 4).
Figure 3.
A, 4-HNE staining in the renal cortex of a high salt (HS) mRen2 female treated with vehicle. B, Staining in a HS+G-1 female. C, Control staining in the absence of the primary antibody. D, Quantification of tubular 4-HNE staining (*P<0.05, n=4).
Figure 4.
A, G-1 reduced urinary 8-isoprostane in high salt (HS) mRen2 females (**P<0.01, n=9). B, Excretion of 8-isoprostane positively associated with proteinuria.
Renal distribution and expression of GPR30
Although we previously demonstrated GPR30 expression in aortic and mesenteric vessels of the peripheral vasculature, distribution of the receptor in the mRen2 kidney is not known.6,7 We utilized the GPR30 antibody LS4272, which was previously shown to have high specificity in immunofluorescent and immunoblot studies.6,12 GPR30 immunostaining was predominant in the renal cortex of HS mRen2 females with less staining in the medulla (Figure 5). GPR30 immunofluorescence was detected specifically in proximal tubules, with no staining evident in distal tubules or the glomerulus (Figure 5B). A higher magnification image using confocal microscopy showed GPR30 localization specifically on the apical surface of proximal tubules (Figure 5C).
Figure 5.
A, In the kidneys of high salt (HS) fed mRen2 females, immunofluorescent GPR30 staining (green) in the medulla (right) was lower than the cortex (left). DAPI (blue) was used to stain nuclei. B, The renal cortex showed a prominent signal primarily on the luminal membrane of proximal tubules with minimal staining in glomeruli and distal tubules. C, Higher magnification using confocal microscopy identifying proximal (P) and distal (D) tubules according to presence of a brush border. D, Preabsorption of primary antibody with the antigenic peptide attenuated immunostaining.
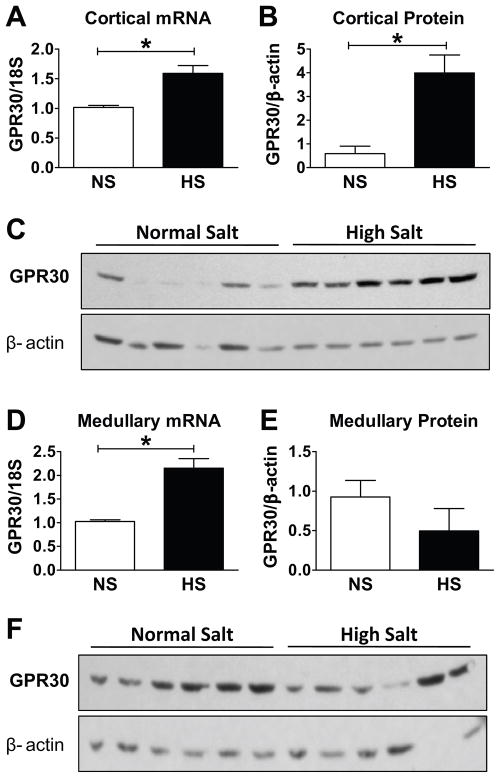
Immunoblot analysis using the same antibody as in immunohistochemistry revealed a single band in cortical (Figure 6C) and medullary (Figure 6F) membrane fractions at a molecular weight of 50 kDa, which is identical to that reported in aortic tissue and brain.6 In the renal cortex, GPR30 mRNA (Figure 6A) and protein (Figure 6B) were both significantly increased with HS. Although medullary GPR30 mRNA was increased in the HS group (Figure 6D), protein analysis did not reveal a significant change (Figure 6E).
Figure 6.
A–B, In comparison to a normal salt diet (NS), high salt (HS) significantly increased renal cortical GPR30 mRNA (*P<0.005, n=6) and protein expression (*P<0.005, n=7). C, Representative immunoblot of renal cortical homogenates. D–E, HS increased medullary GPR30 mRNA (*P<0.001, n=7) but did not influence protein expression (P>0.05, n=6). F, Representative immunoblot of renal medullary homogenates.
We also assessed ERα-36, another purported membrane-bound estrogen receptor, using an antibody directed against amino acid residues 247–261 of the DNA binding domain which is conserved in the various molecular forms of ERα.13,14 In the renal cortex, ERα-66 was the predominant isoform of the receptor while ERα-36 was barely detectable by immunoblot (Figure S1A). Quantification revealed that ERα-36 expression was 50-fold less than ERα-66. The expression of all three ERα isoforms was localized primarily in the soluble fraction of the renal cortex, contrasting with the presence of GPR30 protein exclusively in the 100,000 × g membrane fraction (Figure S1B). Moreover, maintenance on a HS diet did not alter the expression or localization of ERα isoforms in the renal cortex (P>0.20).
GPR30 and Megalin
Since GPR30 immunostaining localized predominantly to the apical surface of proximal tubules, we assessed its relationship with megalin, an endocytic receptor expressed primarily in the brush border which plays a key role in the reabsorption of filtered protein.15 As shown in Figure 7, GPR30 and megalin co-localized in the brush border of proximal tubules. In addition, megalin was significantly reduced with HS and restored with G-1 (Figure 8). Tubular megalin immunofluorescence negatively correlated with proteinuria (Figure 8B). Real-time PCR analysis showed that HS also decreased megalin mRNA (Figure 8C).
Figure 7.
A, GPR30 immunofluorescence in the renal cortex of a HS mRen2 female was localized to the luminal surface of proximal tubules. B, Megalin immunofluorescence was also primarily in the brush border. C, GPR30 and megalin were highly co-localized. D, Bright field image identifying proximal (P) and distal (D) tubules according to presence of a brush border.
Figure 8.
A, Quantification of tubular megalin immunofluorescence showed a significant decrease in high salt (HS) versus normal salt (NS) mRen2 females. G-1 treatment reversed the effect of salt on megalin expression (*P<0.05, **P<0.01, n=4–5). B, Tubular megalin negatively associated with proteinuria. C, HS also decreased megalin mRNA, but G-1 treatment did not significantly alter megalin gene expression (*P<0.05, **P<0.01, n=5–8). D–F, Representative images of tubular megalin staining.
DISCUSSION
Emerging data suggests that GPR30 is widely expressed in various tissues including the brain, heart, vasculature, pancreas, ovary and kidney.16 To our knowledge, the current study is the first to characterize receptor distribution within the kidney of female hypertensive rats. GPR30 immunostaining was most prominent in the proximal tubules of the renal cortex with minimal staining throughout the glomerulus and medulla. The immunoreactive signal for the receptor was predominantly localized to the apical aspect of the epithelium, suggesting that estrogenic signaling may arise from tubular fluid. Although circulating estrogen is predominantly bound to a steroid binding protein, significant concentrations of estradiol and various metabolites are filtered and present in the urine.17 We further find cortical GPR30 upregulation in the HS mRen2 female. It is not known whether salt directly affects GPR30 expression within the renal cortex or whether the increase in GPR30 reflects a compensatory mechanism of enhanced transcription or mRNA stabilization to oppose the damaging effects of salt within the kidney. Indeed, the salt-induced increase in cortical GPR30 may be protective under estrogen-intact conditions but result in an exacerbated response to estrogen loss in the mRen2 female and other animal models.8,9,18,19 In contrast, renal medullary GPR30 did not reflect the increase in mRNA induced by HS. Wang et al. also reported divergent regulation of GPR30 in the hamster ovary in response FSH and LH stimulation.20 The underlying mechanisms for these discrepancies remain to be resolved, and may involve translational regulation of GPR30.
We also assessed the subcellular localization of ERα, the predominant steroid receptor for estrogen in the kidney, using an antibody directed against the DNA binding domain which recognized all molecular forms of the receptor. G-1 does not bind ERα-66 or ERα-46; however, Kang et al. reported that G-1 activates the membrane-associated ERα-36 in the breast cancer cell line SK-BR-3.13 In cortical homogenates, ERα-36 was only detectable in the soluble fraction, was >50-fold lower than ERα-66, and was not altered with HS. In contrast, GPR30 protein was only detected in the membrane fraction, consistent with the protein motif of a G protein-coupled receptor. Although we cannot completely exclude ERα-36 from contributing to the renal actions of G-1, the low level of expression suggests that the renoprotective actions of G-1 in the salt-sensitive mRen2 female are primarily mediated by GPR30.
In the current model of salt-sensitivity, placement of the female mRen2 on a 4% sodium diet for 10 weeks induced a moderate degree of proteinuria and albuminuria despite a high systolic blood pressure (>200 mmHg). Nevertheless, two week G-1 treatment significantly reduced proteinuria without influencing systolic blood pressure, contrasting with our previous studies in the ovariectomized mRen2 on a NS diet in which the same dose of G-1 significantly lowers systolic blood pressure.6 Although telemetry can detect more discrete changes in blood pressure, the tail cuff method has provided consistent detection of changes in pressure to salt or estrogen-depletion in the mRen2, as well as following RAAS blockade.5,6,8,9 We recently demonstrated that G-1 dilates mesenteric vessels from intact and ovariectomized mRen2 to a similar extent as estradiol, and is comprised of both an NO-dependent and -independent component.7 In the current study, G-1 and estradiol relaxation was blunted by >60% in HS vessels, which may contribute to the salt-sensitive increase in blood pressure. Pretreatment of HS vessels with L-NAME completely abolished G-1 vasorelaxation, suggesting that the NO component of this response was still present in HS vessels. We have not identified the mechanism for loss of the G-1 relaxant effects in HS vessels, nor whether other vascular beds including renal resistance vessels also exhibit an attenuated GPR30 response. We speculate that increased salt intake may reduce the vascular expression of GPR30 or attenuate NO-independent signaling pathways involved in vasorelaxation; however, further studies are required to elucidate the influence of high salt on the vascular GPR30 system.
We previously showed that urinary 8-isoprostane is increased in ovariectomized mRen2 maintained on a NS diet and is subsequently reduced by estradiol treatment.5 In this study, urinary excretion of 8-isoprostane in HS females was 40-fold higher than that reported for NS females (120 vs. 3 pmol/day) and was comparable to that of salt-loaded Dahl salt-sensitive rats.21 G-1 administration reduced 8-isoprostane excretion and attenuated renal cortical 4-HNE staining. Although the mechanism for the anti-oxidant effects of GPR30 within the kidney of salt-sensitive mRen2 females remains to be established, estrogenic effects are generally associated with attenuation of oxidative stress. Sandberg and colleagues reported that NADPH oxidase activity in renal wrap hypertension is exacerbated by ovariectomy and reversed with estradiol replacement.22 In addition, estrogen activates several ROS-scavenging pathways including thioredoxin, thiol/disulfide oxidoreductase and glutathione peroxidase.23–25 In this regard, Broughton et al. recently showed that G-1 reduces NADPH-dependent oxidase activity in isolated carotid and intracranial arteries of normotensive Sprague-Dawley rats.26 Elucidation of the underlying mechanisms of GPR30 to attenuate ROS within the proximal tubules, as well as the tissue levels of G-1 that were achieved following chronic administration of the agonist await future studies.
Oxidative stress is linked to tubular damage within the kidney and may contribute to microvilli remodeling and subsequent proteinuria.27 In this study, urinary excretion of the lipid oxidation marker 8-isoprostane highly correlated with proteinuria, and chronic G-1 treatment significantly attenuated oxidative stress and protein excretion. Proteinuria results from glomerular damage and/or a decrease in tubular protein reabsorption mediated by megalin, a large 600 kDa receptor localized primarily to the luminal surface of epithelial cells.28 Megalin is responsible for the endocytosis of multiple ligands, and the marked proteinuria in megalin knockout mice emphasizes its vital role in the reabsorption of filtered proteins.15 We demonstrate co-localization of GPR30 and megalin on the apical surface of proximal tubules, suggesting that GPR30 may influence megalin-mediated protein reabsorption. HS decreased tubular megalin protein and gene expression, indicating regulation at the transcriptional level. While others have shown megalin downregulation in diabetic nephropathy, albumin overload, and in the hypertensive male mRen2(27) fed a NS diet, to our knowledge this is the first report of megalin regulation in response to salt-loading.27,29,30 G-1 increased megalin protein but not mRNA, potentially indicating a posttranscriptional mechanism. The protective effects of GPR30 may result from activation of the receptor-associated protein (RAP), a chaperone for megalin which promotes its localization to the brush border.31 Alternatively, GPR30 may decrease luminal megalin shedding and promote its retention in the tubule, perhaps through the regulation of various secretases or sheddases along the nephon.32,33
PERSPECTIVES
In conclusion, we show that the novel estrogen receptor GPR30 was upregulated within the renal cortex of salt-sensitive mRen2 females following high sodium intake and that chronic administration of the GPR30 agonist reduced renal damage independent of changes in systolic blood pressure. Recent clinical trials reveal that salt-sensitivity increases after menopause, and estradiol treatment reverses this effect.34,35 The HS-induced upregulation of renal GPR30 and the ability of G-1 to ameliorate renal damage further support a protective role for estrogen within the kidney.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
Funding provided by the National Heart Lung and Blood Institute, NIH (HL56973, HL51952, HL103592, HL103974), and the American Heart Association (0825515E) and unrestricted grants from the Unifi Corporation (Greensboro, NC) and the Farley-Hudson Foundation (Jacksonville, NC).
Footnotes
DISCLOSURES: None
References
- 1.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 2.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 3.Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2:207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- 4.Dennis MK, Burai R, Ramesh C, Petrie WK, Alcon SN, Nayak TK, Bologa CG, Leitao A, Brailoiu E, Deliu E, Dun NJ, Sklar LA, Hathaway HJ, Arterburn JB, Oprea TI, Prossnitz ER. In vivo effects of a GPR30 antagonist. Nat Chem Biol. 2009;5:421–427. doi: 10.1038/nchembio.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chappell MC, Gallagher PE, Averill DB, Ferrario CM, Brosnihan KB. Estrogen or the AT1 antagonist olmesartan reverses the development of profound hypertension in the congenic mRen(2).Lewis rat. Hypertension. 2003;42:781–786. doi: 10.1161/01.HYP.0000085210.66399.A3. [DOI] [PubMed] [Google Scholar]
- 6.Lindsey SH, Cohen JA, Brosnihan KB, Gallagher PE, Chappell MC. Chronic treatment with the G protein-coupled receptor 30 agonist G-1 decreases blood pressure in ovariectomized mRen2.Lewis rats. Endocrinology. 2009;150:3753–3758. doi: 10.1210/en.2008-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindsey SH, Carver KA, Prossnitz ER, Chappell MC. Vasodilation in response to the GPR30 agonist G-1 is not different from estradiol in the mRen2.Lewis female rat. J Cardiovasc Pharmacol. 2011;57:598–603. doi: 10.1097/FJC.0b013e3182135f1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chappell MC, Yamaleyeva LM, Westwood BM. Estrogen and salt sensitivity in the female mRen(2).Lewis rat. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1557–R1563. doi: 10.1152/ajpregu.00051.2006. [DOI] [PubMed] [Google Scholar]
- 9.Cohen JA, Lindsey SH, Pirro NT, Brosnihan KB, Gallagher PE, Chappell MC. Influence of estrogen depletion and salt loading on renal angiotensinogen expression in the mRen(2).Lewis strain. Am J Physiol Renal Physiol. 2010;299:F35–F42. doi: 10.1152/ajprenal.00138.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaleyeva LM, Chappell MC. Antifibrotic effects of the nNOS inhibitor L-VNIO are associated with reduced oxidative stress in the kidney. Hypertension. 2008;52:e116. [Google Scholar]
- 11.Jessup JA, Lindsey SH, Wang H, Chappell MC, Groban L. Attenuation of salt-induced cardiac remodeling and diastolic dysfunction by the GPER agonist G-1 in female mRen2.Lewis rats. PLoS One. 2010;5:e15433. doi: 10.1371/journal.pone.0015433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otto C, Rohde-Schulz B, Schwarz G, Fuchs I, Klewer M, Brittain D, Langer G, Bader B, Prelle K, Nubbemeyer R, Fritzemeier KH. G protein-coupled receptor 30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology. 2008;149:4846–4856. doi: 10.1210/en.2008-0269. [DOI] [PubMed] [Google Scholar]
- 13.Kang L, Zhang X, Xie Y, Tu Y, Wang D, Liu Z, Wang ZY. Involvement of estrogen receptor variant ER-alpha36, not GPR30, in nongenomic estrogen signaling. Mol Endocrinol. 2010;24:709–721. doi: 10.1210/me.2009-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. Identification, cloning, and expression of human estrogen receptor-alpha36, a novel variant of human estrogen receptor-alpha66. Biochem Biophys Res Commun. 2005;336:1023–1027. doi: 10.1016/j.bbrc.2005.08.226. [DOI] [PubMed] [Google Scholar]
- 15.Leheste JR, Rolinski B, Vorum H, Hilpert J, Nykjaer A, Jacobsen C, Aucouturier P, Moskaug JO, Otto A, Christensen EI, Willnow TE. Megalin knockout mice as an animal model of low molecular weight proteinuria. Am J Pathol. 1999;155:1361–1370. doi: 10.1016/S0002-9440(10)65238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hazell GG, Yao ST, Roper JA, Prossnitz ER, O’Carroll AM, Lolait SJ. Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J Endocrinol. 2009;202:223–236. doi: 10.1677/JOE-09-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann P, Hartmann MF, Remer T, Zimmer KP, Wudy SA. Profiling oestrogens and testosterone in human urine by stable isotope dilution/benchtop gas chromatography-mass spectrometry. Steroids. 2010;75:1067–1074. doi: 10.1016/j.steroids.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Crofton JT, Share L. Gonadal hormones modulate deoxycorticosterone-salt hypertension in male and female rats. Hypertension. 1997;29:494–499. doi: 10.1161/01.hyp.29.1.494. [DOI] [PubMed] [Google Scholar]
- 19.Harrison-Bernard LM, Schulman IH, Raij L. Postovariectomy hypertension is linked to increased renal AT1 receptor and salt sensitivity. Hypertension. 2003;42:1157–1163. doi: 10.1161/01.HYP.0000102180.13341.50. [DOI] [PubMed] [Google Scholar]
- 20.Wang C, Prossnitz ER, Roy SK. Expression of G protein-coupled receptor 30 in the hamster ovary: differential regulation by gonadotropins and steroid hormones. Endocrinology. 2007;148:4853–4864. doi: 10.1210/en.2007-0727. [DOI] [PubMed] [Google Scholar]
- 21.Meng S, Roberts LJ, Cason GW, Curry TS, Manning RD., Jr Superoxide dismutase and oxidative stress in Dahl salt-sensitive and -resistant rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R732–R738. doi: 10.1152/ajpregu.00346.2001. [DOI] [PubMed] [Google Scholar]
- 22.Ji H, Zheng W, Menini S, Pesce C, Kim J, Wu X, Mulroney SE, Sandberg K. Female protection in progressive renal disease is associated with estradiol attenuation of superoxide production. Gend Med. 2007;4:56–71. doi: 10.1016/s1550-8579(07)80009-x. [DOI] [PubMed] [Google Scholar]
- 23.Ebrahimian T, He Y, Schiffrin EL, Touyz RM. Differential regulation of thioredoxin and NAD(P)H oxidase by angiotensin II in male and female mice. J Hypertens. 2007;25:1263–1271. doi: 10.1097/HJH.0b013e3280acac60. [DOI] [PubMed] [Google Scholar]
- 24.Deroo BJ, Hewitt SC, Peddada SD, Korach KS. Estradiol regulates the thioredoxin antioxidant system in the mouse uterus. Endocrinology. 2004;145:5485–5492. doi: 10.1210/en.2004-0471. [DOI] [PubMed] [Google Scholar]
- 25.Borras C, Gambini J, Gomez-Cabrera MC, Sastre J, Pallardo FV, Mann GE, Vina J. 17beta-oestradiol up-regulates longevity-related, antioxidant enzyme expression via the ERK1 and ERK2[MAPK]/NFkappaB cascade. Aging Cell. 2005;4:113–118. doi: 10.1111/j.1474-9726.2005.00151.x. [DOI] [PubMed] [Google Scholar]
- 26.Broughton BR, Miller AA, Sobey CG. Endothelium-dependent relaxation by G protein-coupled receptor 30 agonists in rat carotid arteries. Am J Physiol Heart Circ Physiol. 2010;298:H1055–H1061. doi: 10.1152/ajpheart.00878.2009. [DOI] [PubMed] [Google Scholar]
- 27.Hayden MR, Chowdhury NA, Cooper SA, Whaley-Connell A, Habibi J, Witte L, Wiedmeyer C, Manrique CM, Lastra G, Ferrario C, Stump C, Sowers JR. Proximal tubule microvilli remodeling and albuminuria in the Ren2 transgenic rat. Am J Physiol Renal Physiol. 2007;292:F861–F867. doi: 10.1152/ajprenal.00252.2006. [DOI] [PubMed] [Google Scholar]
- 28.Christensen EI, Birn H. Megalin and cubilin: multifunctional endocytic receptors. Nat Rev Mol Cell Biol. 2002;3:256–266. doi: 10.1038/nrm778. [DOI] [PubMed] [Google Scholar]
- 29.Tojo A, Onozato ML, Ha H, Kurihara H, Sakai T, Goto A, Fujita T, Endou H. Reduced albumin reabsorption in the proximal tubule of early-stage diabetic rats. Histochem Cell Biol. 2001;116:269–276. doi: 10.1007/s004180100317. [DOI] [PubMed] [Google Scholar]
- 30.Caruso-Neves C, Pinheiro AA, Cai H, Souza-Menezes J, Guggino WB. PKB and megalin determine the survival or death of renal proximal tubule cells. Proc Natl Acad Sci U S A. 2006;103:18810–18815. doi: 10.1073/pnas.0605029103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birn H, Vorum H, Verroust PJ, Moestrup SK, Christensen EI. Receptor-associated protein is important for normal processing of megalin in kidney proximal tubules. J Am Soc Nephrol. 2000;11:191–202. doi: 10.1681/ASN.V112191. [DOI] [PubMed] [Google Scholar]
- 32.Nagai J, Tanaka H, Nakanishi N, Murakami T, Takano M. Role of megalin in renal handling of aminoglycosides. Am J Physiol Renal Physiol. 2001;281:F337–F344. doi: 10.1152/ajprenal.2001.281.2.F337. [DOI] [PubMed] [Google Scholar]
- 33.Zou Z, Chung B, Nguyen T, Mentone S, Thomson B, Biemesderfer D. Linking receptor-mediated endocytosis and cell signaling: evidence for regulated intramembrane proteolysis of megalin in proximal tubule. J Biol Chem. 2004;279:34302–34310. doi: 10.1074/jbc.M405608200. [DOI] [PubMed] [Google Scholar]
- 34.Yamori Y, Liu L, Ikeda K, Mizushima S, Nara Y, Simpson FO. Different associations of blood pressure with 24-hour urinary sodium excretion among pre- and post-menopausal women. WHO Cardiovascular Diseases and Alimentary Comparison (WHO-CARDIAC) Study. J Hypertens. 2001;19:535–538. doi: 10.1097/00004872-200103001-00003. [DOI] [PubMed] [Google Scholar]
- 35.Schulman IH, Aranda P, Raij L, Veronesi M, Aranda FJ, Martin R. Surgical menopause increases salt sensitivity of blood pressure. Hypertension. 2006;47:1168–1174. doi: 10.1161/01.HYP.0000218857.67880.75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.