Abstract
Hybrid materials based on polyvinylpyrrolidone (PVP) with silver nanoparticles (AgNps) were synthesized applying two different strategies based on thermal or chemical reduction of silver ions to silver nanoparticles using PVP as a stabilizer. The formation of spherical silver nanoparticles with diameter ranging from 9 to 16 nm was confirmed by TEM analysis. UV-vis and FTIR spectroscopy were also applied to confirm the successful formation of AgNps. The antibacterial activity of the synthesized AgNPs/PVP against etalon strains of three different groups of bacteria—Staphylococcus aureus (S. aureus; gram-positive bacteria), Escherichia coli (E. coli; gram-negative bacteria), Pseudomonas aeruginosa (P. aeruginosa; non-ferment gram-negative bacteria), as well as against spores of Bacillus subtilis (B. subtilis) was studied. AgNps/PVP were tested for the presence of fungicidal activity against different yeasts and mold such as Candida albicans, Candida krusei, Candida tropicalis, Candida glabrata, and Aspergillus brasiliensis. The hybrid materials showed a strong antimicrobial effect against the tested bacterial and fungal strains and therefore have potential applications in biotechnology and biomedical science.
Keywords: Silver nanoparticles, polyvinylpyrrolidone; Antimicrobial activity; Fungi, spores
Introduction
Silver in its different chemical forms has been known for a long time as an effective antimicrobial agent with high activity against bacteria, viruses, and fungi [1, 2]. Among them, silver nanoparticles (AgNps) are extensively studied in the recent years because of their possible usage as a strong bactericidal material with an application in biomedical field. Their pronounced antibacterial activity can be attributed to the well-developed surface and large surface area to volume ratio providing better contact with the microorganisms [3] and to the strong cytotoxicity to bacterial cells as a result of their interaction with the functional groups on the bacterial cell surface [4–6].
The most common method for synthesis of stable AgNps is the chemical reduction of silver ions in different stabilizers such as polymers and surfactants [7–11]. Among them, polyvinylpyrrolidone (PVP) attracts considerable attention because of its excellent chemical and physical properties making it a good material as a coating or as an additive to different materials. PVP acts not only as a stabilizing agent but also has an impact on the control of the reduction rate of the silver ions and the aggregation process of silver atoms [12, 13]. Beside chemical reduction method, other techniques for synthesis of AgNps stabilized by PVP as γ-irradiation synthesis [14–16] and laser ablation are also employed [17].
The aim of this study was to synthesize AgNps/PVP by thermal reduction of silver ions without using any reducing agent or by chemical reduction using NaBH4 as a reducing agent and to determine the presence of antimicrobial activity of AgNps/PVP against different groups of bacteria, spores of bacteria, yeasts, and molds. There are studies in the literature concerning the antibacterial activity of AgNps stabilized by PVP against Escherichia coli and Staphylococcus aureus [14, 18]. However, the antifungal effect of AgNps has received only scantly attention [19–21]. Therefore, an accent on the antifungal activity of AgNps/PVP obtained by two different methods against different yeasts and molds, such as Candida albicans, Candida krusei, Candida tropicalis, Candida glabrata, and Aspergillus brasiliensis was made. Moreover, there are no published data in the literature concerning the antifungal activity of silver nanoparticles obtained via thermal reduction and stabilized by PVP.
Materials and methods
Materials
PVP (Fluka, Mw = 24,000 mol−1), HNO3 (Riedel de Haën, Standard solution 2 mol/L), silver nitrate (AcrosOrganics) were used as received without further purification. Disks from chromatographic paper (d = 6 mm), Sabouraud-glucose agar, Soya-bean casein digest agar, Mueller-Hinton and Mueller-Hinton + GMB agar media were obtained via Bul Bio-NCIPD.
Methods
Transmission electron microscopy (TEM) images were recorded on a TEM (Philips CM 100). Samples were prepared by placing a drop of the solution on copper-coated grids. UV-vis absorption spectra were recorded at room temperature in the wavelength range from 200 to 800 nm using PerkinElmer Lambda UV-vis spectrophotometer. IR spectra were recorded in transmittance mode in the range from 400 to 4,000 cm−1 using PerkinElmer FTIR.
Synthesis of silver nanoparticles via thermal reduction
Five grams of PVP was dissolved in 250-ml deionized water under stirring at room temperature. Different amount of silver nitrate (0.025, 0.05, and 0.1 g) dissolved in 0.5 mL (water) was added dropwise under stirring to 25 mL of PVP (2%) thus achieving final concentrations of silver nitrate in the solution equal to: 0.98, 1.96, and 3.92 mg/mL. The prepared solutions were heated for 1 h at 100 °C in dark thus leading to the formation of AgNps. The color of the solution depending on the Ag concentration varied from dark yellow to brown. The obtained PVP/AgNps solutions were stable for 1 month. Similar thermal reduction of silver ions to silver nanoparticles stabilized via polyvinyl alcohol has recently been demonstrated [22].
Synthesis of silver nanoparticles via chemical reduction
The silver nanoparticles stabilized by PVP were prepared according to [11].
Five grams of PVP was dissolved in 250-ml deionized water under stirring at room temperature. Different amount of silver nitrate (0.025, 0.05, and 0.1 g) dissolved in 0.5 mL (water) was added dropwise under stirring to 20 mL of PVP (2%). As a reduction agent, NaBH4 was used. Freshly prepared aqueous solution of NaBH4 (5 mL) in different concentrations was added slowly under vigorous stirring to the solution. The samples were stirred for 1 h under stirring thus leading to formation of silver nanoparticles. The color of the solution depending on the Ag concentration varied from dark yellow to brown. The obtained PVP/AgNps solutions were stable for more than 3 months.
Measuring of the antimicrobial activity of the PVP/AgNps
The presence of bactericidal activity was determined using Disks Diffusion Method (DDM). The disks (diameter 6 mm, made from chromatographic paper) were impregnated with 5 μL AgNps/PVP solution on the leveled surface and left to dry at room temperature for 24 h. Then without any sterilization thus prepared disks were used for testing the biological properties of AgNps/PVP as described in CLSI M2-A9 standard, 2008 (Performance Standard for Antimicrobial Disk Susceptibility Tests) and used as a method for proving the antibacterial activity [22]. The results from the investigation are presented as an average value of the diameter of inhibition zones from two petri dishes for each strain.
For testing the presence of sporocidic activity pour agar method was used [23], wherein the impregnated with AgNps/PVP disks were tested against the spore of control microorganism Bacillus subtilis ATCC 6633.
The presence of biological activity of AgNps/PVP was detail accomplished after determination of minimal bactericidal concentration (MBC), minimal sporocidic concentration (MSC), and minimal fungicidal concentration (MFC).
To determine the MFC, standards of CLSI M26-A and M27-A2 [24, 25] were applied which were used in modified variant. Synthetic media RPMI 1640 with glutamine without bicarbonate and containing phenol rot as pH indicator was used. pH of the medium was 7.0 ± 0.1 at 25 °C. The incubation was performed as tubes were incubated at 35 °C for 48 h for yeasts. The accounting of the results was performed as follows: the presence of growth in the tubes with AgNps/PVP was visually compared with the present growth in the control tube without AgNps/PVP. Then control cultivation from each dilution at 48 h after their incubation were made by surface spread agar method on the petri dishes containing Sabouraud-glucose agar or Soya-bean casein digest agar. Influence of the accounting time at M27-A2 method for Candida, the final accounting point was at 48 h. For the most isolates, the difference between the accounting at 24 h or at 48 h is minimal and did not change the interpreting category; however, at 48-h accounting, the colonies were clearer, and the accounting of the results was reliable.
Results and discussion
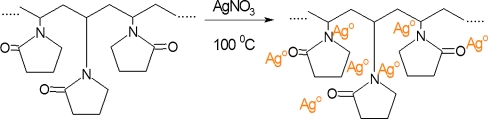
Antibacterial hybrid materials consisting of silver nanoparticles stabilized with PVP were synthesized using two different strategies. The first strategy was based on thermal reduction of silver ions in the presence of PVP as a stabilizer. In comparison to the other techniques for synthesis of silver nanoparticles, thermal reduction of Ag+ ions to Ag0 can be performed at elevated temperature without using reducing agents. This was achieved by adding a silver salt (AgNO3), the precursor for silver ions to PVP solution which lead to coordination of silver ions with −N or −O atoms from PVP [26], followed by boiling the PVP solution at 100 °C for 60 min. In this case, PVP acts as a stabilizer that protects the silver nanoparticles from agglomeration and has impact on the rate of reduction (Scheme 1) [12, 13].
Scheme 1.
Preparation of PVP/AgNps via thermal reduction
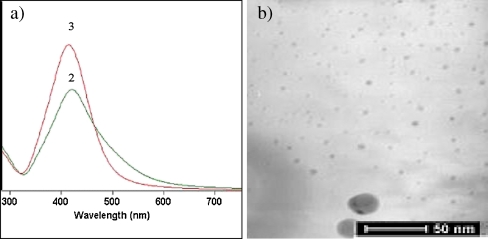
Under the performed thermal reduction, the formation of silver nanoparticles stabilized by PVP was achieved as evidenced by UV-vis spectroscopy and TEM analysis. The UV-vis analysis clearly indicated the formation of silver nanoparticles by the appearance of strong absorption bands at 420 nm (Fig. 1a). The observation of absorption bands at this wavelength demonstrates the formation of spherical AgNPs [19, 20, 27]. Moreover, the absorption of the silver nanoparticles becomes stronger and sharper with increasing the silver concentration, which is indicative for the preparation of silver nanoparticles with narrower particles size distribution.
Fig. 1.
a UV spectra of AgNps/PVP obtained via thermal reduction at concentration of silver precursor: 2 1.96 mg/mL and 3 3.92 mg/mL. b TEM of thermal-reduced silver nanoparticles stabilized by PVP at concentration of silver precursor 3.92 mg/mL
This state is confirmed by TEM analysis. Spherical silver nanoparticles with an average size of 6.4 ± 1.1 nm, which are homogeneously distributed in polymer matrix, were observed after the performed thermal reduction. However, some aggregates were also detected that can be a reason for the absorption at 420 nm and the lower stability of the silver nanoparticles in comparison to the silver nanoparticles prepared via chemical reduction (Fig. 1b).
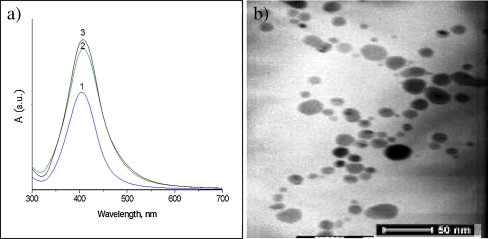
The second strategy consists in the well-known chemical reduction of silver ions. According to this strategy, silver nanoparticles were prepared by adding a strong reduction agent as NaBH4 to the PVP solution in the presence of AgNO3. UV-vis analysis clearly showed the presence of silver nanoparticles by appearance of absorption bands at 410 nm with peaks which intensities increased with silver concentration (Fig. 2a). TEM analysis also confirmed the formation of spherical nanoparticles with an average size of 13.8 ± 3.8 nm (Fig. 2b).
Fig. 2.
a UV spectra of PVP/AgNps obtained via chemical reduction at concentration of silver precursor: 1 0.98 mg/mL, 2 1.96 mg/mL, and 3 3.92 mg/mL. b TEM of silver nanoparticles obtained via chemical reduction of AgNO3 in the presence of NaBH4 at concentration of silver precursor 3.92 mg/mL
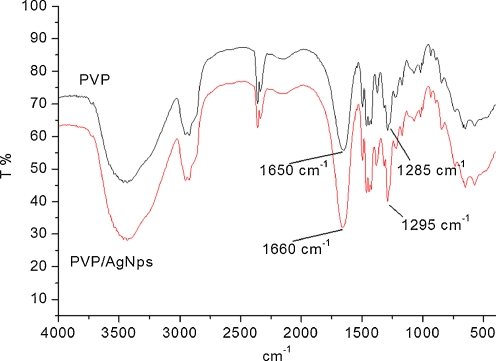
Thus, prepared AgNps stabilized with PVP were further characterized using FTIR spectroscopy. Figure 3 presented FTIR spectra of pure PVP and AgNps/PVP. All characteristic peaks for −CH2 и−CH groups of PVP at 2,923, 1,460, and 1,374 cm−1, respectively, were observed in both cases. The peak at 1,285–1,295 cm−1 typical for —C–N bond in PVP was also presented. In both cases, strong absorption peak, which is characteristic for carbonyl group of PVP at 1,650–1,660 cm−1, respectively, was observed. In the pure PVP, this peak was centered at 1,650 cm−1, and for AgNps/PVP, this peak was shifted toward 1,660 cm−1. The same observation was detected and for the peak typical for—C-N bond—slightly shifting from 1,285 to 1,295 cm−1. These shiftings were associated with the formation of coordination bonds between silver atoms and oxygen or nitrogen atoms arising from PVP units.
Fig. 3.
FTIR spectra of pure PVP and PVP/AgNps obtained via chemical reduction at concentration of silver precursor 1.96 mg/mL
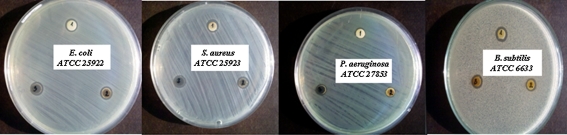
To determine the antibacterial activity by DDM, AgNps/PVP with three different concentrations of silver precursor in the range of 0.98, 1.96, and 3.92 mg/mL were tested against gram-positive bacteria (S. aureus), gram-negative bacteria (E. coli), non-ferment Gram-negative bacteria (P. aeruginosa), and spores of B. subtilis. It was found that all AgNPs stabilized with PVP exhibit bactericidal and sporocidic activity by the appearance of an inhibition zone in the range 7.5–8.5 mm (Fig. 4). Higher antibacterial activity was observed against both gram-negative E. coli and gram-positive S. aureus, as well as against spores of B. subtilis and lowest antibacterial activity was detected for P. aeruginosa. These results were in good agreement with our previous investigations on the antibacterial activity of hybrid material based on PVA/AgNps/TEOS thin films [22]. It was found that the increased silver concentration did not affect the size of the inhibition zones. Probably, this is due to the lower activity of the surface of the disks with which bacteria interact as a result of certain penetration of AgNps/PVP aqueous solution into the disks from chromatographic paper.
Fig. 4.
Antibacterial and sporocidic activity of AgNps/PVP prepared via chemical reduction against S. aureus, E. coli; P. aeruginosa and spore of B. subtilis at different silver concentrations: 1 0.98 mg/mL on the top, 2 1.96 mg/mL on the left, and 3 3.92 mg/mL on the right
To confirm the bactericidal properties, AgNps/PVP (initial concentration of silver precursor 0.98 mg/mL) was tested using macrodilution method. By this method, the MBC corresponding to the lowest concentration without detection of bacterial growth was determined for E. coli ATCC 25922, S. aureus ATCC 25923, and P. aeruginosa ATCC 27853 (Table 1, Fig. 5).
Table 1.
Antibacterial activity of silver nanoparticles stabilized by PVP
| Strain | Ag concentration (mg/mL) | |||||
|---|---|---|---|---|---|---|
| 0.49 | 0.24 | 0.12 | 0.06 | 0.03 | 0.015 | |
| S. aureus ATCC 25923 | − | − | − | − | − | − |
| E. coli ATCC 25922 | − | − | − | − | − | − |
| P. aeruginosa ATCC27853 | − | − | − | − | − | − |
| Spore of B.subtilis ATCC 6633 | + | + | + | + | + | + |
+ (positive) presence of bacterial growth, − (negative) lack of bacterial growth
Fig. 5.
Determination of MBC of AgNps/PVP against aS. aureus, bE. coli, cP. aeruginosa
These results clearly indicated the strong bactericidal activity demonstrated by the lack of any bacterial growth even at lowest silver concentration of 0.015 mg/mL (Table 1, Fig. 5), and this concentration was accepted as MBC for all tested control strains.
These results are in a good agreement with our previous investigations on PVA/PAN-based micelles with embedded silver nanoparticles tested by the same method where the MBC for S. aureus was 2.88 ± 1.75 μg/mL, and for E. coli and P. aeruginosa, MBC was even lower 0.36 ± 0.08 μg/mL [28].
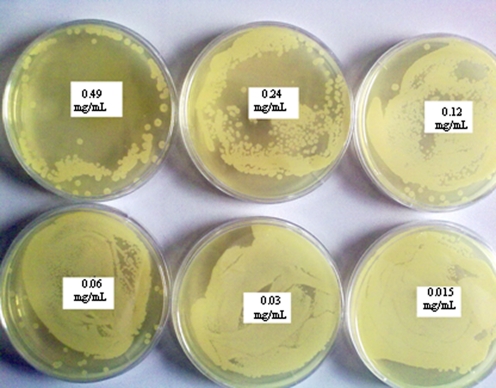
A bacterial growth of B. subtilis was detected in all cases, and therefore, the minimal sporocidic concentration was not established. However, the presence of concentration-dependent sporocidic activity was demonstrated by this method (Fig. 6). The lack of any sporocidic activity of B. subtilis indicated that silver nanoparticles with Ag concentrations ranging from 0.49 to 0.015 mg/mL are not effective antibacterial agent for spores of B. subtilis (Table 1, Fig. 6).
Fig. 6.
Determination of MSC of PVP/AgNps prepared via chemical reduction against spores of Bacillus subtilis ATCC 6633
To examine the antifungal activity of the thermally and non-thermally prepared AgNps stabilized by PVP, the following yeasts and mold C. albicans, C. tropicalis, C. krusei, C. glabrata, and A. brasiliensis were tested. The effect of the type of the material on their antifungal activity was achieved by cultivation of diluted PVP/AgNps solutions with added standardized control microorganisms on petri dishes containing Sabouraud-glucose agar. After incubation at 35 °C, the plates were checked after 48 and 72 h for any growth, and all visible colonies were counted. The presence or the lack of growth was noted as “+” and “−”, respectively. It was found that after 48 h of cultivation, silver nanoparticles prepared via chemical reduction showed lack of yeast growth only for C. krusei and C. tropicalis. The rest of the fungi showed the presence of growth (Table 2). These results allowed determining the MFC for C. tropicalis, which was ≥0.50 mg/mL and for C. krusei ≥0.03 mg/mL.
Table 2.
Fungicidal activity of PVP/AgNps obtained via chemical reduction after 48 h of incubation
| Control Strain | 0.98 mg/mL | 0.50 mg/mL | 0.25 mg/mL | 0.12 mg/mL | 0.06 mg/mL | 0.03 mg/mL | MFC mg/ml |
|---|---|---|---|---|---|---|---|
| 1. | 2. | 3. | 4. | 5. | 6. | ||
| C. albicans | + | + | + | + | + | + | Not determined |
| C. tropicalis | − | − | + | + | + | + | ≥0.5 |
| C. glabrata | + | + | + | + | + | + | Not determined |
| C. krusei | − | − | − | − | − | − | ≥0.03 |
| A. brasiliensis | + | + | + | + | + | + | Not determined |
For C. albicans and C. glabrata, the used silver concentration did not influence their growth, and therefore, their MFC was not determined. These data were confirmed after accounting the results at 72-h incubation. For A. brasiliensis, because of the partial growth in each petri dish, MFC was not determined, but the presence of concentration-dependent activity was observed. This was also visualized with the presence of cotton-like formations in all tests tubes of the twofold dilutions which were most pronounced after 72 h of the experiment. The observed difference in the minimal bactericidal and fungicidal concentrations of the silver nanoparticles is probably derived from the different structures of bacterial and yeast cell since the eukaryotic organisms posses better organized cell structure [29].
For comparison, thermally prepared silver nanoparticles stabilized by PVP showed higher fungicidal activity (Table 3). Cultivations of the tested dilutions were made using surface spread agar method. The results were accounted after 48 h of incubation for C. tropicalis, C. albicans, C. krusei, and C. glabrata. For A. brasiliensis, the results were accounted after 72-h incubation.
Table 3.
Fungicidal activity of AgNps obtained via thermal reduction after 48 h of incubation
| Control strain | 1.96 mg/mL | 0.98 mg/mL | 0.5 mg/mL | 0.25 mg/mL | 0.12 mg/mL | 0.06 mg/mL | 0.03 mg/mL | MFC mg/mL |
|---|---|---|---|---|---|---|---|---|
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | ||
| C. albicans | − | − | − | + | + | + | + | 0.5 |
| C. glabrata | − | + (1 cfu) | + (3 cfu) | + | + | + | + | 0.98 ± 0.5 |
| C. tropicalis | − | − | − | − | − | − | − | <0.03 |
| C. krusei | − | − | − | − | − | − | − | <0.03 |
| A. brasiliensis | − | + | + | + | + | + | + | 1.96 |
The highest antifungal activity for thermally prepared silver nanoparticles is detected against C. krusei and C. tropicalis with MFC 0.03 mg/ml. For C. albicans, MFC was 0.5 mg/mL; for C. glabrata, −0.98 ± 0.5 and for A. brasiliensis −1.96 mg/mL. Apparently, thermally prepared silver nanoparticles showed higher antifungicidal activity in comparison to AgNps prepared via chemical reduction. However, both types of silver nanopartices are more sensitive toward C. krusei in comparison to the rest of the fungi. The higher antifungicidal activity of thermally prepared AgNps is probably due to their smaller size as seen by TEM in comparison to the silver nanoparticles prepared via chemical reduction, thus providing better contact with the environment. Moreover, thermal reduction of silver precursor in PVP solution allows the complete transformation of Ag+ to Ag0 without producing of unwanted by-products of the reducing agent, which can affect their antimicrobial properties.
Conclusions
Silver nanoparticles stabilized by PVP were prepared using thermal and chemical reduction, and their formation was proven by UV-vis spectroscopy and TEM analysis.
The prepared via chemical reduction AgNps stabilized by PVP confirmed the presence of bactericidal activity by determining the MBC of 0.015 mg/mL. The pour agar method was used to establish the sporocidic properties, but the MSC was not determined when macrodilution method was applied. The fungicidal properties of AgNps/PVP were examined via macrodilution method. This method allowed determining the MFC by testing of AgNps/PVP obtained via chemical and thermal reduction. It was established that the MFC value for yeasts and mold by testing the chemical reduced silver nitrate could be determined only for C. tropicalis, which was ≥0.50 mg/mL, and for C. krusei - ≥0.03 mg/mL. For the first time, antifungal activity of thermally prepared AgNps stabilized by PVP was tested. MFC for all tested fungi was determined; as for C. krusei, MFC was ≥0.03; for C. tropicalis was lower ≥0.03 mg/mL, for C. albicans −0.5 mg/mL, for C. glabrata −0.98 mg/mL ± 0.5 and for A. brasiliensis −1.96 mg/mL, respectively. These results demonstrated that the way of preparation of silver nanoparticles has significant impact on their antifungal activity and indicated that AgNps stabilizing by PVP could find potential practical application due to the presence of bactericidal, sporocidal, and fungicidal activity.
Acknowledgments
This work was funded and supported by project no. 10772 of the University of Chemical Technology and Metallurgy Science Fund “Research Investigations”.
References
- 1.Sharma VK, Yngard RA, Lin Y. Adv Colloid Interface Sci. 2009;145:83–96. doi: 10.1016/j.cis.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Sondi I, Salopek-Sondi B. J Colloid Interface Sci. 2004;275:177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 3.Rai M, Yadav A, Gade A. Biotechnol Adv. 2009;27:76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Ray S, Mohan R, Singh JK, Samantaray MK, Shaikh MM, Panda D, Ghosh P. J Am Chem Soc. 2007;129:15042–15053. doi: 10.1021/ja075889z. [DOI] [PubMed] [Google Scholar]
- 5.Lee HY, Park HK, Lee YM, Kim K, Park SB. Chem Commun. 2007;28:2959–2961. doi: 10.1039/b703034g. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Peng H, Huang W, Zhou Y, Yan D. J Colloid Interface Sci. 2008;325:371–376. doi: 10.1016/j.jcis.2008.05.063. [DOI] [PubMed] [Google Scholar]
- 7.Panacek A, Kvitek L, Prucek R, Kolar M, Vecerova R, Pizurova N, Sharma VK, Nevecna T, Zboril R. J Phys Chem B. 2006;110:16248–16253. doi: 10.1021/jp063826h. [DOI] [PubMed] [Google Scholar]
- 8.Pal S, Song JM. Appl Environ Microbiol. 2007;73:1712–1720. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kvitek L, Panacek A, Soukupova J, Kolar M, Vecerova R, Prusek R, Holecova M, Zboril R. J Phys Chem C. 2008;112:5825. doi: 10.1021/jp711616v. [DOI] [Google Scholar]
- 10.Wang H, Qiao X, Chen J, Ding Sh. Colloid Surfaces Physicochem Eng Aspect. 2005;256:111–115. doi: 10.1016/j.colsurfa.2004.12.058. [DOI] [Google Scholar]
- 11.Zielinska A, Skwarek E, Zaleska A, Gazda M, Hupka J. Procedia Chem. 2009;1:1560–1566. doi: 10.1016/j.proche.2009.11.004. [DOI] [Google Scholar]
- 12.Huang HH, Ni XP, Loy GL, Chew CH, Tan KL, Loh FC, Deng JF, Xu GQ. Langmuir. 1996;12:909–912. doi: 10.1021/la950435d. [DOI] [Google Scholar]
- 13.Carotenuto G. Appl Organomet Chem. 2001;15:344. doi: 10.1002/aoc.165. [DOI] [Google Scholar]
- 14.Sheikh N, Akhavan A, Kassaee MZ. Physica E. 2009;42:132–135. doi: 10.1016/j.physe.2009.09.013. [DOI] [Google Scholar]
- 15.Li T, Park HG, Choi S-H. Mater Chem Phys. 2007;105:325–330. doi: 10.1016/j.matchemphys.2007.04.069. [DOI] [Google Scholar]
- 16.Shin HS, Yang HJ, Kim SB, Lee MS. J Colloid Interface Sci. 2004;274:89–94. doi: 10.1016/j.jcis.2004.02.084. [DOI] [PubMed] [Google Scholar]
- 17.Tsuji T, Thang D-H, Okazaki Y, Nakanishi M, Tsuboi Y, Tsuji M. Appl Surf Sci. 2008;254:5224–5230. doi: 10.1016/j.apsusc.2008.02.048. [DOI] [Google Scholar]
- 18.Cho K-H, Park J-E, Osaka T, Park S-G. Elecrochim Acta. 2005;51:956–960. doi: 10.1016/j.electacta.2005.04.071. [DOI] [Google Scholar]
- 19.Falletta E, Bonini M, Fratini E, Lo Nostro A, Pesavento G, Becheri A, et al. J Phys Chem. 2008;112:11758–11766. [Google Scholar]
- 20.Roe D, Karandikar B, Bonn-Savage N, Gibbins B, Roullet JB. J Antimicrob Chemother. 2008;61:869–876. doi: 10.1093/jac/dkn034. [DOI] [PubMed] [Google Scholar]
- 21.Zeng F, Hou C, Wu SZ, Liu XX, Tong Z, Yu SN. Nanotechnology. 2007;18:1–8. doi: 10.1088/0957-4484/18/26/265704. [DOI] [PubMed] [Google Scholar]
- 22.Bryaskova R, Pencheva D, Kale GM, Lad U, Kantardjiev T. J Colloid Interface Sci. 2010;349:77–85. doi: 10.1016/j.jcis.2010.04.091. [DOI] [PubMed] [Google Scholar]
- 23.Pencheva D, Bryaskova R, Kantardjiev T. Clin Microbiol Infect. 2010;16(Suppl.2):S670. [Google Scholar]
- 24.CLSI/NCCLS M26-A - Methods for determining bactericidal activity of antimicrobial agents; Approved Guideline, September (1999).
- 25.CLSI/NCCLS M27- A2 - Reference method for broth dilution antifungal susceptibility testing of yeasts; Approved Standard—Second Edition
- 26.Wiley B, Sun Y, Mayers B, Xia Y. Chem Eur J. 2005;11:454–463. doi: 10.1002/chem.200400927. [DOI] [PubMed] [Google Scholar]
- 27.Pal S, Tak YK, Song JM. Appl Environ Microbiol. 2007;27:1712–1720. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bryaskova R, Pencheva D, Kyulavska M, Bozukova D, Debuigne A, Detrembleur C. J Colloid Interface Sci. 2010;344:424–428. doi: 10.1016/j.jcis.2009.12.040. [DOI] [PubMed] [Google Scholar]
- 29.Panacek A, Kolar M, Vecerova R, Prucek R, Soukupova J, Krystof V, Hamal P, Zboril R, Kvitek L. Biomaterials. 2009;30:6333–6340. doi: 10.1016/j.biomaterials.2009.07.065. [DOI] [PubMed] [Google Scholar]