Abstract
Skin protects itself against infection through a variety of mechanisms. Antimicrobial peptides (AMPs) are major contributors to cutaneous innate immunity, and this system, combined with the unique ionic, lipid and physical barrier of the epidermis is the first line defense against invading pathogens. However, recent studies have revealed that our skin’s innate immune system is not solely of human origin. Staphylococcus epidermidis, a major constituent of the normal microflora on healthy human skin, acts as a barrier against colonization of potentially pathogenic microbes and against overgrowth of already present opportunistic pathogens. Our resident commensal microbes produce their own AMPs, act to enhance the normal production of AMPs by keratinocytes, and are beneficial to maintaining inflammatory homeostasis by suppressing excess cytokine release after minor epidermal injury. These observations indicate that the normal human skin microflora protects skin via various modes of action, a conclusion supported by many lines of evidence associating diseases such as acne, atopic dermatitis, psoriasis and rosacea with an imbalance of the microflora even in the absence of classical infection. This review highlights recent observations on the importance of innate immune systems and the relationship with the normal skin microflora to maintain healthy skin.
Antimicrobial Peptides in Skin Innate Immunity
The skin provides the first line of immune defense against invading pathogens and both passively and actively protects the surface through various modes of action. One critical mechanism for self-protection is the innate production of the epidermis by antimicrobial peptides (AMPs). These molecules are produced in all organs by a variety of cell types and are major contributors to immune defense (Nizet et al., 2001; Zasloff, 2002). In human skin, the main sources of AMPs are keratinocytes, mast cells, neutrophils and sebocytes. Because of their direct antimicrobial action, one mechanism of defense by AMPs is that the secretion or release of these peptides provides innate antibiotic-like action against infectious pathogens. AMPs such as the cathelicidin LL-37 also function by triggering inflammatory cell recruitment and cytokine release. AMPs can be produced constitutively, or actively induced upon sensing of danger, and this system often involves signaling mediated by pattern recognition receptors such as Toll-like receptors (TLRs) or responses to pro-inflammatory cytokines (Lai and Gallo, 2009).
Keratinocytes are the main source of AMPs in normal human skin but once inflamed the recruited leukocytes contribute the majority of antimicrobial activity. There are many AMPs in human skin but cathelicidins and β-defensins are the most well characterized (Lai and Gallo, 2009; Wiesner and Vilcinskas, 2010). Human β-defensin (hBD)-1 is constitutively expressed in keratinocytes, but exhibits only minor antibiotic killing activity in comparison with other defensins (Yadava et al., 2006; Zaalouk et al., 2004). More recently, the reduced form of hBD-1 has been shown to become a potent antimicrobial peptide, of which reduction is catalyzed by thioredoxin expressed in the epidermis (Schroeder et al., 2011). This suggests that the redox regulation is crucial for the innate immune protection by hBD-1. The expression levels of hBD-2, hBD-3, and human cathelicidin in keratinocytes are very low at the steady state and typically upregulated during infection, inflammation and wounding then accumulate in the skin (Froy, 2005; Gallo et al., 2002). This suggests that the increase in AMP is a secondary response to limit the severity of clinical symptoms when the primary line of defense (constitutive expression of AMPs) fails. Human keratinocytes also express proteins with antimicrobial activity that include ribonucleotidases (RNases) 1, 4, 5 and 7. Of these, RNases 5 and 7 exhibit antimicrobial activity against many pathogenic microorganisms independent of the RNase activity, which is due to pore formation and disruption of the bacterial membrane (Abtin et al., 2009; Huang et al., 2007; Zanger et al., 2009). The antimicrobial activity of those RNases is inhibited by RNase inhibitor protein expressed in epidermal keratinocytes, and, in turn, activated when the RNase inhibitor is cleaved by stratum corneum serine proteases (Abtin et al., 2009). An antimicrobial heterodimeric complex, S100A8 / S100A9 (calprotectin), is induced in epidermal keratinocytes during Gram-negative bacteria infection and sensing of bacterial flagellin by TLR5 is critical for the regulation of calprotectin (Abtin et al., 2010). In other aspects of role of AMPs in innate immunity, altered expression of AMPs can play a role in the pathogenesis of chronic inflammatory skin disorders, such as atopic dermatitis, psoriasis and rosacea (see below) (Yamasaki and Gallo, 2008).
Recent evidence has indicated that human sebaceous glands also contribute to skin immune defense by releasing AMPs. Cathelicidin, hBD-2 and antimicrobial histone H4 are detected in the cultured human sebocytes, and their expression levels are upregulated in the presence of Gram positive bacteria (Lee et al., 2009; Lee et al., 2008; Nagy et al., 2006). Large amounts of S100A7 accumulate in the epidermis of sebaceous skin as well as sebaceous glands, suggesting that psoriasin is possibly secreted along with sebum lipids (Glaser et al., 2005). In addition to the AMPs, other molecules also add to the antimicrobial barrier formed by sebocytes. Free fatty acids (FFAs) are ubiquitously found on the surface of human skin and are the most predominant components in human sebum. FFAs are produced by lipases that are secreted from the commensal bacterial flora such as Propionibacterium acnes (P. acnes) and Staphylococcus epidermidis (S. epidermidis) by hydrolyzing sebum triacylglycerides secreted from sebaceous glands(Gotz et al., 1998; Holland et al., 1981; Marples et al., 1971), but can also be produced by sebocytes without the presence of bacteria (Fujie et al., 1996; Zouboulis et al., 1999). Because some middle- to long-chain FFAs (C8–C18) have shown antibacterial activity against a broad range of Gram-positive bacteria (Drake et al., 2008; Nakatsuji et al., 2009; Skrivanova et al., 2005), they have been thought to be responsible for at least a part of the direct antimicrobial activity of the skin surface against pathogen colonization and infection. In addition to the direct antimicrobial activity, lauric acid (C12:0), palmitic acid (16:0) and oleic acid (C18:1, cis-9), representative sebum FFAs, enhance the skin innate immune defense by inducing hBD-2 in the human sebocytes (Nakatsuji et al., 2010). Therefore, the sebaceous glands may play a very significant role in skin in innate immunity by providing antimicrobial agents to the external skin surface.
Eccrine glands are also known as an important supplier of AMPs to the epidermal surface. Dermcidin is an AMP constitutively expressed as a small precursor protein in eccrine sweat glands and secreted into sweat where active AMPs are proteolytically generated (Schittek et al., 2001). Although dermcidin-derived AMPs do not induce pore formation on the bacterial cell membrane in contrast to the mode of action of classic AMPs, they bind to unidentified targets of bacterial cell envelope, resulting in reduced RNA and protein synthesis (Senyurek et al., 2009).
Antimicrobial peptides produced by skin commensal bacteria
A surprising recent revelation is that the AMPs that occupy the surface of our skin are made not only by our own cells, but also in prokaryotic organisms that inhabit our epidermis. A large number of Gram positive bacteria such as Lactococcus, Streptococcus and Streptomyces species have been known to produce factors to inhibit other bacteria (Bastos et al., 2009). Proteinaceous factors produced by bacteria with bactericidal activity against the growth of similar or closely related bacterial strains are called bacteriocins. S. epidermidis, the dominant commensal bacterium found in the skin microflora, produces various types of bacteriocins. Most of these peptides are encoded in plasmids. Epidermin, Pep5 and epilancin K7 are the most characterized bacteriocins isolated from S. epidermidis (Bastos et al., 2009). Because these peptides contain the thioether amino acids lanthionine and/or methyllanthionine, they are classified as lantibiotics. These modified amino acids form three ring structures, which are important for their bactericidal activity. Their bactericidal action is thought to predominantly involve the pore formation on bacterial cell membranes, which is similar to mammalian AMPs like defensins and cathelicidins. Because of their potential to kill pathogens in vitro, these bacteriocins may possess the capacity to provide antimicrobial protection against pathogens on the skin surface.
In spite of a number of studies regarding antimicrobial activity of bacteriocins in vitro, to our knowledge, little effort has been made to demonstrate how bacteriocin-producing bacteria contribute skin innate immune defense. Our group has first proposed that the unique peptides phenol-soluble modulin (PSM)γ and PSMδ produced by S. epidermidis could be beneficial to the host and thus serve as additional AMPs on normal skin surface (Cogen et al., 2010b). These peptides possess two opposing sides organized by their hydrophobic and cationic amino acids with a five-amino acid periodicity, a strategy for action of both a hydrophilic and hydrophobic molecule that resembles that of classic AMPs such as LL-37. Classic AMPs, such as LL-37 and hBDs are also amphipathic molecules that possess clusters of positively charged and hydrophobic charged amino acid chains. This feature is thought to allow them to interact with negatively charged phospholipid head groups and hydrophobic fatty acid chains of microbial membranes, resulting in pore formation on the microbial membrane and releases cytosol components (Glaser et al., 2005; Wimley, 2010). In fact, PSMs caused membrane leakage and membrane perturbation in bacteria, suggesting that these peptides function in a similar mechanistic manner as that of innate cutaneous AMPs. These peptides selectively exhibited bactericidal activity against skin pathogens, such as Staphylococcus aureus (S. aureus), Group A Streptococcus (GAS) and Escherichia coli, whereas they are not active against S. epidermidis. Moreover, inoculating PSMs on the mouse skin surface reduced GAS but not the survival of S. epidermidis. This selective activity is likely to be an important part of a normal microbial defense strategy against colonization. In addition, PSMs will enhance the antimicrobial activity of the host AMPs, such as LL-37, CRAMP and hBDs. More recently, it has been demonstrated that PSMα1 and PSMα 2 isolated from methicillin-resistant S. aureus exhibit only a weak antimicrobial activity, but their antimicrobial activities are in turn considerably enhanced when their N-terminal is proteolytically cleaved, indicating that the N-terminal can act as a negative regulator of antimicrobial activity (Joo et al., 2011). However, it remains unclear how the PSMs are proteolytically activated. In addition, S. aureus PSMs show chemotactic activity for neutrophils through formyl peptide receptor 2 and then induce lysis of the infiltrated neutrophils presumably by a local high concentration of PSMs (micro-molar order), which may contribute to the pathogenicity of S. aureus (Kretschmer et al., 2010; Wang et al., 2007). However, S. epidermidis PSMs do not induce lysis of neutrophils at micro-molar concentrations, but enhance the capacity of their bacteria killing activity as described below (Cogen et al., 2010a). In addition, proinflammatory properties of S. epidermidis PSMs have been reported (Mehlin et al., 1999; Vuong et al., 2004). Thus, staphylococcus PSMs can play roles in both innate immune defense and pathogenesis. Similarly, host AMPs such as LL-37 can also lead to disease when abnormally expressed (described below). It is likely that S. epidermidis PSMs are beneficial when present on the surface of intact skin, but become potentially pathogenic to the host when the interaction between commensals and host innate immunity is imbalanced.
The AMPs released by resident microbes are not a minor component of the epidermal antimicrobial milieu. S. epidermidis PSMγ was abundantly detectable in the normal human epidermis, hair follicle and sparsely in the dermis (Cogen et al., 2010a). PSMs in nano-molar amounts decreased GAS survival on normal human skin by 2–3 log abundance. PSMγ added to the freshly isolated human neutrophils could also be incorporated into the neutrophil extracellular traps (NETs) and facilitated eradication of potentially dangerous bacteria. Incorporated PSMγ into the NETs was colocalized with cathelicidin AMP endogenously released from the cell. Furthermore, addition of PSMγ to cultured neutrophils induced their NET formation. These observations strongly support the concept that S. epidermidis contributes actively to the skin innate immune defense by supplying additional AMPs that act together with the host-derived AMPs.
More recently, it has been demonstrated that the presence of S. epidermidis on the nasal cavity is clinically relevant. The rate of nasal colonization by S. aureus was significantly lower in individuals in the presence of inhibitory S. epidermidis strains that are capable to inhibit biofilm formation by S. aureus (Iwase et al., 2010). These inhibitory S. epidermidis strains secreted a S. epidermidis serine protease that inhibits biofilm formation and destroys biofilms formed by S. aureus. Furthermore, inoculation of inhibitory S. epidermidis in the human nasal cavity eliminated S. aureus colonization. In addition, a thiolactone-containing peptide and its derivatives produced by S. epidermidis blocks the S. aureus agr quorum-sensing system which controls production of various virulence factors (Otto et al., 1999). Because S. epidermidis is the most prevalent of cutaneous resident microflora and S. aureus is a transient resident in healthy skin, such an intraspecies competition may be involved in maintaining the homeostasis of skin microflora.
Microbial symbiosis and immunity: Roles of microbes in skin homeostasis
The human body engages in symbiotic associations with vast and complex microbial communities. The major habitats for human indigenous microbiota are the oral cavity, oropharynx, gastrointestinal tract, vagina, and skin. Recent studies demonstrated that symbiotic factors produced by intestinal commensal bacteria beneficially modulate the host immune systems, decreasing a risk of autoimmune diseases and/ or inflammation induced by infections. Mazmanian et al demonstrated that Polysaccharide A produced by Bacteroides fragilis suppressed pro-inflammatory interleukin (IL)-17 production by intestinal immune cells exposed to pathogenic bacteria, Helicobacter pylori, through a functional requirement for IL-10-producing CD4+ T cells (Mazmanian et al., 2008). Maslowski et al. also demonstrated short-chain fatty acids, which are produced by fermentation of dietary fiber by intestinal microbiota, suppressed inflammation by reducing activity and recruitment of neutrophils through binding to G-protein-coupled receptor 43 (GPR 43, also known as free fatty acid receptor 2) on the cell surface (Maslowski et al., 2009). The GPR43 signaling suppressed colitis, arthritis and allergic airway inflammation on the mouse models.
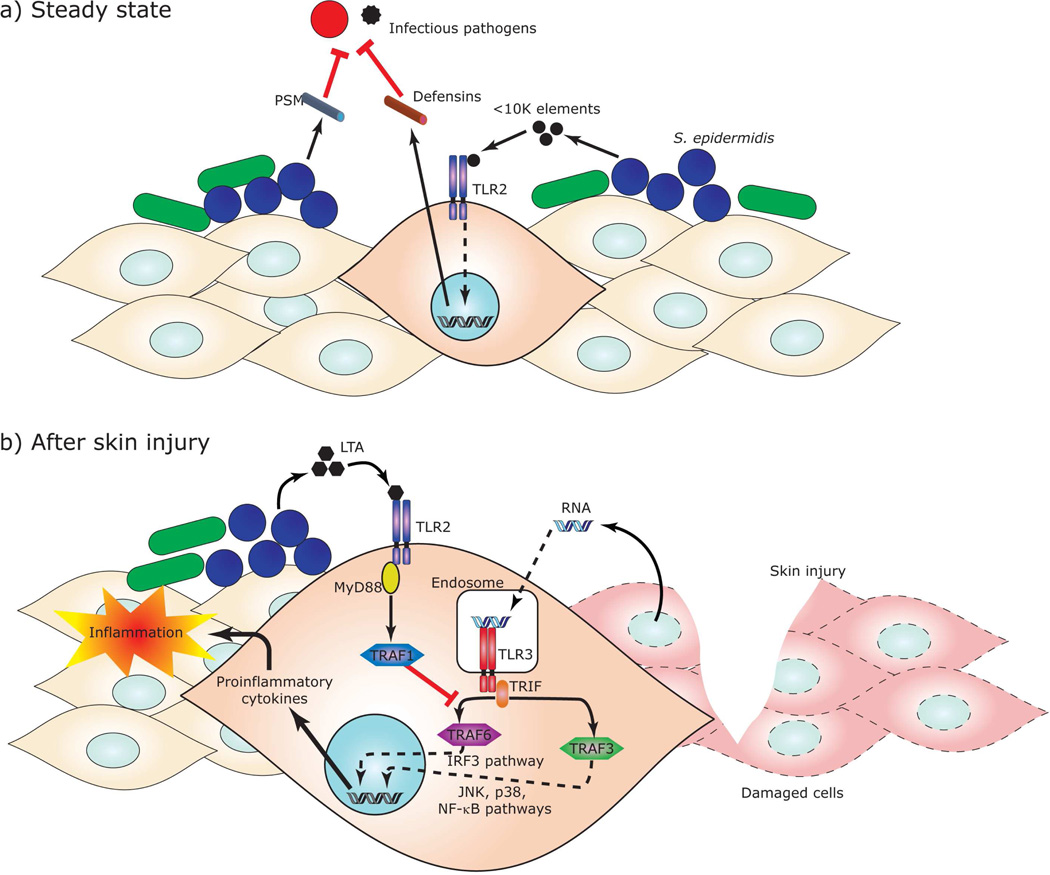
In the skin microenvironment much less research has been directed towards determining whether the resident microbiota of normal human skin, or specific elements from commensal bacteria, influence cutaneous immune systems and host-pathogen interactions. Our group has demonstrated for the first time a mutually beneficial relationship between S. epidermidis and keratinocyte inflammatory responses. In this work it was shown that a unique lipoteichoic acid (LTA) produced by S. epidermidis inhibits uncontrolled skin inflammation during skin injury, which in this case delays wound repair (Lai et al., 2009). After skin injury, the host RNA from damaged cells activates TLR3 in the keratinocytes, which accounts for the release of inflammatory cytokines, resulting in inflammation. Staphylococcal LTA inhibits both inflammatory cytokine release from keratinocytes and inflammation triggered by injury through a TLR2-dependent mechanism (Figure 1). Other mutually beneficial interactions also appear to exist between keratinocytes and S. epidermidis. For example, a small molecule of <10 kDa secreted from S. epidermidis increased expression of hBDs in murine skin or human keratinocytes through TLR2 signaling (Lai et al., 2010). Similarly, co-cultivation of differentiated human primary keratinocytes with live S. epidermidis, but not heat-inactivated bacteria, enhances production of hBD2, hBD3 and RNase7. In addition, the keratinocytes pre-incubated with S. epidermidis-conditioned media strongly enhances AMP production induced by S. aureus, suggesting that S. epidermidis sensitizes human keratinocytes toward pathogenic bacteria and amplifies the innate immune response (Wanke et al., 2011). Thus, the recognition of staphylococcal molecule by TLR2 may be involved in the steady-state production of AMPs in keratinocytes and enhances resistance to infection by bacterial fungal and viral pathogens. Contrary to these reports, live S. epidermidis suppresses AMP expressions in a human living skin equivalent model in vitro for a 24-hour incubation when the inoculated bacteria keep on growing (Holland et al., 2009; Holland et al., 2008). Thus, S. epidermidis may also have capacity to evade innate immune defense during growth phase as it can become an opportunistic pathogen. The balance of evading and stimulating innate immune defense has important consequences for skin homeostasis.
Figure 1. Molecular interactions of microbial symbiosis in skin innate immune systems.
Factors produced by skin commensal bacteria modulate the skin immune system. (a) In a steady state, S. epidermidis, a major constituent of the normal microflora on healthy human skin, produce antimicrobial peptides, PSMδ and PSMγ, which act as a barrier against colonization of potentially pathogenic microbes. In addition, S. epidermidis secretes a small molecule which increases expression of defensins in murine skin or human keratinocytes through TLR2 signaling. (b) After skin injury, host RNA from damaged cells activates TLR3 in keratinocytes. If uncontrolled inflammation occurs this can result in delayed wound healing. Staphylococcal LTA inhibits excess inflammatory cytokine release from keratinocytes and inflammation through a TLR2-dependent mechanism. IFR, IFN-regulatory factor; JNK, C-Jun kinase; MyD88, Myeloid differentiation primary response gene 88; NF-kB, nuclear factor kappa-B; TRAF, TNF receptor-associated factor; TRIF, TIR-domain-containing adapter-inducing interferon-β.
Skin disorders associated with dysbiosis
Because the human skin provides environmental niches that greatly differ in humidity, temperature, oiliness and aerobicity, and these influence the survival of resident microbial flora, the skin-resident microflora are highly diverse. Culture based techniques performed in seminal studies over 30 years ago clearly identified some of the organisms that survive different skin microenvironments (Joo et al., 2011). Newer 16S rRNA gene sequencing approaches have expanded the complexity of the microbial community and further established that the capacity to detect microbes is dependent on the specific characteristics of the skin site sampled. Grice et al. have characterized the topographical and temporal diversity of microbiome in the healthy human skin of a small population of individuals with a 16S rRNA gene phylotyping (Grice et al., 2009). In the sebaceous sites, Propionibacteria species were the most predominant followed by Staphylococci species. In the moist sites, Corynebacteria and Staphylococci species predominated, though β-Proteobacteria also were represented. In the dry sites, a mixed population of bacteria resided with a greater prevalence of β-Proteobacteria and Flavobacteriales species. The microbial profile (Microbiome) observed was of much greater diversity than that revealed by classical culture-based methods, reflecting the increased sensitivity of this technique, the lack of dependence upon culture techniques, and the capacity to detect non-living bacteria that may have only transiently resided at the site. This topographical and temporal survey has provided a baseline for studies that examine which bacterial communities contribute to disease states and the microbial interdependencies required to maintain healthy skin.
Based on even the early information compiled in the last 1–2 years it is reasonable to hypothesize that disrupting the normal skin microbial flora, either by indiscriminant topical cleaners or by systemic antibiotic exposure, could upset skin immune defense. Many lines of evidence associate pathogenicity with an imbalance of microorganisms, known as dysbiosis. Such evidence has been classically assembled in several noninfectious skin diseases such as acne vulgaris, atopic dermatitis, psoriasis and rosacea (Table 1). Recent observations indicate that altered production of AMPs in the skin is implicated in the pathogenesis of psoriasis, rosacea, and atopic dermatitis. Cathelicidins and hBDs are strongly induced in psoriatic lesions in comparison with normal skin, but the induction of AMPs is much lower in atopic dermatitis lesions in comparison to the psoriatic lesion, despite the presence of skin inflammation (Hata et al., 2010; Lande et al., 2007; Ong et al., 2002). In atopic dermatitis lesions, the cathelicidin expression is down-regulated following acute wounding (Mallbris et al., 2010). RNase7 and psoriasin are also induced in atopic dermatitis lesional skin and the AMP induction is upregulated by barrier disruption (Harder et al., 2010). Thus, in atopic disease, the normal induction of some but not all AMPs is inhibited, a phenomenon that is thought to contribute to the susceptibility of atopics to infection. In rosacea, an excess of cathelicidin in the form of LL-37 drives inflammation and abnormal blood vessel growth by mechanisms of cell activation (Yamasaki et al., 2007). In addition, abnormal functions of innate immune pattern recognition by overexpressed TLRs have been observed in the epidermis of the patients with these disorders (Bensch et al., 1995; Jugeau et al., 2005; Yamasaki et al., 2010). These disturbed innate immune systems would impact the homeostasis of skin-resident microflora, which may disrupt skin barrier and induce abnormal inflammatory responses. In fact, although P. acnes is a member of the normal skin commensal bacterial flora, it plays a critical role in the development of inflammatory acne when it overgrows in the pilosebaceous unit (Bojar and Holland, 2004; Cunliffe and Gollnick, 2001). In the psoriatic lesions, the representation of Propionibacterium and Actinobacteria species were lower than normal control skin. In contrast, Firmicutes species were overrepresented in the psoriatic lesions (Gao et al., 2008). Thus, psoriasis may be, in part, associated with substantial alteration in the composition and representation of the cutaneous microflora. However, psoriasis patients are known to rarely suffer from skin infections by pathogens, which may be explained by an over-expression level of AMPs in psoriatic lesions. Although no specific pathogenic organisms were isolated in the lesional skin of rosacea (Marks, 1968), implication of dysbiosis in the pathogenesis of rosacea has been explored. As an example, predominance of S. epidermidis was observed in the lesional skins of pustular and ocular rosacea in comparison to peripheral non-lesional skin, whereas other bacteria were recovered at much lower levels from lesional skin (Whitfeld et al., 2011). Perhaps the best and most well established example of dysbiosis is in atopic dermatitis. Bacterial infections are extremely common in atopic dermatitis patients. Colonization of S. aureus is known to play important roles in the exacerbation of the infection and is correlated with its extent and severity (Miller et al., 2005), which can be explained by defective expression of AMPs in atopic lesions.
Table 1.
Skin diseases associated with dysbiosis: relevance between innate immunity and microbiome
| Diseases | Innate immunity disorder | Abnormality of skin microbiota | References |
|---|---|---|---|
| Inflammatory acne vulgaris |
Up-regulation of hDBs Up-regulation of TLR2 and TLR4 |
Over growth of P. acnes Over-representation of S. epidermidis |
(Jugeau et al., 2005) (Cunliffe and Gollnick, 2001) (Chronnell et al., 2001) |
| Atopic dermatitis |
Lower inductions of cathelicidin and hBDs than those in psoriatic lesional sites despite the presence of inflammation Down-regulation of cathelicidin and hBDs following acute wounding Increased expression of RNase7 and psoriasin |
Frequent bacterial infections S. aureus colonization | (Ong et al., 2002) (Miller et al., 2005) (Hata et al., 2010) (Mallbris et al., 2010) (Harder et al., 2010) (Maintz and Novak, 2011) |
| Psoriasis | Abnormal expression of cathelicidin and hBDs Up-regulation of TLR2 Down-regulation of TLR5 |
Infrequency of Propionibacterium and Actinobacteria species Predominance of Firmicutes species Infrequent bacterial infection |
(Ong et al., 2002) (Bensch et al., 1995) (Miller et al., 2005) (Harder and Schroder, 2005) |
| Rosacea | Abnormal expression of cathelicidin Abnormal expression of kallikrein 5 which proteolytically activate hCAP18 to LL-37 Up-regulation of TLR2 |
No pathogenic bacteria found in lesional site Predominance of S. epidermidis, but inferior representation of other bacteria |
(Yamasaki et al., 2007) (Yamasaki et al., 2010) (Marks, 1968) (Whitfeld et al., 2011) |
Conclusions
Recent studies have revealed that the skin innate immune system work is together with the cutaneous microbiota to act as a barrier against pathogenic microbes and against overgrowth of opportunistic pathogens. Antibiotics are conventionally used as treatment for various skin infectious diseases, such as acne vulgaris and atopic dermatitis. However, antibiotic therapy nonspecifically kills a variety of bacteria, which may impact the homeostasis derived from the beneficial microflora. This may result in short term improvement but long term enhancement of the risks of subsequent colonization by harmful bacteria. Restoration and maintenance of normal microflora may be important to maintain healthy skin and for management of skin diseases associated with dysbiosis. As microbial AMPs specifically exert antimicrobial activity against skin pathogens, but not against commensals itself, these peptides may have potential to be safely used as a pathogen-specific antibiotic therapy for skin infections. In the future expect exciting new revelations of the molecular functions of the normal microflora and better understanding of symbiotic processes between host and our essential prokaryotic inhabitants.
References
- Abtin A, Eckhart L, Glaser R, Gmeiner R, Mildner M, Tschachler E. The antimicrobial heterodimer S100A8/S100A9 (calprotectin) is upregulated by bacterial flagellin in human epidermal keratinocytes. J Invest Dermatol. 2010;130:2423–2430. doi: 10.1038/jid.2010.158. [DOI] [PubMed] [Google Scholar]
- Abtin A, Eckhart L, Mildner M, Ghannadan M, Harder J, Schroder JM, et al. Degradation by stratum corneum proteases prevents endogenous RNase inhibitor from blocking antimicrobial activities of RNase 5 and RNase 7. J Invest Dermatol. 2009;129:2193–2201. doi: 10.1038/jid.2009.35. [DOI] [PubMed] [Google Scholar]
- Bastos MC, Ceotto H, Coelho ML, Nascimento JS. Staphylococcal antimicrobial peptides: relevant properties and potential biotechnological applications. Curr Pharm Biotechnol. 2009;10:38–61. doi: 10.2174/138920109787048580. [DOI] [PubMed] [Google Scholar]
- Bensch KW, Raida M, Magert HJ, Schulz-Knappe P, Forssmann WG. hBD-1: a novel beta-defensin from human plasma. FEBS Lett. 1995;368:331–335. doi: 10.1016/0014-5793(95)00687-5. [DOI] [PubMed] [Google Scholar]
- Bojar RA, Holland KT. Acne and Propionibacterium acnes. Clin Dermatol. 2004;22:375–379. doi: 10.1016/j.clindermatol.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Chronnell CM, Ghali LR, Ali RS, Quinn AG, Holland DB, Bull JJ. Human beta defensin-1 and -2 expression in human pilosebaceous units: upregulation in acne vulgaris lesions. J Invest Dermatol. 2001;117:1120–1125. doi: 10.1046/j.0022-202x.2001.01569.x. [DOI] [PubMed] [Google Scholar]
- Cogen AL, Yamasaki K, Muto J, Sanchez KM, Crotty Alexander L, Tanios J, et al. Staphylococcus epidermidis antimicrobial delta-toxin (phenol-soluble modulingamma) cooperates with host antimicrobial peptides to kill group A Streptococcus. PLoS One. 2010a;5:e8557. doi: 10.1371/journal.pone.0008557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogen AL, Yamasaki K, Sanchez KM, Dorschner RA, Lai Y, MacLeod DT, et al. Selective antimicrobial action is provided by phenol-soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. J Invest Dermatol. 2010b;130:192–200. doi: 10.1038/jid.2009.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunliffe WJ, Gollnick HP. Microbiology of acne. In: Cunliffe WJ, Gollnick HP, editors. Acne. Kent: Martin Dunitz; 2001. pp. 29–36. [Google Scholar]
- Drake DR, Brogden KA, Dawson DV, Wertz PW, et al. Thematic review series: skin lipids. Antimicrobial lipids at the skin surface. J Lipid Res. 2008;49:4–11. doi: 10.1194/jlr.R700016-JLR200. [DOI] [PubMed] [Google Scholar]
- Froy O. Regulation of mammalian defensin expression by Toll-like receptordependent and independent signalling pathways. Cell Microbiol. 2005;7:1387–1397. doi: 10.1111/j.1462-5822.2005.00590.x. [DOI] [PubMed] [Google Scholar]
- Fujie T, Shikiji T, Uchida N, Urano Y, Nagae H, Arase S. Culture of cells derived from the human sebaceous gland under serum-free conditions without a biological feeder layer or specific matrices. Arch Dermatol Res. 1996;288:703–708. doi: 10.1007/BF02505281. [DOI] [PubMed] [Google Scholar]
- Gallo RL, Murakami M, Ohtake T, Zaiou M. Biology and clinical relevance of naturally occurring antimicrobial peptides. J Allergy Clin Immunol. 2002;110:823–831. doi: 10.1067/mai.2002.129801. [DOI] [PubMed] [Google Scholar]
- Gao Z, Tseng CH, Strober BE, Pei Z, Blaser MJ. Substantial alterations of the cutaneous bacterial biota in psoriatic lesions. PLoS One. 2008;3:e2719. doi: 10.1371/journal.pone.0002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R, Harder J, Lange H, Bartels J, Christophers E, Schroder JM. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat Immunol. 2005;6:57–64. doi: 10.1038/ni1142. [DOI] [PubMed] [Google Scholar]
- Gotz F, Verheij HM, Rosenstein R. Staphylococcal lipases: molecular characterisation, secretion, and processing. Chem Phys Lipids. 1998;93:15–25. doi: 10.1016/s0009-3084(98)00025-5. [DOI] [PubMed] [Google Scholar]
- Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, et al. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324:1190–1192. doi: 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder J, Dressel S, Wittersheim M, Cordes J, Meyer-Hoffert U, Mrowietz U, et al. Enhanced expression and secretion of antimicrobial peptides in atopic dermatitis and after superficial skin injury. J Invest Dermatol. 2010;130:1355–1364. doi: 10.1038/jid.2009.432. [DOI] [PubMed] [Google Scholar]
- Hata TR, Kotol P, Boguniewicz M, Taylor P, Paik A, Jackson M, et al. History of eczema herpeticum is associated with the inability to induce human beta-defensin (HBD)-2, HBD-3 and cathelicidin in the skin of patients with atopic dermatitis. Br J Dermatol. 2010;163:659–661. doi: 10.1111/j.1365-2133.2010.09892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland DB, Bojar RA, Farrar MD, Holland KT. Differential innate immune responses of a living skin equivalent model colonized by Staphylococcus epidermidis or Staphylococcus aureus. FEMS Microbiol Lett. 2009;290:149–155. doi: 10.1111/j.1574-6968.2008.01402.x. [DOI] [PubMed] [Google Scholar]
- Holland DB, Bojar RA, Jeremy AH, Ingham E, Holland KT. Microbial colonization of an in vitro model of a tissue engineered human skin equivalent--a novel approach. FEMS Microbiol Lett. 2008;279:110–115. doi: 10.1111/j.1574-6968.2007.01021.x. [DOI] [PubMed] [Google Scholar]
- Holland KT, Ingham E, Cunliffe WJ. A review, the microbiology of acne. J Appl Bacteriol. 1981;51:195–215. doi: 10.1111/j.1365-2672.1981.tb01234.x. [DOI] [PubMed] [Google Scholar]
- Huang YC, Lin YM, Chang TW, Wu SJ, Lee YS, Chang MD, et al. The flexible and clustered lysine residues of human ribonuclease 7 are critical for membrane permeability and antimicrobial activity. J Biol Chem. 2007;282:4626–4633. doi: 10.1074/jbc.M607321200. [DOI] [PubMed] [Google Scholar]
- Iwase T, Uehara Y, Shinji H, Tajima A, Seo H, Takada K, et al. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature. 2010;465:346–349. doi: 10.1038/nature09074. [DOI] [PubMed] [Google Scholar]
- Joo HS, Cheung GY, Otto M. Antimicrobial activity of community-associated methicillin-resistant Staphylococcus Aureus is caused by phenol-soluble modulin derivatives. J Biol Chem. 2011 doi: 10.1074/jbc.M111.221382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jugeau S, Tenaud I, Knol AC, Jarrousse V, Quereux G, Khammari A, et al. Induction of toll-like receptors by Propionibacterium acnes. Br J Dermatol. 2005;153:1105–1113. doi: 10.1111/j.1365-2133.2005.06933.x. [DOI] [PubMed] [Google Scholar]
- Kretschmer D, Gleske AK, Rautenberg M, Wang R, Koberle M, Bohn E, et al. Human formyl peptide receptor 2 senses highly pathogenic Staphylococcus aureus. Cell Host Microbe. 2010;7:463–473. doi: 10.1016/j.chom.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Cogen AL, Radek KA, Park HJ, Macleod DT, Leichtle A, et al. Activation of TLR2 by a small molecule produced by Staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J Invest Dermatol. 2010;130:2211–2221. doi: 10.1038/jid.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Di Nardo A, Nakatsuji T, Leichtle A, Yang Y, Cogen AL, et al. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat Med. 2009;15:1377–1382. doi: 10.1038/nm.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Gallo RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009;30:131–141. doi: 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- Lee DY, Huang CM, Nakatsuji T, Thiboutot D, Kang SA, Monestier M, et al. Histone H4 Is a Major Component of the Antimicrobial Action of Human Sebocytes. J Invest Dermatol. 2009 doi: 10.1038/jid.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DY, Yamasaki K, Rudsil J, Zouboulis CC, Park GT, Yang JM, et al. Sebocytes express functional cathelicidin antimicrobial peptides and can act to kill propionibacterium acnes. J Invest Dermatol. 2008;128:1863–1866. doi: 10.1038/sj.jid.5701235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maintz L, Novak N. Modifications of the innate immune system in atopic dermatitis. J Innate Immun. 2011;3:131–141. doi: 10.1159/000323963. [DOI] [PubMed] [Google Scholar]
- Mallbris L, Carlen L, Wei T, Heilborn J, Nilsson MF, Granath F, et al. Injury downregulates the expression of the human cathelicidin protein hCAP18/LL-37 in atopic dermatitis. Exp Dermatol. 2010;19:442–449. doi: 10.1111/j.1600-0625.2009.00918.x. [DOI] [PubMed] [Google Scholar]
- Marks R. Concepts in the pathogenesis of rosacea. Br J Dermatol. 1968;80:170–177. doi: 10.1111/j.1365-2133.1968.tb12288.x. [DOI] [PubMed] [Google Scholar]
- Marples RR, Downing DT, Kligman AM. Control of free fatty acids in human surface lipids by Corynebacterium acnes. J Invest Dermatol. 1971;56:127–131. doi: 10.1111/1523-1747.ep12260695. [DOI] [PubMed] [Google Scholar]
- Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- Mehlin C, Headley CM, Klebanoff SJ. An inflammatory polypeptide complex from Staphylococcus epidermidis: isolation and characterization. J Exp Med. 1999;189:907–918. doi: 10.1084/jem.189.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LS, Sorensen OE, Liu PT, Jalian HR, Eshtiaghpour D, Behmanesh BE, et al. TGF-alpha regulates TLR expression and function on epidermal keratinocytes. J Immunol. 2005;174:6137–6143. doi: 10.4049/jimmunol.174.10.6137. [DOI] [PubMed] [Google Scholar]
- Nagy I, Pivarcsi A, Kis K, Koreck A, Bodai L, McDowell A, et al. Propionibacterium acnes and lipopolysaccharide induce the expression of antimicrobial peptides and proinflammatory cytokines/chemokines in human sebocytes. Microbes Infect. 2006;8:2195–2205. doi: 10.1016/j.micinf.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Nakatsuji T, Kao MC, Fang JY, Zouboulis CC, Zhang L, Gallo RL, et al. Antimicrobial Property of Lauric Acid Against Propionibacterium Acnes: Its Therapeutic Potential for Inflammatory Acne Vulgaris. J Invest Dermatol. 2009 doi: 10.1038/jid.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuji T, Kao MC, Zhang L, Zouboulis CC, Gallo RL, Huang CM. Sebum free fatty acids enhance the innate immune defense of human sebocytes by upregulating beta-defensin-2 expression. J Invest Dermatol. 2010;130:985–994. doi: 10.1038/jid.2009.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- Otto M, Sussmuth R, Vuong C, Jung G, Gotz F. Inhibition of virulence factor expression in Staphylococcus aureus by the Staphylococcus epidermidis agr pheromone and derivatives. FEBS Lett. 1999;450:257–262. doi: 10.1016/s0014-5793(99)00514-1. [DOI] [PubMed] [Google Scholar]
- Schittek B, Hipfel R, Sauer B, Bauer J, Kalbacher H, Stevanovic S, et al. Dermcidin: a novel human antibiotic peptide secreted by sweat glands. Nat Immunol. 2001;2:1133–1137. doi: 10.1038/ni732. [DOI] [PubMed] [Google Scholar]
- Schroeder BO, Wu Z, Nuding S, Groscurth S, Marcinowski M, Beisner J, et al. Reduction of disulphide bonds unmasks potent antimicrobial activity of human beta-defensin 1. Nature. 2011;469:419–423. doi: 10.1038/nature09674. [DOI] [PubMed] [Google Scholar]
- Senyurek I, Paulmann M, Sinnberg T, Kalbacher H, Deeg M, Gutsmann T, et al. Dermcidin-derived peptides show a different mode of action than the cathelicidin LL-37 against Staphylococcus aureus. Antimicrob Agents Chemother. 2009;53:2499–2509. doi: 10.1128/AAC.01679-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrivanova E, Marounek M, Dlouha G, Kanka J. Susceptibility of Clostridium perfringens to C-C fatty acids. Lett Appl Microbiol. 2005;41:77–81. doi: 10.1111/j.1472-765X.2005.01709.x. [DOI] [PubMed] [Google Scholar]
- Vuong C, Durr M, Carmody AB, Peschel A, Klebanoff SJ, Otto M. Regulated expression of pathogen-associated molecular pattern molecules in Staphylococcus epidermidis: quorum-sensing determines pro-inflammatory capacity and production of phenol-soluble modulins. Cell Microbiol. 2004;6:753–759. doi: 10.1111/j.1462-5822.2004.00401.x. [DOI] [PubMed] [Google Scholar]
- Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, et al. Identification of novel cytolytic peptides as key virulence determinants for communityassociated MRSA. Nat Med. 2007;13:1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- Wanke I, Steffen H, Christ C, Krismer B, Gotz F, Peschel A, et al. Skin commensals amplify the innate immune response to pathogens by activation of distinct signaling pathways. J Invest Dermatol. 2011;131:382–390. doi: 10.1038/jid.2010.328. [DOI] [PubMed] [Google Scholar]
- Whitfeld M, Gunasingam N, Leow LJ, Shirato K, Preda V. Staphylococcus epidermidis: A possible role in the pustules of rosacea. J Am Acad Dermatol. 2011;64:49–52. doi: 10.1016/j.jaad.2009.12.036. [DOI] [PubMed] [Google Scholar]
- Wiesner J, Vilcinskas A. Antimicrobial peptides: the ancient arm of the human immune system. Virulence. 2010;1:440–464. doi: 10.4161/viru.1.5.12983. [DOI] [PubMed] [Google Scholar]
- Wimley WC. Describing the mechanism of antimicrobial Peptide action with the interfacial activity model. ACS Chem Biol. 2010;5:905–917. doi: 10.1021/cb1001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadava P, Zhang C, Sun J, Hughes JA. Antimicrobial activities of human beta-defensins against Bacillus species. Int J Antimicrob Agents. 2006;28:132–137. doi: 10.1016/j.ijantimicag.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975–980. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Gallo RL. Antimicrobial peptides in human skin disease. Eur J Dermatol. 2008;18:11–21. doi: 10.1684/ejd.2008.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki K, Kanada K, Macleod DT, Borkowski AW, Morizane S, Nakatsuji T, et al. TLR2 Expression Is Increased in Rosacea and Stimulates Enhanced Serine Protease Production by Keratinocytes. J Invest Dermatol. 2010 doi: 10.1038/jid.2010.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaalouk TK, Bajaj-Elliott M, George JT, McDonald V. Differential regulation of beta-defensin gene expression during Cryptosporidium parvum infection. Infect Immun. 2004;72:2772–2779. doi: 10.1128/IAI.72.5.2772-2779.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanger P, Holzer J, Schleucher R, Steffen H, Schittek B, Gabrysch S. Constitutive expression of the antimicrobial peptide RNase 7 is associated with Staphylococcus aureus infection of the skin. J Infect Dis. 2009;200:1907–1915. doi: 10.1086/648408. [DOI] [PubMed] [Google Scholar]
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- Zouboulis CC, Seltmann H, Neitzel H, Orfanos CE. Establishment and characterization of an immortalized human sebaceous gland cell line (SZ95) J Invest Dermatol. 1999;113:1011–1020. doi: 10.1046/j.1523-1747.1999.00771.x. [DOI] [PubMed] [Google Scholar]