Abstract
This study aims to evaluate in detail the biological osteoconductive properties of the low-temperature synthetic porous calcium-deficient hydroxyapatite and to compare it with the biological apatite. Bone reactions to granules of similar sizes of the low-temperature hydroxyapatite and commercially available non-sintered deproteinized bovine bone were compared. Two different temperatures were used to fabricate two batches of newly developed porous hydroxyapatite with different carbonate groups content and specific surface area. The histological analysis of specimens with histomorphometry was performed at different time after in vivo implantation. Based on histological analysis, the level of bone formation in the spaces between the implanted granules and through the interconnected pores of all implanted materials within a cortical region (bone area ingrowth 72–85 %) was several-fold higher than within a cancellous bone site (bone area ingrowth 16–28 %) at three and six months after implantation. Within the cancellous bone site, bone coverage of the implanted material at six months was significantly higher in hydroxyapatite material fabricated using low-temperature synthesis and subsequent processing at 150°C than in hydroxyapatite scaffold developed using low-temperature synthesis with subsequent processing at 700°C or deproteinized bovine bone. According to our study, the bioactive properties of the low-temperature calcium-deficient hydroxyapatite are comparable with the biological apatite. The favourable influence of a high specific surface area of a low-temperature calcium-deficient hydroxyapatite on in vivo bone formation was emphasized.
Introduction
There is a need for treatment of bone defects throughout the whole spectrum of orthopaedic surgery—to replace bone substance which has been lost due to congenital disorders, traumatic and inflammatory events, for reconstructive surgical procedures in hip arthroplasty revision surgery and surgery for bone tumours and tumour-like lesions. Bone remodelling allows the skeleton to achieve the optimal architecture dependent on applied load. Bone tissue regeneration and repair processes can be effective in small defects (under 60 cm3) but larger defects usually need filling [1]. Autologous bone grafting is the current gold standard in this situation because autografts have osteogenic, osteoconductive, and osteoinductive properties. However, there is a limited quantity of autologous bone grafts as harvesting requires a further operation with prolongation of operative times, and there is a potential for donor site morbidity. An allograft bonegraft has to be both safe in use and to possess acceptable mechanical and biological properties. In an effort to overcome the limitations of autografts and allografts, a variety of synthetic bone-graft substitutes have been developed [2].
The bone-graft substitutes include a spectrum of products that have various effects on bone healing. Growth factors, principally bone morphogenetic proteins (BMPs), present within the osteoinductive materials induce the process of differentiation of undifferentiated mesenchymal cells into the chondro-osteogenic pathway [3]. Enhanced bone healing induced by BMPs has been shown in preclinical and clinical studies [4]. Growth-factor therapy with two BMPs available for clinical applications—recombinant human BMP-2 and recombinant human BMP-7 (osteogenic protein 1)—has been approved for specific indications [5]. However, the effects of BMPs in clinical studies have revealed safety and efficacy complying with autologous bone grafts without the intensive response that was seen in animal studies [6].
Calcium phosphate biomaterials are frequently used for bone defect reconstructions, because of their bone-like chemical composition. They are bioactive and highly osteoconductive. These properties are dependent on the chemical composition of the material, the technique of fabrication, the level of crystallinity, the morphological and structural parameters that have a direct or indirect effect on the cells’ migration, and the adhesion, proliferation and differentiation with subsequent new bone formation. Hydroxyapatite (HA) seems to be one of the most important calcium phosphate materials for bone substitution. In contrast to stoichiometric calcium HA Ca10(PO4)6(OH)2 with a Ca/P molar ratio of 1.67, biological hydroxyapatites contain minor substituents in their structure (CO2-3,Cl-, Mg++, K+, Na+), are usually calcium-deficient with a Ca/P molar ratio lower than 1.67 and CO2-3 substitutes primarily for PO3-4 groups (B-type substitution) [7]. The synthetic porous B-type carbonated HA prepared by low-temperature synthesis (precipitation) resembles the structure and chemical composition of the biological bone HA and can successfully substitute non-sintered bovine apatite prepared by deproteinisation of bovine bone with absolute elimination of risk caused by residual antigenic proteins of xenogeneic bone [8].
To date there have been no reports which compare the in vivo bioactivity of the synthetic porous carbonated HA with non-sintered bovine apatite after implantation into defects of long bones. In this study, we investigated two different types of scaffolds; bone reaction to granules of similar sizes of the new low-temperature calcium-deficient HA and commercially available deproteinised bovine bone (DBB) were compared. Two different temperatures were used to create two batches of newly developed porous HA with different carbonate groups content and specific surface area. The aim of this study was to evaluate the biological osteoconductive properties of the low-temperature synthetic porous calcium-deficient HA and to compare it with biological apatite–non-sintered deproteinised bovine bone.
Materials and methods
Implant materials
The porous HA used in this study was prepared using low-temperature synthesis and subsequent processing at 150°C (HAp1) or 700°C (HAp2) with hydrogen peroxide as a pore-creating additive (Fig. 1). Both synthetic low-temperature HA bioceramics had different material properties and were produced by Lasak Praha (Prague, Czech Republic) (Table 1). The third implanted biomaterial was DBB Bio-Oss® (Geistlich Pharma AG, Wolhusen, Switzerland). The porous granules of the tested materials measuring 0.6–1.0 mm in diameter were implanted into tibial defects of 3.7 mm diameter and 8 mm depth.
Fig. 1.
Scanning electron micrograph of the tested material HAp1 showing its macro- (a) and nanostructure (b)
Table 1.
Physical and chemical characteristics of the implanted materials
| Characteristics | HAp 1 | HAp 2 | DBB |
|---|---|---|---|
| Size of granules (mm) | 0.6–1 | 0.6–1 | 0.6–1 |
| Size of macropores (μm) | >100 | >100 | > 100 |
| Mean size of micropores (nm) | 5 | 20 | 12.4 |
| Specific surface area (m2/g) | 78.3 (±0,34) | 25.4 (±0.11) | 74.4 (±0.27) |
| Porosity (%) | 83 | 74 | 54.8 |
| Crystal size (nm) | 30 | 40 | 30 |
| Ca/P molar ratio | 1.65 | 1.66 | 1.57 |
| Rel CO3″ „A“ (I1545/I1041) a | 1.10−6 | 2.10−6 | 1.9.10−6 |
| Rel CO3″ „B“ (I1420/I1041) a | 0.023 | 0.006 | 0.051 |
a Values expressing a relative carbonate content using phosphate band at 1,041 cm−1as standard
Material characteristics
The phases present were identified by X-ray diffraction analysis (XRD 3000P, Seifert, Germany). The X-ray diffraction patterns of the synthetic samples (HAp1, HAp2) and DBB showed apatitic patterns (PDF database card 9-342) with low crystallinity. The diffuse characteristic of X-ray diffraction peaks increases with increasing carbonate content. The X-ray diffraction pattern of DBB has been almost identical to that of HAp1 (Fig. 2).
Fig. 2.
The X-ray diffraction patterns of DBB, HAp1 and HAp2
Carbonate substitution in the structure of samples was distinguished by infrared spectroscopy. A-type carbonate substitution was detected by the presence of a band observed at 1,545 cm-1 and B-type at wave number 1,415 cm-1. Infrared spectra were recorded on a Nicolet 7600 FTIR spectrophotometer (Thermo Nicolet Instruments Co., Madison, USA). The specific surface area of the implanted materials was measured using BET method (ASAP 2010 M, Micromeritics, USA). The porosity was determined by mercury intrusion porosimetry (Autopore III Micromeritics, USA), standard light microscopy (Olympus BX60, Japan) with image analysis and scanning electron microscopy (Vega II-LSU, Tescan, Czech Republic). The phosphorous content was analysed colorimetrically. The complexation titration method was used for the calcium determination. High structural and morphological resemblance of the synthetic biomaterials (HAp1, HAp2) to nanocrystalline non-sintered biological hydroxyapatite (DBB) was found (Table 1).
Implantation procedure
Twenty-seven implantations were performed on two-year-old Beagle dogs weighing between 12 and 16 kg. The tested materials were implanted into the tibia of the hind legs of the dogs. Each material composition (HAp1, HAp2, DBB) was applied in three implantations for a period of three months and in six implantations for a period of six months. The study was performed in agreement and with the approval of the competent ethical committee.
The animals were anaesthetised with Narkamon 5% (15 mg/kg) and Rometar 2% (2 mg/kg) and maintained with a mixture of oxygen, nitrous oxide and halothane after administration of Atropin by way of premedication. The left and right hind limb were clipped, scrubbed and draped for aseptic surgery. An incision was made adjacent to the right tibial diaphysis and the soft tissue was dissected to reveal the underlying bone. Defects, 3.7 mm in diameter and 8 mm in depth, were created within the bone using drill bits and a battery drill. The defect sites were subsequently irrigated with sterile saline solution and the granules of the tested materials were implanted into the defects. After implantation, the incisions were closed using interrupted suturing. The process was repeated on the opposite hind leg with granules of a different composition. Analgesics (Novalgin 16 mg/kg/day) were administered for three days by intramuscular injections; no antibiotics were given.
Histological evaluation and histomorphometry
The dogs were put to sleep at three and six months after surgery by an intravenous overdose of barbiturates and both tibiae were dissected. Radiographs of the tibiae were obtained perpendicular to the direction in which the implants were placed. The trimmed tibiae containing the implants were placed in 10% w/v of paraformaldehyde solution for fixation. Tibial bone defects filled with the tested materials were separated as tissue blocks. The samples were dehydrated using increasing concentrations of alcohol and embedded in an acrylate resin. The EXACT technology was used for preparation of the longitudinal undecalcified thin cut sections [9]. A total of three sections were cut perpendicular to the longitudinal axis of the tibia per implant area.
The sections with a thickness of 30–50 μm were stained at 60°C for one hour with 0.9% w/v toluidine blue solution for histology and histomorphometry. The amount of newly-formed bone was assessed using an Olympus BX 60 optical microscope and quantified using the image analysis software Image-Pro Plus 5.1. In the cortical bone region, the bone ingrowth in the spaces between granules (BAI%-bone area ingrowth) for all implant compositions and time points was evaluated using the quantification of the material/new bone/soft tissue amount. In the cancellous bone region, the osteoconductive properties of materials were assessed using the coverage of the material surface (bone implant contact as BIC%).
Statistical analysis
The values were presented as mean ± standard deviation (SD). The Mann-Whitney U test was used to determine differences between the implanted materials. A P value of less than 0.05 was considered significant.
Results
All animals survived without complications, the wounds were primarily healed and the range of movement in adjacent joints was full. At the retrieval of the tibiae three months postoperatively, normal healing of the soft tissues had occurred and postoperative examination revealed only mild swelling of the soft tissues over the site of the operation. The fascia and periosteum were firmly fixed to the surface of the bone and no signs of hyperaemia were found. In most cases the defect site was mildly prominent over the surface of bone. Several entry apertures were covered with bone tissue. At the retrieval of the tibiae six months postoperatively, normal healing of the soft tissues was observed without swelling over the anteromedial aspect of the tibia. The defect sites were indistinguishable from the surrounding unoperated bone due to new bone overgrowth.
Contact radiographs made prior to the histological processing three and six months postoperatively demonstrated no radiolucent zones between the implanted ceramic and the surrounding bone, no thickening of the cortex, and no signs of periosteal reaction. The bone trabeculae growing in close proximity to the surface of the implanted material were seen in cancellous bone sites. No differences at the implanted material-recipient bone interface were observed for any implant composition (HAp1, HAp2, DBB) after the two different time points within both the cortical and cancellous bone sites.
Bone healing in the cortical bone sites—histomorphometry
In the cortical bone sites, the amount of new bone formation, remaining material volume (HAp1, HAp2, DBB), and amount of soft tissue (marrow spaces) were determined using histomorphometry after three and six months postoperatively. Histological evaluation indicated that bone bridging had occurred between the new bone and the surface of the implanted granules with no evidence of fibrous encapsulation for all implant compositions and time points (Fig. 3). No evidence of giant cell foreign body reaction was found in any biopsy.
Fig. 3.
Light micrograph (a) of 3-month biopsies, the tibial defect filled with granules of synthetic hydroxyapatite (HAp1). Dashed line indicates the border of the created bone defect, thicker line indicates the border between the cortical and cancellous site of bone. Overview showing the pre-existing bone area (OB), residual hydroxyapatite particles and newly-formed bone (NB). High magnification image (b) of HAp1 implant at 3 months in vivo showing direct contact of newly-formed bone to the surface of granule and deposition of bone tissue from the edge of ceramic pore towards the centre. Arrows indicate newly-formed bone (NB), dashed lines indicate osteoid depositions (O). BM = bone marrow. Toluidin blue staining. Original magnification (a) × 20; (b) × 200
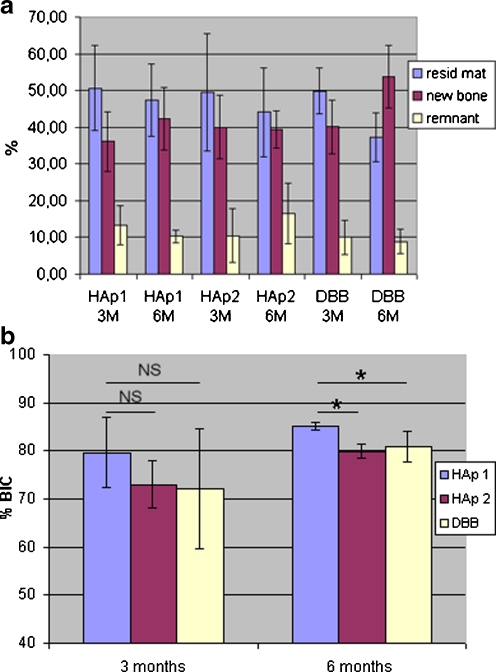
The newly-formed bone tissue in the cortical sites of the HAp1 implants had reached 36.07% (±8.04) of the total cortical defect after three months in vivo; in HAp2 and DBB the newly-formed bone was 39.99% (±8.70) and 40.08% (±7.35) of the total cortical defect, respectively (Fig. 4a). The bone formation in the spaces between implanted granules of HAp1 within the cortical region (BAI % -Bone Area Ingrowth) had reached 73.2% (± 11.6); in HAp2 and DBB the newly-formed bone filled 79.2% (±15.8) and 79.9% (±7.3), respectively. At three months in vivo, the newly-formed bone between the implanted particles connected them into a mass of mostly mineralised tissue.
Fig. 4.
Tissue volumes of remaining material, newly-formed bone and marrow spaces (a) at 3 and 6 months after surgery within the cortical bone site. Each double bar represents one implant material and one time point. Values are mean ± SD of three or six biopsies. Bone coverage of different implanted materials (b) at 3 and 6 months after surgery within the cancellous bone site. The significant (*) and non-significant (NS) differences are marked
The amount of remaining material (HAp1, HAp2, and DBB) within the cortical sites of implantation decreased in the period between three and six months after the operation (a relative decrease of 3.2%, 5.4%, and 12.5%, respectively). At the same time the amount of new bone formation increased (a relative increase of 6.2%, 0.5%, and 13.6%, respectively). The bone formation in the spaces between the implanted granules within the cortical region (BAI % -Bone Area Ingrowth) had reached 80.49%, 72.46%, and 85.90% in HAp1, HAp2, and DBB, respectively. However, there was no significant difference in BAI %. The new bone tissue was viable, organised into Haversian systems and completely mineralised at six months.
Bone healing in the cancellous bone sites—histomorphometry
In the cancellous bone sites, the bone implant contact (BIC%) was determined using histomorphometry after three and six months postoperatively. The bone implant contact of the tested materials (HAp1, HAp2 a DBK) was >70% after three months and increased to >80% after six months independently of the granules’ size (Fig. 4b). These histomorphometrical findings indicate that the in vivo osteoconductivity of HAp1 material was greater than that for HAp2 and DBB at both three and six months.
The thickness of the new bone layer formed by osteoconduction on the surface of granules was relatively unified and ranged from 100 to 300 μm. The interconnections which had a size above 100 μm were partially or completely filled with vital bone. The bone bridging up to 2000 μm in size was observed but the spaces between granules were not completely filled by bone tissue (Fig. 5). The bone formation in the spaces between the implanted granules within the cancellous region (bone area ingrowth as BAI%) had reached 22 ± 6% without significant differences between different implanted materials and time points.
Fig. 5.
High magnification image of HAp1 implant showing that new bone (NB) has formed around the implanted granules. (a) Notice the bone bridge that connects the granules. Arrows indicate the osteoid layer at the periphery of the newly-formed bone. (b) Note the central osteoid area in case of thinner and longer bone bridge. BM bone marrow. Toluidin blue staining. Original magnification: (a) × 500, (b) × 200
Discussion
It remains a great challenge to design the ideal bone graft substitute that emulates nature’s bone structure and function. Consequently, the first generation of biomaterials consisted of bioinert materials which reduced the immune response and foreign body reaction to a minimum. Between 1980 and 2000 the field of development began to shift to bioactive and resorbable biomaterials. Bioactive components could interact with the biological environment to enhance the tissue/surface bonding. Resorbable materials undergo a progressive degradation while new tissue regenerates and heals. The third generation of biomaterials are able to stimulate specific cellular response at the molecular level, and the bioactivity and biodegradability concepts are combined. A temporary three-dimensional porous structure that stimulates the cells’ invasion, adhesion, proliferation, differentiation and extracellular matrix production is required for clinical applications [10].
DBB, which was used in our study as a control, is calcium-deficient carbonate apatite, chemically and physically identical to human bone with high osteoconductive properties. The granules provide an ideal scaffold for new bone formation [11]. DBB is a xenogeneic material from which all organic components have been removed. The testing of materials of bovine origin was recommended despite a complete absence of proteins to guarantee that they are free of infectious prion particles [12]. Xenograft bone substitutes have been successfully applied in stomatology and traumatology [13, 14]. Histological evaluations have shown that DBB is a highly osteoconductive material with no adverse effects, such as an inflammatory cell infiltrate or foreign body response even at long-term follow-up [15]. The presence of DBB particles perfectly integrated into the bone architecture but without complete resorption could be explained by the influence of a microenvironment at the bone–xenograft interface on the recipient osteoclastic activity [16]. The chemical composition, solubility, and surface morphology were reported as significantly important factors regulating osteoclast activity. Integrins play a critical role as mediators of osteoclastic adhesion to the surface of the implanted material with subsequent acid secretion into the resorption lacuna. The erosion of the material surface via a mineral release causes a high concentration of Ca+2 ions in this area that could inhibit osteoclastic activity [15].
HA (Ca10(PO4)6(OH)2), β-tricalcium phosphate (TCP) (Ca3(PO4)2), their derivatives and their combinations are the most commonly used ceramic materials in bone surgery. The requirements for an ideal synthetic bone substitute appear deceptively simple. It should be a porous matrix with interconnecting porosity that promotes rapid bone ingrowth. The microstructure of an implant should allow a formation of environment optimal for cellular settlement, growth and function. At the same time, the ideal matrix should possess a sufficient strength to prevent its crushing under physiological loads during integration and healing. The pore size and interconnection pathway play a crucial role in ceramic biomaterials [17–20]. A minimal pore size of ∼ 100 μm appropriate for bone growth into ceramic has been described. The porosity must be interconnected to allow the protein adhesion, ingrowth of cells and formation of effective blood supply that is necessary for new bone formation and subsequent bone remodelling. The ideal material should form a secure bond with the surrounding tissues by encouraging new cells to grow and penetrate. The implantation of HA ceramics with porosities range from 35% to 75%, and pore sizes between 50 μm and 400 μm have been reported in the literature [17–20]. There are few reports about quantitative analysis of bone formation with respect to different particle sizes of implanted bioceramics. Kuroda reported that the percentages of bone in contact with the HA surfaces did not differ significantly after implantation of granules with three different sizes into osseous tissue [21]. In recent years, considerable attention has been paid to the development of fabrication methods to prepare porous ceramic scaffolds suitable for bone tissue regeneration.
From the presented histological analysis, it is evident that the level of bone formation in the spaces between implanted granules and through the interconnected pores of implanted materials (HAp1, HAp2, DBB) within a cortical region (BAI% 72–85%) was several-fold higher than within a cancellous bone site (BAI% 16–28%) at three and six months after implantation. Relatively great differences in the bone area ingrowth within cortical and cancellous regions may be influenced by simultaneously running new bone formation and spontaneous healing of the bone defect that is greater in the cortical site than in the cancellous site [22]. Within the cancellous bone site, the bone coverage of the implanted material at six months was significantly higher in HAp1 than in HAp2 and DBB. The level of bioactivity may be relative to the time interval (t0.5bb in days) that is necessary for bone coverage more than 50% of implant surface and is represented by the index of bioactivity Ib = 100/t0.5bb [23]. The time interval t0.5bb was estimated and the index of bioactivity was calculated using a logarithmic regression analysis of the bone implant contact correlated to the period after implantation (Table 2).
Table 2.
The index of bioactivity of the implanted materials
| Implanted material | t0.5bb (days) | Ib = 100/t0.5bb (days -1) |
|---|---|---|
| HAp1 | 12 | 8,3 |
| HAp2 | 17 | 5,9 |
| DBK | 18 | 5,6 |
| HAa | 32 | 3,1 |
a Values expressing parameters of the synthetic sintered hydroxyapatite produced at high temperature according to [19]
With regard to the calculated index of bioactivity, the level of osteoconductive properties is greater in HAp1 than in HAp2. This may be caused by different structural parameters and chemical composition of both materials particularly the content of carbonate substituents, specific surface area and porosity (Table 1). These parameters were described as important factors in osteogenesis and osteoconduction [24]. Compared to non-sintered bovine apatite with nanometric-size crystals, the osteoconductive properties of synthetic porous HA prepared at low temperature are comparable in HAp2 and higher in HAp1. The findings presented are preliminary as the number of implantations was small. Larger experimental groups and a longer follow-up are necessary to verify these results. This is planned in subsequent trials.
Although bone grafts are used in all facets of orthopaedic surgery, nearly 50% of all bone graft material, including allograft, demineralised bone matrix and synthetic bone graft substitutes, are applied in spinal surgery. Revision arthroplasty and trauma cases each make up approximately 20% [25]. HA ceramics have been successful in filling bone voids resulting from the reduction of tibial plateau fractures and in surgery for bone tumours; biphasic HA/TCP material has been beneficial for the reconstruction in hip arthroplasty revision surgery [2, 26, 27].
In recent years, human mesenchymal stem cells have aroused great interest as a potential source of cell-based therapeutic strategies. Mesenchymal stem cells are pluripotent cells capable of differentiating into several lineages such as osteoblasts, chondrocytes and adipocytes. The association of mesenchymal stem cells differentiated with respect to osteoblasts and ceramics is an important treatment strategy developed by tissue engineering for large bone defects. To induce in vivo bone formation, mesenchymal stem cells were associated with an appropriate matrix. The effect of the particle size and shape on bone formation has been published [28]. Although ceramic is chemically very close to the natural mineral structure of bone, high-temperature porous HA with a low specific surface area is very far from bone structure at the cellular level [29]. Low-temperature calcium-deficient HA, biphasic HA/TCP and β-TCP could be suitable materials for the application of mesenchymal stem cells [28, 30, 31].
According to our study, the bioactive properties of the low-temperature synthetic porous HA are comparable with the non-sintered DBB that is successfully used in traumatology and stomatology. The favourable influence of a high specific surface area of a low-temperature calcium-deficient HA on in vivo bone formation was confirmed. This new bioactive HA matrix, produced at low temperature, appears to be a successful bone replacement material in orthopaedic surgery.
Acknowledgments
Conflict of interest statement None of the authors have a conflict of interest in relation to the content of this manuscript.
References
- 1.Hirn M, Silva U, Sidharthan S, Grimer RJ, Abudu A, Tillman RM, Carter SR. Bone defects following curettage do not necessarily need augmentation. A retrospective study of 146 patients. Acta Orthop. 2009;80:4–8. doi: 10.1080/17453670902804505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sponer P, Urban K, Urbanova E, Karpas K, Mathew PG. Behavior of nonresorbable bioactive glass-ceramic implanted into long bone defects: comparison with cancellous allografts. Arch Orthop Trauma Surg. 2009;129:1353–1360. doi: 10.1007/s00402-009-0839-3. [DOI] [PubMed] [Google Scholar]
- 3.Pecina M, Vukicevic S. Biological aspects of bone, cartilage and tendon regeneration. Int Orthop. 2007;31:719–720. doi: 10.1007/s00264-007-0425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKay WF, Peckham SM, Badura JM. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE® Bone Graft) Int Orthop. 2007;31:729–734. doi: 10.1007/s00264-007-0418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White AP, Vaccaro AR, Hall JA, Whang PG, Friel BC, McKee MD. Clinical applications of BMP-7/OP-1 in fractures, nonunions and spinal vision. Int Orthop. 2007;31:735–741. doi: 10.1007/s00264-007-0422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bishop GB, Einhorn TA. Current and future clinical applications of bone morphogenetic proteins in orthopaedic trauma surgery. Int Orthop. 2007;31:721–727. doi: 10.1007/s00264-007-0424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wopenka B, Pasteris JD. A mineralogical perspective on the apatite in bone. Mater Sci Eng C Biomim Mater Sens Syst. 2005;25:131–143. doi: 10.1016/j.msec.2005.01.008. [DOI] [Google Scholar]
- 8.Honig JF, Merten HA, Heinemann DE. Risk of transmission of agents associated with Creutzfeldt-Jakob disease and bovine spongiform encephalopathy. Plast Reconstr Surg. 1999;103:1324–1325. doi: 10.1097/00006534-199904040-00041. [DOI] [PubMed] [Google Scholar]
- 9.Donath K, Breuner GA. A method for the study of undecalcified bones and teeth with attached soft tissues—the sage-schliff (sawing and grinding) technique. J Oral Pathol. 1982;11:318–326. doi: 10.1111/j.1600-0714.1982.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 10.Hench LL, Polak JM. Third-generation biomedical materials. Science. 2002;295:1014–1017. doi: 10.1126/science.1067404. [DOI] [PubMed] [Google Scholar]
- 11.Wetzel AC, Stich H, Caffesse RG. Bone apposition onto oral implants in the sinus area filled with different grafting materials. A histological study in beagle dogs. Clin Oral Implants Res. 1995;6:155–163. doi: 10.1034/j.1600-0501.1995.060304.x. [DOI] [PubMed] [Google Scholar]
- 12.Hartl A, Bitzan P, Wanivenhaus A, Kotz R. Faster integration of human allograft bone than of the bovine substitute Lubboc. Non-randomized evaluation of 20 cases with benign tumors or tumor-like conditions. Acta Orthop Scand. 2004;75:217–220. doi: 10.1080/00016470412331294485. [DOI] [PubMed] [Google Scholar]
- 13.Briem D, Linhart W, Lehmann W, Meenen NM, Rueger JM. Long-term results after application of a porous hydroxyapatite ceramic (Endobon) in proximal tibia fractures. Unfallchirurg. 2002;105:128–133. doi: 10.1007/s001130100303. [DOI] [PubMed] [Google Scholar]
- 14.Meyer S, Floerkemeier T, Windhagen H. Histological osseointegration of Tutobone®: First results in human. Arch Orthop Trauma Surg. 2008;128:539–544. doi: 10.1007/s00402-007-0402-z. [DOI] [PubMed] [Google Scholar]
- 15.Traini T, Valentini P, Iezzi G, Piattelli A. A histologic and histomorphometric evaluation of anorganic bovine bone retrieved 9 years after a sinus augmentation procedure. J Periodontol. 2007;78:955–961. doi: 10.1902/jop.2007.060308. [DOI] [PubMed] [Google Scholar]
- 16.Ramaswamy Y, Haynes DR, Berger G, et al. Bioceramics composition modulate resorption of human osteoclasts. J Mater Sci Mater Med. 2005;16:1199–1205. doi: 10.1007/s10856-005-4729-0. [DOI] [PubMed] [Google Scholar]
- 17.Flautre B, Descamps M, Delecourt C, Blary MC, Hardouin P. Porous HA ceramic for bone replacement: role of the pores and interconnections—experimental study in the rabbit. J Mater Sci Mater Med. 2001;12:679–682. doi: 10.1023/A:1011256107282. [DOI] [PubMed] [Google Scholar]
- 18.Itala AI, Ylanen HO, Ekholm C, Karlsson KH, Aro HT. Pore diameter of more than 100 mm is not requisite for bone ingrowth in rabbits. J Biomed Mater Res. 2001;58:679–683. doi: 10.1002/jbm.1069. [DOI] [PubMed] [Google Scholar]
- 19.Pilliar RM, Filiaggi MJ, Wells JD, Grynpas MD, Kandel RA. Porous calcium polyphosphate scaffolds for bone substitute applications—in vitro characterization. Biomaterials. 2001;22:963–972. doi: 10.1016/S0142-9612(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 20.Tamai N, Myoui A, Tomita T, Nakase T, Tanaka J, Ochi T, et al. Novel hydroxyapatite ceramics with an interconnective porous structure exhibit superior osteoconduction in vivo. J Biomed Mater Res. 2002;59:110–117. doi: 10.1002/jbm.1222. [DOI] [PubMed] [Google Scholar]
- 21.Kuroda T. Bone formation and mechanical properties of the cancellous bone defect site filled with hydroxyapatite granules. Nippon Seikeigeka Gakkai Zasshi. 1995;69:1037–1049. [PubMed] [Google Scholar]
- 22.Lu JX, Gallur A, Flautre B, Anselme K, Deschamps M, Thierry B, Hardouin P. Comparative study of tissue reactions to calcium phosphate ceramics among cancellous, cortical, and medullar bone sites in rabbits. J Biomed Mater Res. 1998;42:357–367. doi: 10.1002/(SICI)1097-4636(19981205)42:3<357::AID-JBM3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 23.Hench LL. Bioactive glasses and ceramics. In: Yamamuro T, Hench LL, Wilson J, editors. Handbook of bioactive ceramics. CRC Press: Boca Raton; 2000. [Google Scholar]
- 24.Afonso A, Santos JD, Vasconcelos M, Branco R, Cavalheiro J. Granules of osteopatite and glass-reinforced hydroxyapatite implanted in rabbit tibiae. J Mater Sci Mater Med. 1996;7:507–510. doi: 10.1007/BF00705433. [DOI] [Google Scholar]
- 25.Bostrom MPG, Seigerman DA. The clinical use of allografts, demineralized bone matrice, synthetic bone graft substitutes and osteoinductive growth factors: A survey study. HSS J. 2005;1:9–18. doi: 10.1007/s11420-005-0111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogose A, Hotta T, Kawashima H, Kondo N, Gu W, Kamura T, Endo N. Comparison of hydroxyapatite and beta tricalcium phosphate as bone substitutes after excision of bone tumors. J Biomed Mater Res B Appl Biomater. 2005;72B:94–101. doi: 10.1002/jbm.b.30136. [DOI] [PubMed] [Google Scholar]
- 27.Schwarz C, Bornei CR. Biphasic phospho-calcium ceramics used as bone substitutes are efficient in the management of severe acetabular bone loss in revision total hip arthroplasties. Eur J Orthop Surg Traumatol. 2005;15:191–196. doi: 10.1007/s00590-005-0244-8. [DOI] [Google Scholar]
- 28.Mankani MH, Kuznetsov SA, Fowler B, Kingman A, Gehron Robey P. In vivo bone formation by human bone marrow stromal cells: effect of carrier particle size and shape. Biotechnol Bioeng. 2001;72:96–107. doi: 10.1002/1097-0290(20010105)72:1<96::AID-BIT13>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 29.Cordonnier T, Layrolle P, Gaillard J, Langonne A, Sensebe L, Rosset P, Sohier J. 3D environment on human mesenchymal stem cells differentiation for bone tissue engineering. J Mater Sci Mater Med. 2010;21:981–987. doi: 10.1007/s10856-009-3916-9. [DOI] [PubMed] [Google Scholar]
- 30.Kasten P, Beyen I, Niemeyer P, Luginbühl R, Bohner M, Richter W. Porosity and pore size of β-tricalcium phosphate scaffold can influence protein production and osteogenic differentiation of human mesenchymal stem cells: An in vitro and in vivo study. Acta Biomater. 2008;4:1904–1915. doi: 10.1016/j.actbio.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 31.Kasten P, Vogel J, Luginbühl R, Niemeyer P, Tonak M, Lorenz H, Helbig L, Weiss S, Fellenberg J, Leo A, Simank HG, Richter W. Ectopic bone formation associated with mesenchymal stem cells in a resorbable calcium deficient hydroxyapatite carrier. Biomaterials. 2005;26:5879–5889. doi: 10.1016/j.biomaterials.2005.03.001. [DOI] [PubMed] [Google Scholar]