Abstract
Two-stage revisions with antibiotic-loaded spacers have gained popularity for treating infected hip-joint arthroplasties. The aim of this prospective study was to assess patient functionality between stages and treatment impact on duration of hospital stay and to describe related complications. Sixty-one consecutive patients with infected hip arthroplasties underwent two-stage revision with preformed spacer implantation. Mean Harris Hip and Merle d’Aubigné scores between the two stages were 39.9 and 7.6, respectively. Forty-six patients (75.4%) were able to leave hospital between stages. Spacer dislocation occurred in 16.4%. No cases of spacer breakage were noted. Preformed cement spacers provide acceptable functional outcome between revision hip arthroplasty stages and facilitate the surgical procedure without increasing mechanical complication rates.
Introduction
Deep periprosthetic infection is one of the most devastating and costly complications of total hip replacement, occurring in 0.3–1.7% of patients [1]. The number of infections is increasing as the rate of primary hip arthroplasties increases. In the literature, there is no general consensus on the optimal treatment for these infections. Different therapeutic approaches have been advocated, the most frequently used being suppression with antibiotics, surgical debridement, one-stage revision with an antibiotic-loaded cemented prosthesis and two-stage revision either with a Girdlestone resection alone or combined with a temporary antibiotic cement spacer or beads. Resection arthroplasty and arthrodesis are successful techniques for eradicating infection but result in a poor functional outcome and are only used as a salvage operation when other methods have failed. [2].
Antibiotic-loaded cement spacers have gained popularity over recent decades, with reported infection eradication rates ranging from 90% to 100% [3–7]. The main advantages of hip-spacer implantation are immediate treatment of the infection by temporarily attaining high local antibiotic levels, maintenance of joint mobility, limitation of scar formation and soft-tissue contraction and ease of reimplantation [8]. Different types of cement spacers can be used: commercially available preformed spacers and custom made with or without the insertion of a metallic endoskeleton [9]. A custom-made spacer has the advantage of creating an optimal fit, theoretically resulting in better stability. However, spacer manufacturing is time consuming, increasing the duration of surgery. In this respect, a preformed spacer, although more costly than a custom-made one and only available in a limited range of sizes, could be beneficial by standardising the implant technique while decreasing surgical effort and operating time. Little information is available on the specific complications of the different cement spacers and patient functional abilities and discomfort during the interim period. The aim of this study was to assess patient functionality and discomfort after commercially available preformed spacer implantation and the impact on the length of hospital stay, and to describe related complications and how to manage them.
Materials and methods
Between April 2003 and June 2009, 61 consecutive patients (31 women and 30 men) were treated according to a two-stage revision protocol using an antibiotic cement spacer for deep infection at the site of the hip implant. Mean patient age at spacer implantation was 65.4 (range 17.4 – 84.7) years. Gram-positive micro-organisms were responsible for the infection in most hips (Table 1). The mean interval between the two stages was 7.5 (range six to 20) weeks. The length of hospital stay ranged from ten to 111 days, mainly depending on the degree of comorbidity or the need for prolonged IV administration of antibiotics, with a mean of 27 days.
Table 1.
Microorganisms isolated during first stage surgery
| Microorganism | Number |
|---|---|
| Staphylococcus aureus, oxacillin sensitive | 14 |
| Staphylococcus aureus, oxacillin resistant | 8 |
| Coagulase-negative staphylococcus | 13 |
| Proprionibacterium species | 3 |
| Streptococcus viridans | 2 |
| Streptococcus agalactia | 2 |
| Streptococcus milleri | 1 |
| Streptococcus pneumoniae | 1 |
| Enterococcus | 1 |
| Gram-positive anaerobic coccus, unspecified | 1 |
| Polymicrobial | 4 |
| Negative cultures | 11 |
All patients signed an informed consent form, as required by the local university ethics committee. The diagnosis of deep infection was based on a combination of technical and clinical criteria, including the presence of a sinus tract communicating with the prosthesis, haematological screening tests, culture from hip aspirates and radiological findings. In both stages, surgery was performed via the posterior approach by the same senior surgeon. At the first stage, all foreign material was removed, followed by a thorough debridement of all necrotic or infected tissue. Standard deep-tissue specimens of synovium, acetabular and femoral prosthesis–bone interface were obtained for microbiological analysis. A pressurised pulse-lavage system was repeatedly used throughout the procedure. All patients received a commercially available monoblock Spacer G loaded with gentamicin (Tecres, Verona, Italy). The inner part of the spacer consists of a stainless steel rod that provides mechanical stability. These spacers are available in six versions: three head sizes ( 46, 54 and 60 mm) in a short- (153–168 mm) and long- (275–290 mm) stem version (Fig. 1). The latter versions are usually reserved for large osseous defects of the proximal femur or when a femoral osteotomy has been performed for implant removal (Fig. 2). The cement spacer was inserted using the press-fit technique. In selected cases, additional limited cementation of the proximal femur was required to enhance rotational stability of the spacer (Fig. 3).
Fig. 1.
Preformed Spacer G is available in six versions
Fig. 2.
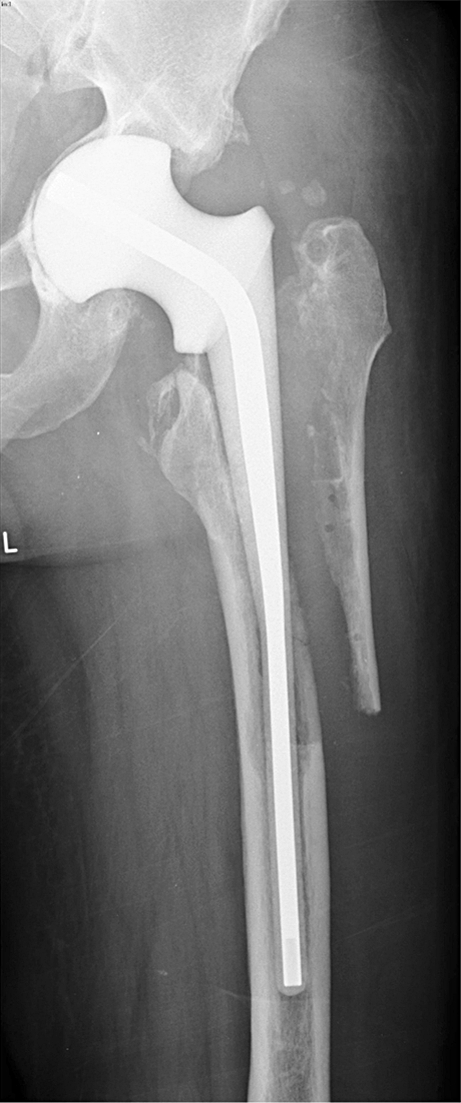
Long version of Spacer G used in combination with femoral osteotomy
Fig. 3.
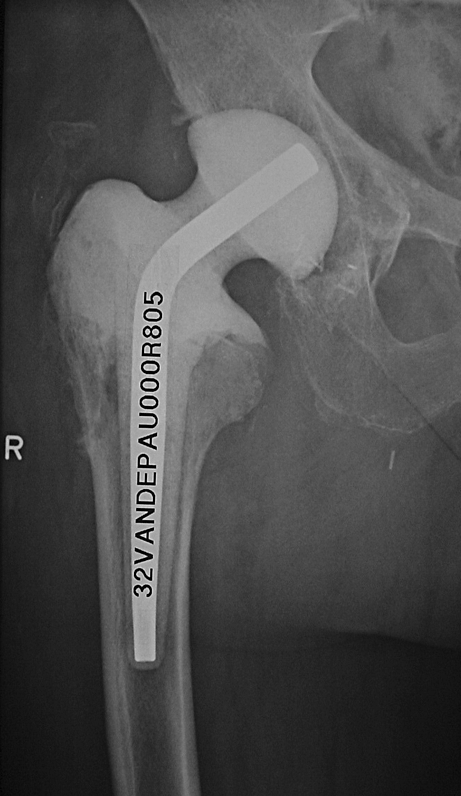
Limited cementation of the proximal femur enhances rotational stability of the spacer
Postoperatively, patients were encouraged to be mobile, and partial weightbearing was allowed. All patients received intravenous antibiotics during their hospital stay. Antibiotic treatment was adjusted to culture results from deep-tissue samples obtained at the first surgical stage. Between the two stages, patients were discharged from hospital if they were comfortable and medically healthy and were prescribed antibiotics if the appropriate antibiotic was available in oral form. Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels were monitored every two weeks. Patients were examined six weeks after spacer implantation before the second stage, and functional outcome was assessed using the Harris Hip and the Merle d’Aubigne Scores. Radiographs were taken at the beginning and end of spacer interval. The Paprosky classification was used to determine osseous femoral and acetabular defects (Table 2). Spacer-related adverse events—in particular, spacer dislocations—were evaluated in relation to the Paprosky score using the Mann–Whitney U rank-sum test. Level of significance was set at α = 0.05.
Table 2.
Paprosky classification
| Paprosky classification | |
| Femoral defects | |
| Type I | Minimal loss of metaphyseal cancellous bone, intact diaphysis |
| Type II | Extensive loss of metaphyseal cancellous bone, intact diaphysis |
| Type IIIa | Metaphysis not supportive, > 4 cm bone in the diaphysis for distal fixation |
| Type IIIb | Metaphysis not supportive, < 4 cm bone in the diaphysis for distal fixation |
| Type IV | Extensive metaphyseal and diaphyseal damage in conjunction with a widened femoral canal |
| Acetabular defects | |
| Type I | Indicates an intact and supportive acetabular rim, with no migration of the component, no evidence of osteolysis in the ischium or tear drop and no violation of the Köhler line. |
| Type II | Indicates adequate host bone remaining to support a cementless acetabular component and >50 % host bone support, with <2 cm of superior migration of the hip centre from the superior obturator line and no major osteolysis of the ischium or tear drop (ischial osteolysis of <7 mm below the obturator line) |
| Type IIIa | Indicates > 2 cm of superior and lateral migration of the component above the obturator line with mild to moderate ischial lysis. The component is at or lateral to the Köhler line. and the ilioischial and iliopubic lines are intact. The failed component migrates superiorly and laterally |
| Type IIIb | indicates more extensive ischial osteolysis (> 15 mm below the obturator line), complete destruction of the tear drop, migration medial to the Köhler line, and >2 cm of superior migration of the component cephalad to the obturator line. The failed component migrates superiorly and medially |
The second stage was usually performed at six weeks, but only if blood infection parameters had returned to normal. When there was insufficient response to antibiotic treatment, the second stage was delayed, and repeat debridement with spacer exchange was performed. At the second stage, the spacer was removed, samples for microbiological examination were taken and extensive debridement with lavage was again carried out before reimplantation. Patients received antibiotics orally for an additional six weeks to three months, depending on their clinical and haematological assessments.
Results
Duration of IV antibiotic treatment averaged 20 (range 9–99) days. Forty-six patients (75.4 %) were able leave hospital between the two stages after a mean stay of 17.8 days. Forty-one (67.2%) of them were able to ambulate with crutches, and five were wheelchair-bound. Mean Harris Hip and Merle d’Aubigné scores at six weeks after spacer implantation were 39.9 (median 40, range 6–79) and 7.6 (median eight, range two to 16), respectively. Thirty-two patients received a short cement spacer and the other 29 the long-stem version. In six patients who showed insufficient response to antibiotic treatment, the second stage was delayed, and repeat debridement with spacer exchange was performed.
Spacer dislocation occurred in ten patients (16.4%), of whom eight were treated conservatively with restricted weightbearing (Fig. 4). Five of the patients who suffered spacer dislocation were able to leave hospital between stages. One had an open reduction due to sciatic nerve palsy, which resolved after the intervention, and another was treated by open reduction and skin traction due to severe hip pain.
Fig. 4.
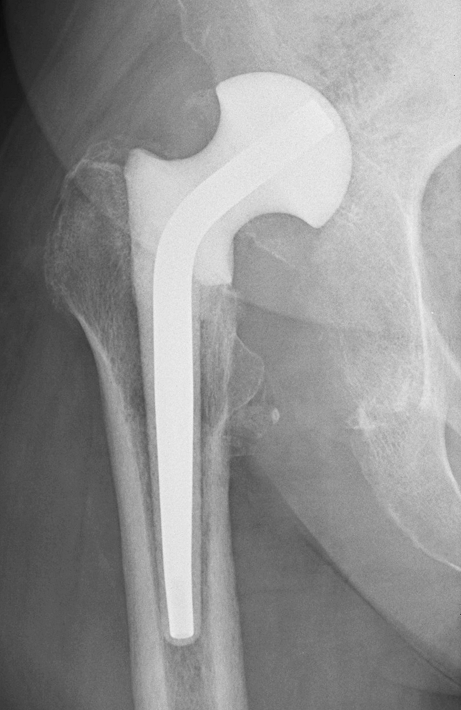
Dislocated spacer. Patient was put on a restricted weightbearing regimen
Seven femoral fractures (11.4 %) were encountered in our series; six were of the proximal femur that occurred during the first stage and were successfully treated by fixation with cable wires at the time of hip prosthesis implantation during the second stage; the remaining one was a diaphyseal fracture that occurred four weeks after the first stage (Fig. 5), and although there was no marked displacement, we debrided the haematoma and exchanged the spacer–as there was apparently insufficient response to antibiotics–followed by a nonweightbearing regimen. We encountered no spacer breakage in this group of patients (Table 3).
Fig. 5.
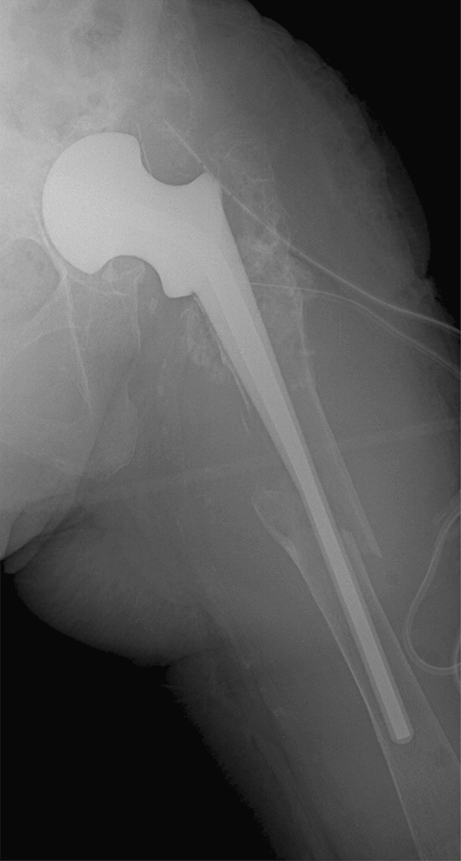
Diaphyseal fracture occurred 4 weeks after spacer implantation
Table 3.
Mechanical complications
| Mechanical complications | Number |
|---|---|
| Spacer fractures | 0 |
| Spacer dislocations | 10 |
| - Sciatic nerve palsy | 1 |
| - Extreme pain requiring reintervention | 1 |
| Femoral fractures | 7 |
| - During first stage | 6 |
| - Between stages | 1 |
| - During second stage | 0 |
Two patients (3.3%) had a reinfection after the second-stage procedure and were successfully treated with a repeat, two-stage revision. At the latest follow-up (mean 36 months, range nine to 84 months), three hips (4.9%) required revision due to complications other than infection: two were recurrent dislocations that required cup revision; the other was a metal-on-metal implant that was converted to a ceramic friction couple to treat metallosis. Spacer-related adverse events were evaluated with the Paprosky score. Only large acetabular defects—Paprosky scores IIIa and IIIb—were significantly related with an increased dislocation risk (P = 0.02).
Discussion
Operative treatment of a chronically infected total hip replacement remains a controversial issue. One-stage revision, if successful, provides the best benefit for patient and and is the most cost effective [10]. A literature review by Jackson et al. reported an 83% infection eradication rate after one-stage revision arthroplasty with the use of almost exclusively cemented implants [11]. However, Hanssen et al. reported higher incidences of recurrent infection after single-stage revisions without antibiotic-loaded cement [12]. For these reasons, two-stage revision with local antibiotic delivery has been recommended by a number of authors as the preferred treatment for late infection of a total hip prosthesis [8, 13, 14]. Two-stage reimplantations have been found to successfully eradicate infection in 90–100% of cases when local antibiotic cement beads or spacers are used [3–7, 15]. Additional advantages of a cement spacer are maintenance of a functional joint, limitation of scar formation and ease of reimplantation of the final prosthesis. Spacers allow the use of a cementless reconstruction and allografts, as suggested by Berry et al. and Gustillo et al., which is an advantage when dealing with large osseous defects such as those frequently encountered after an infected hip arthroplasty [16, 17]. Cement spacers provide greater patient comfort during the interim period and allow ambulation, which facilitates their discharge from hospital. In our study group, 46 patients (75.4%) were able to leave hospital between the two stages, decreasing the costs of treatment. Most of them were independent and able to walk with crutches.
The optimal protocol for studying patient functionality would be to compare cement spacers with a Girdlestone procedure in a randomised fashion. To our knowledge, Hsieh et al. are the only authors to compare the Girdlestone procedure with the use of antibiotic-loaded beads and cement spacer implantation and to study functional results during the interim period of a two-stage protocol [18]. They found that antibiotic-loaded cement prosthesis was associated with better functional results in the interim period, a lower complication rate and less problems at the time of reimplantation. The Merle d’ Aubigné score was 13.3 with the use of a custom-made hip spacer in the interim period compared with 10.2 when cement beads were used. With the PROSTALAC articulated spacer, Haddad et al. reported an average Harris Hip Score of 56 during this period [14]. In our series, Harris Hip and Merle d’Aubigné Scores were 39.9 and 7.6, respectively, using preformed commercially available cement spacers.
Despite these obvious functional advantages, several mechanical complications may occur when cement spacers are used. Spacer fractures, dislocations and femoral fractures have frequently been reported, although their exact incidence is still unknown. The reasons for mechanical problems are multifactorial. Some are related to the spacer itself (manually formed vs. preformed, spacer geometry, femoral fixation); others are patient-related (bone quality, femoral and acetabular defects, soft-tissue insufficiency and compliance with the partial weightbearing regimen). Spacer dislocation is one of the most common mechanical complications. Widely divergent dislocation rates have been reported in the literature. Some authors encountered >20% dislocation rate, whereas others observed none [3, 19–21]. In general, hip-spacer dislocation rates are higher if the patient is noncompliant or cannot tolerate partial weightbearing, if the size of the spacer is too small, if the spacer is insufficiently fixed onto the proximal femur, if muscular insufficiency is present and if large osseous defects of the acetabulum do not allow for normal spacer articulation [22]. The average dislocation rate is estimated to be between 10% and 20% [23]. Results are difficult to compare due to the variety in cement spacers. Customised spacers are more constrained, whereas preformed spacers are only available in a select number of sizes, with a risk of undersizing. This potentially results in a less rigid fixation, increasing the theoretical risk of instability and dislocation. For proximal femoral spacer fixation, we used the press-fit technique. In nearly all cases, we obtained sufficient fixation at the initial operation. In only four cases (6.6%) of large proximal femoral defects, where rotational stability was doubtful, was additional cementation onto the proximal femur required.
Spacer dislocation occurred in ten cases (16.4%), which is consistent with other reports in the literature [5, 19, 20, 24]. The dislocation rate was significantly higher in patients with large acetabular bone defects. Anagnostakos et al. suggested that a spacer cup should be implanted in these unstable cases so that the hip spacer would act as a total arthroplasty rather than as a hemiarthroplasty [23]. This could decrease the risk of spacer dislocation and prevent spacer migration into the pelvis. Data on managing hip-spacer dislocations are scarce in the literature. Because closed reduction can be difficult to achieve and dislocation may recur, treatment with orthosis or skin traction has been advocated [22]. In our empirical experience, most cases can be comfortably managed conservatively. In our series of ten dislocation cases, only two patients were treated operatively. Half of the patients (5/10) with dislocated spacer were comfortable enough without treatment to leave hospital between stages. One patient required an open reduction for pain, and another developed sciatic nerve palsy, which resolved after open reduction.
Femoral fractures are a common finding when dealing with hip-joint infections. Most patients have poor bone quality due to bone resorption, osteoporosis, disuse of the affected limb and previous operations. The majority of femoral fractures occur at the time of implant removal and are not related to the use of a cement spacer. In our series, six of seven femoral fractures were observed at the first stage. These fractures do not require immediate treatment and are usually managed at the second stage with the use of modular revision stems and cable wires.
An important finding in our series was the absence of spacer fractures. Several hip-spacer fractures are reported in the literature. For this reason, and to improve the mechanical properties, metallic endoskeletons can be inserted into the spacers. Thielen et al. compared the mechanical stability of reinforced and nonreinforced hip spacers in an in vitro study [25]. Nonreinforced spacers failed at loads of 400–600 N, whereas full-stem reinforced spacers only failed at 2,380–4,311 N. Kummer et al. compared the strength of a commercially available preformed hip spacer with that of three hip-spacer constructions: Steinmann pins, an intramedullary nail and a Charnley prosthesis [26]. The preformed hip spacer and the Charnley prosthesis were equivalent in strength and did not fail at 3,000 N, whereas the other constructions failed at significantly lower loads. These mechanical studies and the results of our series and others support the use of a metallic endoskeleton because it allows partial weightbearing with a minimal risk of spacer breakage [5, 27].
A limitation of preformed gentamicin-loaded spacers is that specific antibiotics specific to the causative organism cannot be added during surgery. However, preoperative culture of aspirated synovial fluid identifies the infecting micro-organism in 45–100% of cases [28]. In order to deal with methicillin-resistant organisms, vancomycin-loaded spacers have recently become available (Vancogenx Space Hip, Tecres).
In conclusion, the results of our series confirm that chronic hip infections can successfully be managed with a two-stage revision. Commercially available preformed spacers reduce the operating time and provide good functional outcome between stages. More than 75% of our patients were able to leave hospital in the interim period, offering important social and economic advantages. Spacer dislocation is the most frequently encountered mechanical complication (16.4%), which seems to be consistent with rates reported in the literature. Most dislocations can be managed conservatively. Femoral fractures usually occur during implant removal and are managed at the second stage. The risk of breakage seems negligible with the use of preformed hip spacers.
Acknowledgments
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Pozo JL, Patel R. Infection associated with prosthetic joints. N Engl J Med. 2009;361:787–794. doi: 10.1056/NEJMcp0905029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanssen AD, Spangehl MJ. Treatment of infected hip replacement. Clin Orthop Relat Res. 2004;420:63–71. doi: 10.1097/00003086-200403000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Koo KH, Yang JW, Cho SH, Song HR, Park HB, Ha YC, Chang JD, Kin SY, Kim YH. Impregnation of vancomycin, gentamycin, and cefotaxime in the cement spacer for two-stage cementless reconstruction in infected total hip arthroplasty. J Arthroplasty. 2001;16:882–892. doi: 10.1054/arth.2001.24444. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto K, Miyagawa N, Masoaka T, Katori Y, Shisido T, Imakiire A. Cement spacer loaded with antibiotics for infected implants of the hip joint. J Arthroplasty. 2009;24:83–89. doi: 10.1016/j.arth.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 5.Younger ASE, Duncan CP, Masri BA, McGraw RW. The outcome of two-stage arthroplasty using a custom-made interval spacer to treat the infected hip. J Arthroplasty. 1997;12(6):615–623. doi: 10.1016/S0883-5403(97)90133-9. [DOI] [PubMed] [Google Scholar]
- 6.Lieberman JR, Callaway GH, Salvatti EA, Brause BD. Treatment of the infected total hip arthroplasty with a two-stage reimplantation protocol. Clin Orthop. 1994;301:205–212. [PubMed] [Google Scholar]
- 7.Masri BA, Panagiotopoulos KP, Greidanus NV, Garbuz DS, Duncan CP. Cementless two-stage exchange arthroplasty for infection after total hip arthroplasty. J Arthroplasty. 2007;22(1):72–78. doi: 10.1016/j.arth.2006.02.156. [DOI] [PubMed] [Google Scholar]
- 8.Anagnostakos K, Fürst O, Kelm J. Antibiotic-impregnated PMMA hip spacers: current status. Acta Orthop. 2006;77:628–637. doi: 10.1080/17453670610012719. [DOI] [PubMed] [Google Scholar]
- 9.Kent M, Rachha R, Sood M. A Technique for the fabrication of a reinforced moulded articulating cement spacer in two-stage revision total hip arthroplasty. Int Orthop. 2010;34(7):949–953. doi: 10.1007/s00264-009-0847-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoo JJ, Kwon YS, Koo KH, Yoon KS, Kim YM, Kim HJ. One-stage cementless revision arthroplasty for infected hip replacements. Int Orthop. 2009;33(5):1195–1201. doi: 10.1007/s00264-008-0640-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson WO, Schmalzreid TP. Limited role of direct exchange arthroplasty in the treatment of infected hip replacements. Clin Orthop Relat Res. 2000;381:101–105. doi: 10.1097/00003086-200012000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Hanssen AD, Rand JA. Evaluation and treatment of infection site of a total hip or knee arthroplasty. J Bone Joint Surg Am. 1998;80-A:910–922. [Google Scholar]
- 13.Garvin KL, Hansen AD. Infection after total hip artroplasty. Past, present and future. J Bone Joint Surg Am. 2005;77-A:1576–1588. doi: 10.2106/00004623-199510000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Haddad FS, Masri BA, Garbuz DS, Duncan CP. The treatment of the infected hip replacement. The complex case. Clin Orthop Relat Res. 1999;369:144–156. doi: 10.1097/00003086-199912000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Haddad FS, Muirhead-Allwood SK, Manktelow AR, Bacarese Hamilton I. Two-stage uncemented revision hip arthroplasty for infection. J Bone Joint Surg Br. 2000;82-B(5):689–694. doi: 10.1302/0301-620X.82B5.9668. [DOI] [PubMed] [Google Scholar]
- 16.Berry DJ, Chandler HP, Reilly DT. The use of bone allografts in two-stage reconstruction after failure of hip replacement due to infection. J Bone Joint Surg Am. 1991;73-A:1460–1468. [PubMed] [Google Scholar]
- 17.Gustilo RB, Pasternak HS. Revision total hip arthroplasty with titanium ingrowth prosthesis and bone grafting for failed cemented femoral component loosening. Clin Orthop Relat Res. 1998;235:111–119. [PubMed] [Google Scholar]
- 18.Hsieh PH, Shih CH, Chang YH, Lee MD, Shih HN, Yang WE. Two-stage revision hip arthroplasty for infection: comparison between the interim use of antibiotic-loaded cement beads and a spacer prosthesis. J Bone Joint Surg Am. 2004;86-A:1989–1997. [PubMed] [Google Scholar]
- 19.Jahoda D, Sosna A, Landor I, Vavrik P, Pokorny D. Canulated spacer in the treatment of deep infection of total hip arthroplasty using a two-stage reimplantation. Oper Orthop Traumatol. 2003;1:57–69. doi: 10.1007/s00064-003-1063-x. [DOI] [Google Scholar]
- 20.Leunig M, Chosa E, Speck M, Ganz R. A cement spacer for two-stage revision of infected implants of the hip-joint. Int Orthop. 1998;22(4):209–214. doi: 10.1007/s002640050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin SS, Della Valle CJ, Ong BC, Meere PA. A simple method for construction of an articulating antibiotic loaded spacer. J Arthroplasty. 2002;17(6):785–787. doi: 10.1054/arth.2002.33568. [DOI] [PubMed] [Google Scholar]
- 22.Anagnostakos K, Jung J, Schmid NV, Schmitt E, Kelm J. Mechanical complications and reconstruction strategies at the site of hip spacer implantation. Int J Med Sci. 2009;6(5):274–279. doi: 10.7150/ijms.6.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anagnostakos K, Köhler D, Schmitt E, Kelm J. The “glove” technique: a modified method for femoral fixation of antibiotic-loaded hip spacers. Acta Orthop. 2009;80(3):386–388. doi: 10.3109/17453670903039551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung J, Schmid NV, Kelm J, Schmitt E, Anagnostakos K. Complications after spacer implantation in the treatment of hip joint infections. Int J Med Sci. 2009;6(5):265–273. doi: 10.7150/ijms.6.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thielen T, Maas S, Zuerbes A, Waldmann D, Anagnostakos K, Kelm J. Development of a reinforced PMMA based hip spacer adapted to patients’ needs. Med Eng Phys. 2009;31(8):930–936. doi: 10.1016/j.medengphy.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Kummer FJ, Strauss E, Wright K, Kubiak EN, Cesare PE. Mechanical evaluation of unipolar hip spacer constructs. Am J Orthop. 2008;37(10):517–518. [PubMed] [Google Scholar]
- 27.Romanò CL, Romanò D, Logoluso N, Meani E. Long-stem versus short-stem preformed antibiotic-loaded cement spacers for two-stage revision of infected total hip arthroplasty. Hip Int. 2010;20(1):26–33. doi: 10.1177/112070001002000104. [DOI] [PubMed] [Google Scholar]
- 28.Trampuz A, Hanssen AD, Osmon DR, Mandrekar J, Steckelberg JM, Patel R. Advances in the laboratory diagnosis of prosthetic joint infection. Rev Med Microbiol. 2003;14:1–14. [Google Scholar]



