Abstract
Purpose
The purpose of this study was to investigate (a) whether pre-operative serum CRP is a predictor of survival in patients with high-grade osteosarcoma, (b) whether post-operative infection is a predictor of survival in these patients and (c) whether CRP is a predictor of post-operative infection, and especially deep prosthetic infection.
Methods
In this retrospective single-centre study, pre-operative serum CRP levels in 79 patients (37 females, 42 males; average age, 18 years; mean follow-up, 46 months) undergoing resection of an osteosarcoma were correlated with clinical data and survival.
Results
The mean pre-operative serum CRP level of all 79 patients was 0.53 mg/dl (SD, 1.27 mg/dl). Patients dying of their underlying disease had significantly higher CRP levels compared to patients surviving throughout the follow-up period (1.09 mg/dl ± 2.02 mg/dl versus 0.32 mg/dl ± 0.75 mg/dl, respectively; p = 0.015). CRP levels were significantly correlated with survival (Pearson’s correlation coefficient = −0.25; p = 0.026) and histological subtype (Pearson’s correlation coefficient = −0.42; p < 0.001), but not with sex, age, histological response, tumour size or metastatic disease. In uni- and multivariate survival analysis, age, response to chemotherapy and serum CRP were associated with disease-specific survival. Patients with a CRP level over 1 mg/dl had a significantly lower disease-specific five-year survival of 36.7% compared to 73.8% in patients with normal CRP values (p = 0.020). Infection was not correlated with disease-specific survival. Pre-operative serum CRP levels were not correlated with post-operative infection or deep prosthetic infection.
Conclusions
Pre-operative serum CRP seems to be an independent predictor of survival in patients with high-grade osteosarcoma. Further studies are needed to confirm these results on a large-scale basis.
Introduction
Osteosarcoma is the most frequent primary malignant bone tumour [1]. Standard therapy today comprises neo-adjuvant multidrug chemotherapy, wide surgical resection and adjuvant chemotherapy. It is well established that patients with poor histological response to chemotherapy have an adverse outcome [2, 3]. Even though efforts have been made to improve the survival of these patients by dose intensification or second-line therapies, effects of such changes are limited [4]. Therefore, many studies have tried to define different prognostic factors not only to improve outcome evaluation and potentially stratify risk before starting therapy, but also to investigate new targets for alternative treatment regimens [2, 5–9]. In osteosarcoma, definition of such factors, both on a clinical and molecular basis, remains controversial due to the rarity of the disease, and there is an ongoing search for further, easily available markers predicting survival. In this context, even post-operative infection in patients undergoing surgery for osteosarcoma has been raised as a potential predictor of survival [10, 11].
C-reactive protein (CRP), an acute phase protein, is produced mainly by hepatocytes as a response to inflammation [12]. Thus, the measurement of circulating serum CRP is an important monitor of inflammatory disease in clinical routine [13]. Moreover, there is a rapidly increasing body of evidence that CRP levels can also be elevated in patients with chronic infections, artherosclerosis or malignant disease when compared to healthy controls [14–18]. Additionally, it has been shown that in many cancer types overall survival is strongly linked to and can reliably be predicted by pre-operative serum CRP levels [19]. To date there is no published evidence that this is also the case in patients with high-grade osteosarcoma.
Finally, elevated pre-operative CRP levels have been linked to an elevated peri-operative risk for complication and post-operative infection in patients undergoing surgery [20–22]. Infection represents one of the most severe complications in patients undergoing limb salvage surgery for musculoskeletal tumours by endoprosthetic reconstruction, which today is regarded the gold standard of extremity osteosarcoma treatment. Infection in these patients has a lifelong incidence of 12% and often leads to frequent revision surgery, pain, immobility or even amputation [23]. CRP was shown to predict deep infection in standard arthroplasty [24]; however, no such data is available for the orthopaedic-oncological patient population.
The aim of this retrospective single-centre study was to investigate (a) whether pre-operative serum CRP is a predictor of survival in patients with high-grade osteosarcoma, (b) whether post-operative infection is a predictor of survival in these patients and, furthermore, (c) whether CRP is a predictor of post-operative infection, and especially of deep prosthetic infection.
Material and methods
To evaluate the prognostic impact of pre-operative serum CRP levels on survival, database analysis within the Vienna Bone and Soft Tissue Tumour Registry identified 192 patients who underwent surgical treatment for an osteosarcoma between 1998 and 2008. Inclusion criteria for this retrospective single centre study of histologically confirmed high-grade osteosarcoma, were treatment after 1998 to provide standardised and technically consistent CRP measurement; a minimum follow-up of two years, unless death from disease had occurred within those two years; and pre-operative in-house assessment of serum CRP. Patients were not allowed to have a history of nicotine consumption or cardio-vascular disease at the time of treatment. A concomitant infection was ruled out by clinical and radiological investigation. Patients over the age of 60 years and patients with an osteosarcoma of the soft tissues were excluded. Consequently, a total of 79 patients (41.1%) were included in this analysis. Data were collected with retrospective review of patient files in our registry. Approval of the respective institutional review board was obtained prior to this investigation and informed consent was routinely obtained from the subjects and/or their guardians at the time of treatment to further use their files and registry data for scientific analyses.
Patients
The study group included 37 female (46.8%) and 42 male (53.2%) patients with a mean age of 18 years (median, 16 years; range 6–50 years). Tumour distribution included the femur in 42 patients (53.2%), the tibia in 20 (25.3%), the humerus in seven (8.9%) and other bones in ten (12.7%). Mean tumor size was 10.6cm in longest diameter (range, 1.0–27.5cm). All diagnoses were histologically confirmed by open biopsy and all patients were managed with curative intention by multi-modal treatment including wide tumour resection and (neo-)adjuvant chemotherapy according to the COSS-96 protocol (66 patients, 83.5%), the EURAMOS-1 protocol (seven patients, 8.9%) or other protocols (six patients, 7.6%). Fifty-nine patients (74.7%) underwent resection and endoprosthetic reconstruction, 14 (17.7%) had a resection with or without biological reconstruction, five (6.3%) had a resection-replantation or rotationplasty and one patient (1.3%) was amputated. Resection margins were wide in 77 patients (97.5%) and marginal in two (2.5%). Histological tumour subtypes comprised conventional osteosarcoma (73 patients, 92.4%), teleangiectatic osteosarcoma (four, 5.1%) and high-grade parosteal osteosarcoma (two, 2.5%). Response to chemotherapy was rated as Salzer-Kuntschik grades 1 to 6 [25]. Accordingly, responders were defined as grades 1 to 3 (23 patients, 29.1%), non-responders as grades 4 to 6 (56 patients, 70.9%). Patients were followed up by clinical and radiological examination. Our standard follow-up protocol for sarcoma patients consists of clinical and radiographic examination of the tumour site and thoracic/abdominal computertomography scans every four months for three years, every six months for further three years and yearly post-operatively thereafter, as well as yearly technetium bone scans.
CRP
Patients’ blood was obtained before tumour resection by venous puncture. CRP measurements were not expressly ascertained for study purposes but performed as part of a pre-operative clinical routine by a latex-enhanced immuno-turbidimetric test (Olympus AU 2700 CRP Latex, Beckman Coulter Inc., Brea, California) according to the manufacturer’s instructions. The respective hardware specifications indicate a coefficient of variation of 1.65–3.79% in a normal-sensitivity setting, covering serum CRP levels ranging from 0.02–45 mg/dl. A serum CRP level of <1 mg/dl was regarded normal corresponding to our clinical routine practice.
Statistical analysis
Statistical analyses of the data focused on disease-specific survival in relation to CRP levels or infection, respectively. CRP values are indicated as means with standard deviation (SD). Values were compared using the t-test, correlations calculated by the Pearson’s correlation coefficient. Demographic (sex and age), surgical (endoprosthetic or other reconstruction) and clinico-pathological variables (serum CRP-level, response to chemotherapy, histological subtype, tumour size, post-operative infection, metastatic disease and death of disease) were examined. Age, tumour size and serum CRP levels were regarded as continuous variables; all other covariates were modelled as categorical variables. Investigated endpoints of the study were death of disease, metastatic disease and post-operative infection. Actuarial survival was estimated using the Kaplan-Meier method. Significance between survival functions was identified by the log-rank test. According to previous reports investigating the prognostic value of post-operative infection on survival [10, 11], landmark analysis was performed additionally in order to better control confounding factors. For this, a landmark was defined at 12 months post-operatively and patients dying from metastases before this landmark as well as patients developing infection after this landmark were excluded before survival analysis. Uni- and multivariate analyses were calculated by a Cox-regression model. All statistical tests were two-sided. A p-value of <0.05 was considered significant. All calculations were made with the SPSS® (SPSS Inc., Chicago, Illinois; version 13.0, 2004) software package, graphical visualisation was performed by use of GraphPad Prism® (GraphPad Software Inc., LaJolla, California; version 4.00, 2003).
Results
A total of 30 patients (38.0%) developed metastatic disease at a mean of 12 months post-operatively (median, ten months; range, 1–44 months). Twenty-six patients (32.9%) developed metastatic disease within 24 months of diagnosis. Twenty-two patients (27.8%) died of their underlying disease 21 months after operation (median, 17 months; range, 1–60 months). There were no concurrent fatal events throughout the follow-up period. Disease-specific survival of all patients was 81.0% at two years and 67.5% at five and ten years, respectively. The mean overall follow-up was 46 months (median, 33 months; range, 1–126 months); the mean follow-up for patients surviving their disease was 56 months (median, 44 months; range, 24–126 months).
CRP and survival
The mean pre-operative serum CRP level of all 79 patients was 0.53 mg/dl (SD, 1.27 mg/dl). Fourteen patients (17.1%) had pre-operative serum CRP levels above the defined cut-off of 1 mg/dl, ranging from 1.20 mg/dl to 8.50 mg/dl. Table 1 gives a detailed description of patient characteristics and corresponding CRP levels. Patients dying of their underlying disease had significantly higher CRP levels compared to patients surviving throughout the follow-up period (1.09 mg/dl ± 2.02 mg/dl versus 0.32 mg/dl ± 0.75 mg/dl; p = 0.015). Also, patients with teleangiectatic or high-grade parosteal osteosarcoma had significantly higher CRP levels than patients with the conventional subtype (2.40 mg/dl ± 3.31 mg/dl versus 0.38 mg/dl ± 0.82 mg/dl; p < 0.001). Thus, CRP levels were significantly correlated with survival (Pearson’s correlation coefficient = −0.25; p = 0.026) and histological subtype (Pearson’s correlation coefficient = −0.42; p < 0.001), but not with sex, age, histological response, tumour size or metastatic disease. In a univariate survival analysis age, histological response to neo-adjuvant chemotherapy and pre-operative serum CRP were associated with disease-specific survival. These factors remained statistically significant in the multivariate Cox regression model. Table 2 represents the respective test results.
Table 1.
Clinico-pathological characteristics and respective pre-operative serum CRP levels of 79 patients with high-grade osteosarcoma
| Variable | N (%) | Mean pre-operative serum CRP level (SD) | p value (t-test) |
|---|---|---|---|
| Sex | |||
| Male | 42 (53.2%) | 0.42 (0.94) | |
| Female | 37 (46.8%) | 0.66 (1.57) | 0.417 |
| Age | |||
| <40 years | 74 (93.7%) | 0.55 (1.31) | |
| >40 years | 5 (6.3%) | 0.19 (0.27) | 0.545 |
| Post-operative infection | |||
| Yes | 13 (16.5%) | 0.25 (0.90) | |
| No | 66 (83.5%) | 0.59 (1.33) | 0.395 |
| Histological response to chemotherapy | |||
| Yes | 23 (29.1%) | 0.37 (0.80) | |
| No | 56 (70.9%) | 0.60 (1.42) | 0.478 |
| Tumor size | |||
| <10 cm | 46 (58.2%) | 0.50 (1.01) | |
| >10 cm | 33 (41.8%) | 0.58 (1.59) | 0.778 |
| Histological subtype | |||
| Conventional | 73 (92.4%) | 0.38 (0.82) | |
| Others | 6 (7.6%) | 2.40 (3.31) | <0.001 |
| Metastatic disease | |||
| Yes | 30 (38.0%) | 0.81 (1.78) | |
| No | 49 (62.0%) | 0.36 (0.80) | 0.132 |
| Death of Disease | |||
| Yes | 22 (27.8%) | 1.09 (2.02) | |
| No | 57 (72.2%) | 0.32 (0.75) | 0.015 |
CRP C-reactive protein, SD standard deviation
Table 2.
Univariate and multivariate survival analysis in 79 patients with high-grade osteosarcoma
| Variable | Univariate p | Multivariate p | Multivariate hazard ratio (95% confidence interval) |
|---|---|---|---|
| Sex | 0.281a | 0.410 | 1.5 (0.6–3.7) |
| Age | 0.003b | 0.003 | 1.1 (1.0–1.1) |
| Post-operative infection | 0.600a | 0.815 | 1.1 (0.4–3.3) |
| Histological response to chemotherapy | 0.002a | 0.024 | 10.8 (1.4–85.6) |
| Tumour size | 0.153b | 0.267 | 1.0 (1.0–1.1) |
| Histological subtype (conventional vs. other) | 0.052a | 0.519 | 0.6 (0.1–3.3) |
| Pre-operative serum CRP level | <0.001b | 0.031 | 1.4 (1.0–1.8) |
CRP C-reactive protein
a Log-rank test
bUnivariate Cox regression
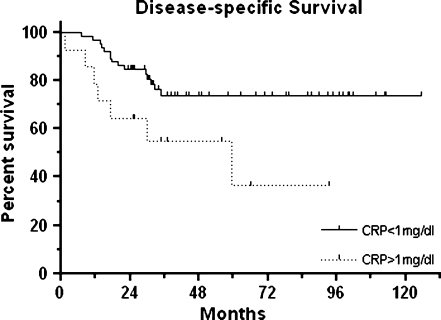
Patients with a pre-operative serum CRP level over 1 mg/dl had a significantly lower disease-specific two-year survival of 64.2% compared to 84.6% in patients with CRP values below the cut-off (p = 0.020). The respective disease-specific five-year survival rates were 36.7% versus 73.8% (see Fig. 1). Given our total patient number of 79, a power analysis revealed a power of 0.95 to detect a survival difference of 28.4% or higher.
Fig. 1.
Kaplan-Meier curves for disease-specific survival of 79 patients with high-grade osteosarcoma according to pre-operative serum C-reactive protein (CRP) levels
Infection and survival
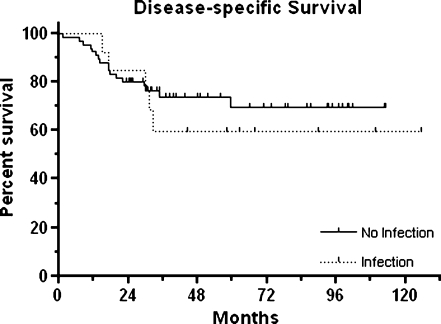
A total of 13 patients (16.5%) developed an infection at a mean of 17 months post-operatively (median, eight months; range, one to 53 months). Infection only occurred in patients with endoprosthetic reconstruction. Infection was not correlated with disease-specific survival. The disease-specific two-year and five-year survival rates of patients with infection versus patients without infection were 84.6% versus 80.3% and 59.8% versus 69.5%, respectively (p = 0.600; see Fig. 2).
Fig. 2.
Kaplan-Meier curves for disease-specific survival of 79 patients with high-grade osteosarcoma according to the event of post-operative infection
For landmark analysis, 22 patients (27.8%) were excluded as they either developed metastatic disease within 12 months post-operatively or had infection more than one year after index surgery. This did not reveal significant differences in disease-specific survival. The respective disease-specific survival rates were 100% for patients with infection versus 96.2% for patients without infection at two years and 75.0% versus 82.5% at five years, respectively (p = 0.740).
CRP and infection
Pre-operative serum CRP levels were not correlated with post-operative infection. Conversely, patients without post-operative infection had higher pre-operative CRP levels than patients with septic complications; however, this was not statistically significant (see Table 1). CRP levels had no significant impact on infection-free survival. Two-year and five-year infection-free survival of patients with a pre-operative serum CRP level over 1 mg/dl versus patients with normal levels were 100% versus 87.5% and 88.9% versus 73.9%, respectively (p = 0.368).
Endoprosthetic reconstruction
Finally, a subgroup analysis for the 59 patients with endoprosthetic reconstruction was performed. Pre-operative serum CRP levels were not significantly different between patients with or without deep prosthetic infection (p = 0.530). Infection-free survival was independent of pre-operative CRP levels (p = 0.399). Nor did infection have significant influence on disease-specific survival of patients with endoprosthetic reconstruction (p = 0.471) irrespective of landmark analysis (p = 0.771). There was, however, a significant difference in disease-specific survival between patients with CRP levels over 1 mg/dl versus patients with normal levels (two-year disease-specific survival, 70.0% versus 85.7%; five-year disease-specific survival, 37.3% versus 74.8%; p = 0.038) and again patients dying of their disease had significantly higher pre-operative serum CRP levels compared to those surviving throughout the follow-up period (0.80 mg/dl ± 1.32 mg/dl versus 0.24 mg/dl ± 0.64 mg/dl; p = 0.034).
Discussion
This study, to our best knowledge, represents the first attempt to investigate CRP as a prognostic factor in patients with high-grade osteosarcoma. We have been able to show that pre-operative serum CRP levels before tumour resection correlate with disease-specific outcome, i.e. patients with CRP levels above the cut-off of 1 mg/dl had a significantly worse survival. Furthermore, we have investigated the impact of infection on survival in these patients, but have failed to identify significant correlations. Finally, we were unable to link pre-operative CRP levels to the likelihood of developing post-operative infection. This was also the case in a subset of patients with endoprosthetic reconstruction.
There certainly are limitations to this study. First, this is an analysis with a retrospective, single-centre design. Osteosarcoma is a rare disease and for evaluation of prognostic factors long-term results are preferable. Therefore, most reports in this field are entirely based on retrospective series [6], as prospective trials would require a vast time span. Second, we only were able to include a small group of 79 patients. In total, 192 patients with osteosarcoma have been treated throughout the recruitment period; however, a high exclusion rate was dictated by the necessity of a reasonable follow-up for event-free patients and the elimination of clinical factors potentially influencing serum CRP levels. Nevertheless, we have identified a high power (0.95) in our survival analysis. The patients we have included form a representative spectrum of patients with high-grade osteosarcoma. The predominant age was around the second decade of life, there was a male preponderance and the anatomical tumour distribution reflects that of large scale studies [2, 3, 7]. As 87% of all metastatic events occurred within 24 months of diagnosis, our follow-up should provide adequate evidence. Also, the overall survival rate of 68% at five and ten years goes along with pooled results of the Cooperative Osteosarcoma Study Group [2]. Third, we have only investigated pre-operative CRP levels before tumour resection and not before commencement of chemotherapy. We are unable to draw any conclusions from these results concerning the value of CRP as a pre-treatment predictor before neo-adjuvant treatment. The rational for this primarily was that this study was designed to investigate not only the correlation between CRP and survival but also between CRP and post-operative infection as well as between infection and survival, and thus required the use of pre-operative CRP values as in previous investigations [20–22]. Furthermore, CRP levels before biopsy or induction therapy were only available in a small subset of our patients as in clinical routine of a tertiary referral centre many of these patients presented for tumour resection only and had undergone (neo-)adjuvant therapy at other institutions or presented with institutional CRP measurements, that were technically not comparable to our in-house assessments. Finally, due to the retrospective design we also were unable to assess the prognostic relevance of other acute phase proteins, as they were not a consistent part of standardised pre-operative diagnostic routine. The fact that elevated CRP levels before surgical treatment can predict survival raises the question of whether this marker could serve as a potential surveillance tool of tumour activity before and during chemotherapy [19] and has encouraged us to further investigate pre-biopsy CRP levels and other acute phase proteins such as fibrinogen.
There are many studies investigating demographic, clinicopathological, serological and molecular markers in osteosarcoma [2, 5–9, 26]. A variety of less common clinical factors such as disease onset, relative tumour burden, skip lesions, tumour pattern, tumour necrosis rate and/or volume under chemotherapy have shown prognostic evidence [26–28], while others such as pathological fracture have failed to do so or remain controversial [29, 30]. This has allowed the definition of risk stratification models [4] and even nomograms [26] throughout single-centre studies. Many of these factors, however, have lost their significance when analysed in large study cohorts or pooled data analyses and have been closely connected or outweighed by histological necrosis (i.e. response rate to neo-adjuvant chemotherapy) [5–7]. Well-established clinical factors are rather confined to age [2, 3, 7], tumour site [2, 7], size [3, 6] and response rate [2–7], with the latter being observed as the most reliable indicator [5]. Similarly, controversial evidence is reported for serological markers such as alkaline phosphatase or lactate dehydrogenase [3, 6, 8] while different molecular factors prove to be of rather reproducible predictive value, including, among others, human epidermal growth factor receptor 2 (HER2), vascular endothelial growth factor (VEGF), p-glycoprotein, transmembrane glycoprotein ErbB-2, chemokine receptor, matrix metalloproteinases (MMPs), livin, ezrin or heat shock proteins [8, 9, 31–33]. Though, their measurement is to some extent time-consuming and complex and therefore often not integrated into clinical practice.
CRP is evaluated in daily routine. Measurement is relatively cheap and performed by commercially available and standardised test systems. Elevated levels of CRP have been described as a prognostic factor in various human malignancies including like gastro-esophageal, ovarian, and several others [34, 35]. Significantly higher levels of CRP in cancer patients compared to healthy controls have been linked to carcinogenesis as expression of the host defence or as paraneoplastic syndrome due to the up-regulation of inflammatory pathways by the tumour microenvironment [18, 19]. Some of the malignancies investigated in this context have been closely tied to inflammatory disease [34, 35]. Such connections have not yet been described in osteosarcoma.
In this study, we have observed significantly higher levels of pre-operative CRP in patients dying of osteosarcoma. All patients in this series died of metastatic disease, and there certainly was a trend towards higher levels in patients developing metastases (see Table 1), possibly linking CRP to aggressive tumour behaviour. CRP levels were also higher in non-responders to chemotherapy; however, this was not statistically significant. CRP levels were not correlated to tumour size. Therefore, it remains unclear, whether CRP is elevated, because of aggressive tumour biology, or if this is the consequence of local inflammatory host reactions to a tumour with a high rate of viable cells despite chemotherapy (i.e. non-responder).
CRP was additionally elevated in patients with other than conventional histological subtype, but subtype had no influence on survival. Besides, the low number of this subgroup (six out of 73) limits the usefulness of this finding. In the uni- and multivariate survival analysis, age, response rate and CRP levels were the only independent prognostic variables (see Table 2 and Fig. 1).
Post operative infection was also shown to be of prognostic value in patients undergoing surgery for osteosarcoma. A study of 412 patients with osteosarcoma has shown that the ten-year survival of 85% for patients with infection was significantly better compared to 62% in the non-infected group [10]. Controversially, this data could not be confirmed by another report on 31 infected patients out of a total cohort of 347 after matched control group analysis and the authors attributed positive effects of infection on survival to clinical characteristics of infected patients rather than to an anti-tumour effect of infection itself [11]. Our study did not find a significant impact of infection on survival (see Fig. 2). Patients with infection had even lower pre-operative CRP levels compared to non-infected patients (not significant; see Table 1), which leaves the impact of infection unclear.
Several surgical studies have linked high pre-operative CRP levels to later surgical complications [20, 21] or post-operative infection, especially after orthopaedic interventions such as arthroplasty [22, 24]. No such data has so far been reported for orthopaedic-oncological patients or patients receiving megaprostheses. In this series, post-operative infection was not predictable from elevated pre-operative CRP levels, despite a similar methodological approach. It is noteworthy that, all patients with infection had also received megaprostheses, and it is well established that these implants are accompanied by a remarkable risk of infection [23]. We therefore conclude that this factor (i.e. the implantation of a megaprosthesis) outweighs a possible predictive effect of elevated CRP levels or similar prognosticators of post-operative infection. We also performed a sub-group analysis of patients with megaprostheses, as deep infection of standard total joint arthroplasties was described to depend on elevated pre-operative CRP [22]. A recent report has shown that optimised CRP cut-off levels to predict infection in standard arthroplasties range from >1 mg/dl to 7 mg/dl [24]. This was not the case in our study and we failed to predict prosthetic infection, again most likely due to the elevated risk of infection in megaprostheses per se. Obviously, in oncological patients with megaprostheses, there are a variety of different additional factors influencing the risk of implant infection such as immuno-suppression, long operating times and extensive soft tissue loss. Strikingly, in this subset of patients with megaprostheses (i.e. extremity osteosarcoma only), CRP was still significantly predicting disease-specific survival.
In conclusion, pre-operative serum CRP seems to be an independent predictor of survival in patients with high-grade osteosarcoma. CRP levels do not predict the likelihood of a post-operative infection or deep prosthetic infection in these patients. The impact of infection on survival remains unclear. Further studies are required to confirm these results on a large-scale basis and to investigate CRP and other acute phase proteins as a pre-treatment predictor in osteosarcoma patients before induction chemotherapy.
Acknowledgments
Disclosure The authors declare that they have no conflict of interest.
References
- 1.Dahlin DC, Coventry MB. Osteogenic sarcoma—a study of six hundred cases. J Bone Joint Surg Am. 1967;49:101–110. [PubMed] [Google Scholar]
- 2.Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790. doi: 10.1200/JCO.20.3.776. [DOI] [PubMed] [Google Scholar]
- 3.Bacci G, Longhi A, Versari M, Mercuri M, Briccoli A, Picci P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer. 2006;106:1154–1161. doi: 10.1002/cncr.21724. [DOI] [PubMed] [Google Scholar]
- 4.Lee JA, Kim MS, Kim DH, et al. Risk stratification based on the clinical factors at diagnosis is closely related to the survival of localized osteosarcoma. Pediatr Blood Cancer. 2009;52:340–345. doi: 10.1002/pbc.21843. [DOI] [PubMed] [Google Scholar]
- 5.Davis AM, Bell RS, Goodwin PJ. Prognostic factors in osteosarcoma: a critical review. J Clin Oncol. 1994;12:423–431. doi: 10.1200/JCO.1994.12.2.423. [DOI] [PubMed] [Google Scholar]
- 6.Bramer JA, Linge JH, Grimer RJ, Scholten RJ. Prognostic factors in localized extremity osteosarcoma: a systematic review. Eur J Surg Oncol. 2009;35:1030–1036. doi: 10.1016/j.ejso.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Pakos EE, Nearchou AD, Grimer RJ, et al. Prognostic factors and outcomes for osteosarcoma: an international collaboration. Eur J Cancer. 2009;45:2367–2375. doi: 10.1016/j.ejca.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Trieb K, Kotz R. Proteins expressed in osteosarcoma and serum levels as prognostic factors. Int J Biochem Cell Biol. 2001;33:11–17. doi: 10.1016/S1357-2725(00)00066-2. [DOI] [PubMed] [Google Scholar]
- 9.Clark JC, Dass CR, Choong PF. A review of clinical and molecular prognostic factors in osteosarcoma. J Cancer Res Clin Oncol. 2008;134:281–297. doi: 10.1007/s00432-007-0330-x. [DOI] [PubMed] [Google Scholar]
- 10.Jeys LM, Grimer RJ, Carter SR, Tillman RM, Abudu A. Post operative infection and increased survival in osteosarcoma patients: are they associated? Ann Surg Oncol. 2007;14:2887–2895. doi: 10.1245/s10434-007-9483-8. [DOI] [PubMed] [Google Scholar]
- 11.Lee JA, Kim MS, Kim DH, et al. Postoperative infection and survival in osteosarcoma patients. Ann Surg Oncol. 2009;16:147–151. doi: 10.1245/s10434-008-0184-8. [DOI] [PubMed] [Google Scholar]
- 12.Marnell L, Mold C, Clos TW. C-reactive protein: ligands, receptors and role in inflammation. Clin Immunol. 2005;117:104–111. doi: 10.1016/j.clim.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Deodhar SD. C-reactive protein: the best laboratory indicator available for monitoring disease activity. Cleve Clin J Med. 1989;56:126–130. doi: 10.3949/ccjm.56.2.126. [DOI] [PubMed] [Google Scholar]
- 14.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 15.Jialal I, Devaraj S, Venugopal SK. C-reactive protein: risk marker or mediator in atherothrombosis? Hypertension. 2004;44:6–11. doi: 10.1161/01.HYP.0000130484.20501.df. [DOI] [PubMed] [Google Scholar]
- 16.Devaraj S, Singh U, Jialal I. The evolving role of C-reactive protein in atherothrombosis. Clin Chem. 2009;55:229–238. doi: 10.1373/clinchem.2008.108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kushner I, Rzewnicki D, Samols D. What does minor elevation of C-reactive protein signify? Am J Med. 2006;119(166):e17–e28. doi: 10.1016/j.amjmed.2005.06.057. [DOI] [PubMed] [Google Scholar]
- 18.Heikkilä K, Ebrahim S, Lawlor DA. A systematic review of the association between circulating concentrations of C reactive protein and cancer. J Epidemiol Community Health. 2007;61:824–833. doi: 10.1136/jech.2006.051292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang CS, Sun CF. C-reactive protein and malignancy: clinico-pathological association and therapeutic implication. Chang Gung Med J. 2009;32:471–482. [PubMed] [Google Scholar]
- 20.Woeste G, Müller C, Bechstein WO, Wullstein C. Increased serum levels of C-reactive protein precede anastomotic leakage in colorectal surgery. World J Surg. 2010;34:140–146. doi: 10.1007/s00268-009-0304-z. [DOI] [PubMed] [Google Scholar]
- 21.Haupt W, Hohenberger W, Mueller R, Klein P, Christou NV. Association between preoperative acute phase response and postoperative complications. Eur J Surg. 1997;163:39–44. [PubMed] [Google Scholar]
- 22.Pfitzner T, Krocker D, Perka C, Matziolis G. C-reactive protein. An independent risk factor for the development of infection after primary arthroplasty. Orthopade. 2008;37:1116–1120. doi: 10.1007/s00132-008-1342-1. [DOI] [PubMed] [Google Scholar]
- 23.Jeys LM, Grimer RJ, Carter SR, Tillman RM. Periprosthetic infection in patients treated for an orthopaedic oncological condition. J Bone Joint Surg Am. 2005;87:842–849. doi: 10.2106/JBJS.C.01222. [DOI] [PubMed] [Google Scholar]
- 24.Piper KE, Fernandez-Sampedro M, Steckelberg KE, et al. C-reactive protein, erythrocyte sedimentation rate and orthopedic implant infection. PLoS ONE. 2010;5:e9358. doi: 10.1371/journal.pone.0009358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salzer-Kuntschik M, Brand G, Delling G. Determination of the degree of morphological regression following chemotherapy in malignant bone tumors. Pathologe. 1983;4:135–141. [PubMed] [Google Scholar]
- 26.Kim MS, Lee SY, Lee TR, et al. Prognostic nomogram for predicting the 5-year probability of developing metastasis after neo-adjuvant chemotherapy and definitive surgery for AJCC stage II extremity osteosarcoma. Ann Oncol. 2009;20:955–960. doi: 10.1093/annonc/mdn723. [DOI] [PubMed] [Google Scholar]
- 27.Kager L, Zoubek A, Kastner U, et al. Skip metastases in osteosarcoma: experience of the Cooperative Osteosarcoma Study Group. J Clin Oncol. 2006;24:1535–1541. doi: 10.1200/JCO.2005.04.2978. [DOI] [PubMed] [Google Scholar]
- 28.Kim MS, Lee SY, Cho WH, et al. Tumor necrosis rate adjusted by tumor volume change is a better predictor of survival of localized osteosarcoma patients. Ann Surg Oncol. 2008;15:906–914. doi: 10.1245/s10434-007-9779-8. [DOI] [PubMed] [Google Scholar]
- 29.Bramer JA, Abudu AA, Grimer RJ, Carter SR, Tillman RM. Do pathological fractures influence survival and local recurrence rate in bony sarcomas? Eur J Cancer. 2007;43:1944–1951. doi: 10.1016/j.ejca.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Kim MS, Lee SY, Lee TR, et al. Prognostic effect of pathologic fracture in localized osteosarcoma: a cohort/case controlled study at a single institute. J Surg Oncol. 2009;100:233–239. doi: 10.1002/jso.21265. [DOI] [PubMed] [Google Scholar]
- 31.Nedelcu T, Kubista B, Koller A, et al. Livin and Bcl-2 expression in high-grade osteosarcoma. J Cancer Res Clin Oncol. 2008;134:237–244. doi: 10.1007/s00432-007-0276-z. [DOI] [PubMed] [Google Scholar]
- 32.Trieb K, Lehner R, Stulnig T, Sulzbacher I, Shroyer KR. Survivin expression in human osteosarcoma is a marker for survival. Eur J Surg Oncol. 2003;29:379–382. doi: 10.1053/ejso.2002.1415. [DOI] [PubMed] [Google Scholar]
- 33.Trieb K, Gerth R, Holzer G, Grohs JG, Berger P, Kotz R. Antibodies to heat shock protein 90 in osteosarcoma patients correlate with response to neoadjuvant chemotherapy. Br J Cancer. 2000;82:85–87. doi: 10.1054/bjoc.1999.0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nozoe T, Saeki H, Sugimachi K. Significance of preoperative elevation of serum C-reactive protein as an indicator of prognosis in esophageal carcinoma. Am J Surg. 2001;182:197–201. doi: 10.1016/S0002-9610(01)00684-5. [DOI] [PubMed] [Google Scholar]
- 35.Hefler LA, Concin N, Hofstetter G, et al. Serum C-reactive protein as independent prognostic variable in patients with ovarian cancer. Clin Cancer Res. 2008;14:710–714. doi: 10.1158/1078-0432.CCR-07-1044. [DOI] [PubMed] [Google Scholar]